Abstract
Chronic infection with hepatitis B virus (HBV) is a major risk factor for the development of hepatocellular carcinoma (HCC). The pathogenesis of HBV-associated HCC involves both viral and host factors. The latter include a functionally inefficient CD8+ T-cell response that fails to clear the infection from the liver but sustains a chronic necroinflammatory process that contributes to the development of HCC. According to this scenario, amelioration of immune-mediated chronic liver injury may prevent HCC. Because platelets facilitate immune-mediated liver injury by promoting the hepatic accumulation of virus-specific CD8+ T cells, we evaluated the long-term consequences of antiplatelet therapy in an HBV transgenic mouse model of chronic immune-mediated necroinflammatory liver disease that progresses to HCC. Treatment with aspirin and clopidogrel during the chronic phase of the disease diminished the number of intrahepatic HBV-specific CD8+ T cells and HBV-nonspecific inflammatory cells, the severity of liver fibrosis, and the development of HCC. Antiplatelet therapy improved overall survival without causing significant side effects. In contrast, the same antiplatelet regimen had no antitumor effect when HCC was induced nonimmunologically by chronic exposure to a hepatotoxic chemical. The unprecedented observation that antiplatelet therapy inhibits or delays immune-mediated hepatocarcinogenesis suggests that platelets may be key players in the pathogenesis of HBV-associated liver cancer and supports the notion that immune-mediated necroinflammatory reactions are an important cause of hepatocellular transformation during chronic hepatitis.
Keywords: antiplatelet drugs, viral hepatitis, CD8 T cells
Hepatocellular carcinoma (HCC) is the fifth most frequently diagnosed cancer and the third most common cause of death from cancer worldwide (1). Over 50% of HCC cases are attributable to underlying chronic hepatitis B infection (1). Although the potential mechanisms whereby the hepatitis B virus (HBV) causes HCC include viral factors (e.g., insertional mutagenesis, expression of viral gene products with transforming potential), host factors associated with the antiviral immune response are thought to play a significant role (2). Indeed, almost all cases of HCC occur after decades of immune-mediated chronic hepatitis, which is characterized by a functionally inefficient virus-specific CD8+ T-cell response that fails to eliminate HBV from the liver (3). In this situation, the virus-specific CD8+ T-cell response appears to maintain continuous cycles of low-level hepatocellular injury both directly [through the killing of hepatocytes expressing viral antigens (4)] and indirectly [through the hepatic recruitment of virus-nonspecific inflammatory cells exacerbating pathology (5, 6)]. This process promotes hepatocellular proliferation and exposes proliferating hepatocytes to inflammatory mutagens (3). Over time, repetitive cycles of immune-mediated hepatocellular necrosis, regeneration, and inflammation are thought to trigger the multiple genetic alterations that typify HCC (3).
In mouse models of CD8+ T cell-mediated acute viral hepatitis, we have previously shown that platelets are present at sites of tissue damage and that platelet depletion ameliorates disease severity by reducing the accumulation of hepatic virus-specific CD8+ T cells (7). In the ongoing effort to explain mechanistically why platelets are required to support CD8+ T cell-induced liver pathology, we found that this process is influenced by two specific inhibitors of platelet activation pathways: aspirin (Asp), which blocks thromboxane (TX) A2 production, and clopidogrel (Clo), which blocks the P2Y12 ADP receptor (8). Indeed, treating mice with Asp, Clo, or a combination of the two (Asp/Clo) attenuates acute liver injury by reducing the hepatic accumulation of virus-specific CD8+ T cells and virus-nonspecific inflammatory cells (9).
With the expectation that Asp and Clo may also blunt the hepatic accumulation of pathogenic immune cells under conditions of sustained liver injury, we adapted a mouse model of chronic immune-mediated hepatitis B that progresses to HCC (10, 11) to evaluate the impact of these drugs on hepatocarcinogenesis. We show here that antiplatelet therapy suppresses hepatic immunopathology and prevents or delays the development of HCC, as well as improving survival.
Results
Study Design and Platelet Response to Asp and/or Clo.
The HBV transgenic mouse model of chronic hepatitis used herein has been described (10) and is summarized in Materials and Methods and SI Materials and Methods. Groups of hepatitis B surface antigen (HBsAg)-expressing transgenic mice (lineage 107-5) that had been reconstituted ∼30 d earlier with an HBsAg-primed WT immune system and displayed histological evidence of chronic hepatitis (10) were treated orally (as described in Materials and Methods) with one of the following: (i) diluents (Vehicle), (ii) Asp, (iii) Clo, or (iv) the latter two combined (Asp/Clo) (Fig. S1). Age- and sex-matched lineage 107-5 transgenic mice that were reconstituted with an HBsAg-tolerant transgenic immune system (Sham) and unmanipulated control (Ctrl) mice were included in the study (Fig. S1); a total of 540 animals were studied, including 110 (Ctrl), 110 (Vehicle), 110 (Sham), 50 (Asp), 50 (Clo), and 110 Asp/Clo) mice (Fig. S2 A and B). Three days after initiation of Asp, Clo, or Asp/Clo treatment (and at monthly intervals thereafter), Asp-treated mice showed reduced platelet production of TX-A2 (measured as the stable inactive metabolite TX-B2) and Clo-treated mice displayed impaired ADP-induced platelet aggregation (Fig. S2C).
Treatment with Asp, Clo, or Asp/Clo Suppresses Hepatic Immunopathology.
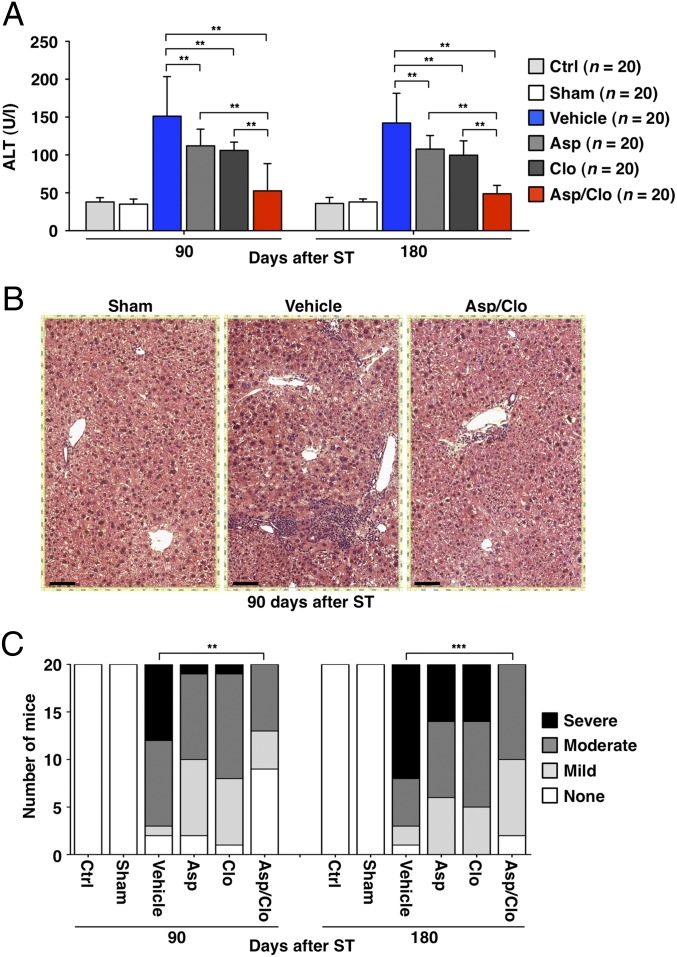
By 90 or 180 d after spleen transfer (ST) (i.e., before the appearance of HCC), mice that had been treated with Asp, Clo, or Asp/Clo displayed serum alanine aminotransferase (ALT) activity lower than that observed in Vehicle-treated mice (Fig. 1A); these differences were reflected in histological differences in the corresponding livers. Livers harvested from Vehicle-treated mice at day 90 revealed abundant portal inflammatory infiltrates, which were much less prominent in antiplatelet-treated mice, particularly in animals receiving Asp/Clo (Fig. 1B). Portal inflammation and other parameters of chronic hepatitis were used to produce an Ishak-based (12) scoring system that cumulatively graded liver necroinflammatory activity (Materials and Methods). The analysis indicated that the number of Vehicle-treated mice displaying signs of severe liver injury increased with time and significantly exceeded that of animals receiving dual antiplatelet therapy (Fig. 1C). Of note, neither Vehicle-treated mice nor Asp-, Clo-, or Asp/Clo-treated mice showed evidence of liver infarction, and the detection of platelet aggregates in these livers was confined within necroinflammatory foci.
Fig. 1.
Treatment with Asp, Clo, or Asp/Clo suppresses hepatic immunopathology. (A) Mean serum ALT activity (U/L ± SD, 20 mice per group per time point) was measured at the indicated time points in groups of mice that received the indicated treatment. Vehicle-treated mice showed serum ALT activity approximately threefold above that detected in Ctrl or Sham-treated mice. Compared with the former, mice treated with Asp, Clo, or Asp/Clo showed a reduction in serum ALT activity of ∼25%, ∼30%, and ∼65%, respectively. (B) Micrographs of representative histological liver preparations from the indicated groups of mice at day 90 after ST are shown. H&E staining. (Scale bar = 150 μm.) (C) Livers from the indicated groups of mice were graded histologically (Materials and Methods) at the indicated time points (20 livers per group per time point) to express the relative number of mice per group that had severe, moderate, or mild liver disease. The number of mice displaying signs of severe liver injury at days 90 and 180, respectively, was as follows: 0 of 20 (0%) and 0 of 20 (0%) Ctrl, Sham-treated, or Asp/Clo-treated mice; 7 of 20 (35%) and 12 of 20 (60%) Vehicle-treated mice; and 1 of 20 (5%) and 6 of 20 (30%) Asp- or Clo-treated mice. The statistical significance of differences in the assays shown in A and C is indicated (**P < 0.001; ***P < 0.0001).
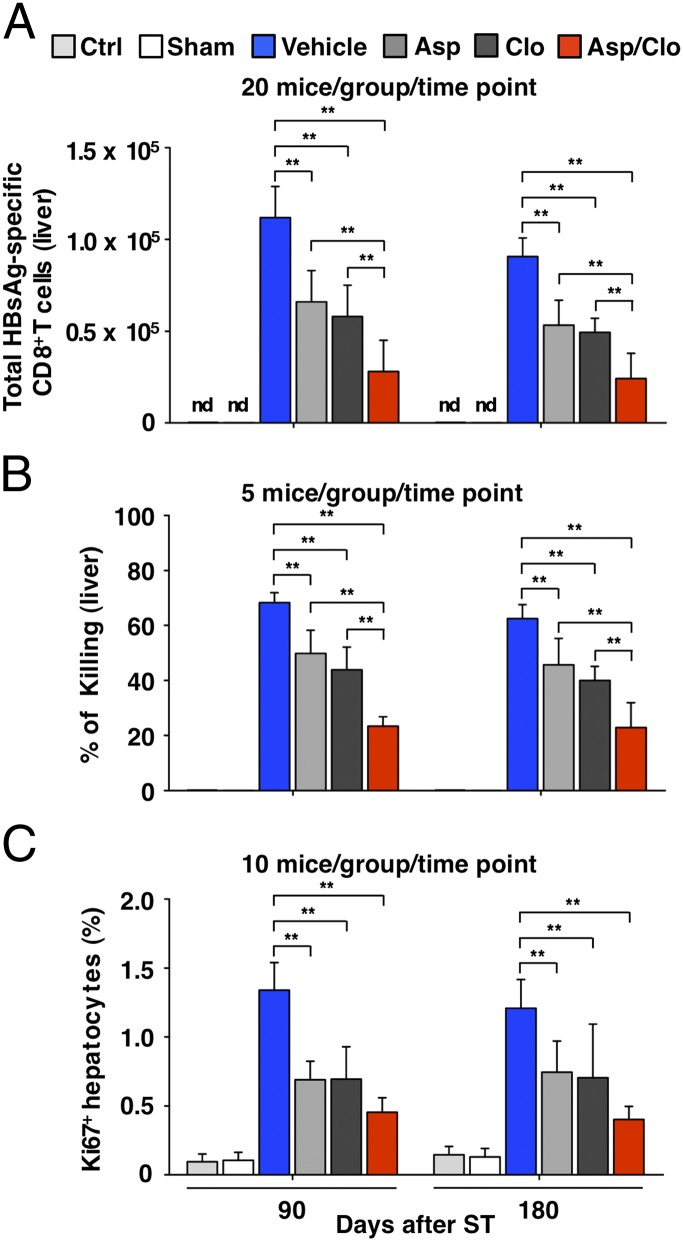
Next, we asked whether antiplatelet treatment could influence the pathogenic potential of the HBsAg-specific CD8+ T cells that trigger liver disease in this model (10, 11). At both day 90 and day 180, the absolute number of hepatic HBsAg-specific CD8+ T cells (Fig. 2A) and antigen-nonspecific inflammatory cells they recruit (Fig. S3) was lower in mice that received antiplatelet drugs compared with Vehicle-treated mice, and the relative values reflected the serum ALT activity in those animals (compare with Fig. 1A). In contrast, the capacity of the CD8+ T cells to kill target cells in vivo (SI Materials and Methods) was comparable in the different groups of mice (Fig. 2B); that is, it paralleled the serum ALT activity (Fig. 1A) and the number of intrahepatic CD8+ T cells (Fig. 2A) in each group. Importantly, virtually no HBsAg-specific CD8+ T cells were detected in the spleen or in liver-draining (portal) lymph nodes (13) of mice receiving vehicle or antiplatelet drugs, suggesting that the rejection of target cells in these animals occurred within the liver. In accordance with the diminished number of hepatic HBsAg-specific CD8+ T cells and the diminished liver injury, the magnitude of compensatory hepatocellular regeneration (quantified by counting Ki67-labeled hepatocytes) was reduced in mice receiving antiplatelet treatment (Fig. 2C). Collectively, these results suggest that antiplatelet treatment reduced the number of HBsAg-specific CD8+ T cells and nonspecific inflammatory cells that accumulate in the liver but that it had no impact on their cytolytic activity. Because mice receiving Asp/Clo consistently showed the greatest difference in serum ALT activity, liver disease severity, liver CD8+ T-cell accumulation, and hepatocellular regeneration compared with Vehicle-treated mice, beyond the 180-d time point, we studied only the effect of Asp/Clo treatment.
Fig. 2.
Treatment with Asp, Clo, or Asp/Clo reduces the number but not the killing function of hepatic HBsAg-specific CD8+ T cells. (A) Assessment by dimer staining (SI Materials and Methods) of the absolute number of HBsAg-specific CD8+ T cells recovered from the liver of the same mice described in Fig. 1 (20 mice per group per time point). (B) Hepatic in vivo cytotoxicity assay of five mice from the indicated groups at the indicated time points (SI Materials and Methods). (C) Mean percentage (±SD) of Ki67+ hepatocytes detected in the liver of the same mice described in A (10 mice per group per time point). The statistical significance of differences in the assays shown in A and C is indicated (**P < 0.001). nd, not detectable.
Treatment with Asp/Clo Reduces the Severity of Liver Fibrosis.
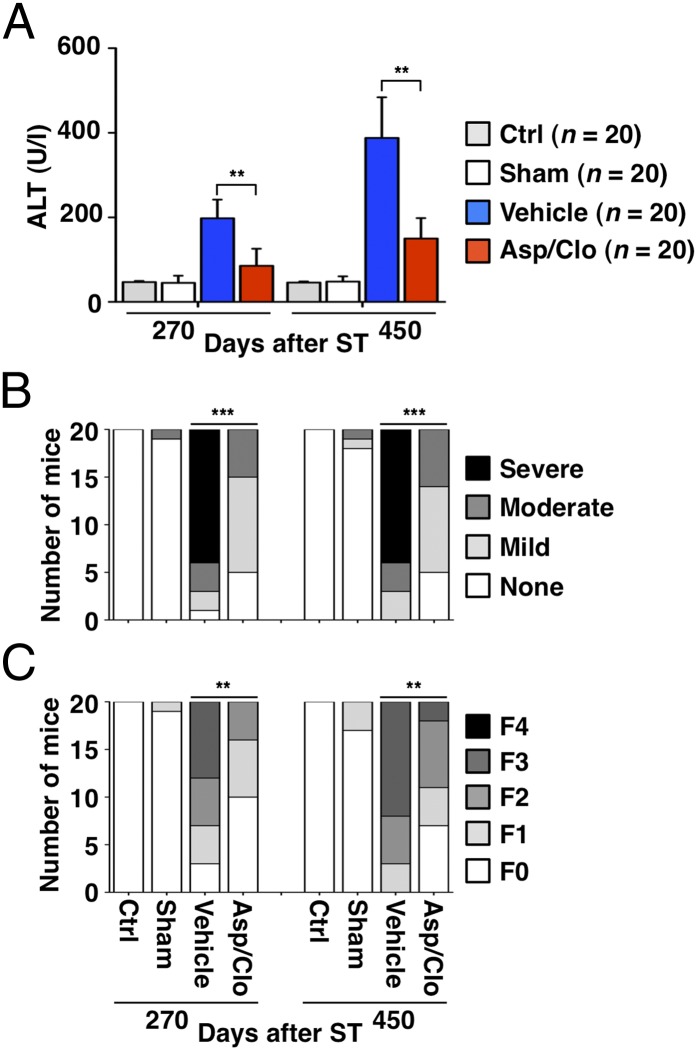
At day 270, serum ALT activity in Ctrl, Sham-, Vehicle-, and Asp/Clo-treated mice was similar to that detected at earlier time points (compare Figs. 3A and 1A). At day 450, serum ALT activity was approximately eightfold higher in Vehicle-treated compared with Sham-treated mice (Fig. 3A), likely reflecting the presence of tumors compressing normal liver parenchyma and/or becoming partly necrotic. The percentage of mice displaying signs of severe chronic hepatitis increased further on days 270 and 450 in Vehicle-treated mice but not in Asp/Clo-treated mice (Fig. 3B). Persistence of severe hepatitis in Vehicle-treated mice resulted in abundant liver deposition of collagen, which often formed fibrous septa bridging portal areas (Fig. S4). Treatment with Asp/Clo reduced collagen deposition, which, at worst, was associated with the appearance of short fibrous septa (Fig. S4). Staging liver fibrosis in all animals at both time points (SI Materials and Methods) indicated that none of the mice became cirrhotic (F4), but the percentage of Vehicle-treated mice progressing to bridging fibrosis (F3) was significantly higher than that of Asp/Clo-treated mice (Fig. 3C).
Fig. 3.
Treatment with Asp/Clo suppresses hepatic immunopathology and reduces the severity of liver fibrosis. (A) Mean serum ALT activity (U/L ± SD, 20 mice per group per time point) was measured at the indicated time points in mice that received the indicated treatment. (B) Relative number of mice per group displaying varying degrees of liver necroinflammatory activity is indicated. (C) Livers from the indicated groups (20 livers per group per time point) were staged histologically (Materials and Methods) at the indicated time points to express the number of mice per group with the following degrees of liver fibrosis: F0, no fibrosis; F1, fibrous expansion of some portal areas with or without short fibrous septa; F2, fibrous expansion of most portal areas with or without short fibrous septa; F3, bridging fibrosis without cirrhosis; and F4, cirrhosis with architectural distortion. This analysis revealed that 8 of 20 (40%) and 12 of 20 (60%) Vehicle-treated mice displayed signs of bridging fibrosis (F3) at day 270 or day 450, respectively. Bridging fibrosis was never detected in Ctrl or Sham-treated mice, and only 2 of 20 (10%) Asp/Clo-treated mice reached this stage at the later time point. The statistical significance of differences in the assays shown in A–C is indicated (**P < 0.001; ***P < 0.0001).
Treatment with Asp/Clo Prevents or Delays the Development of HCC.
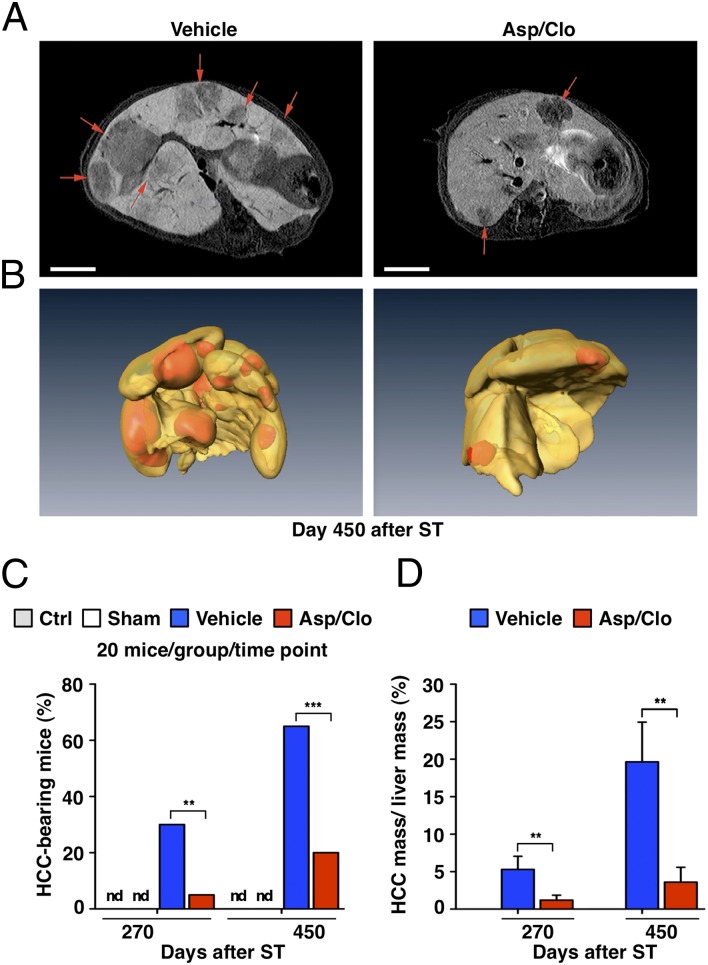
Shortly before being killed, mice from the Ctrl group as well as Sham-, Vehicle-, and Asp/Clo-treated groups were subjected to 7-T MRI (SI Materials and Methods). Regions of interest were identified (a representative example is shown in Fig. 4A and Movies S1 and S2) and serially sectioned at times of autopsy. In virtually all cases, MRI detected liver tumors (hepatomas) that, at autopsy, displayed the classic histological features of well-differentiated HCCs associated with chronic HBV infection (14), consistent with what has been previously described by Nakamoto et al. (10) in this experimental system. The hepatomas detected in both Vehicle- and Asp/Clo-treated groups were characterized by trabecular cords composed of several layers of neoplastic hepatocytes (Fig. S5A) usually expressing no HBsAg (Fig. S5B). Large HCCs (>7 mm in diameter), frequently detected in the liver of Vehicle-treated mice, also displayed neoplastic hepatocytes that were highly mitotic (Ki67+) and covered by a thin lining of CD34+ endothelial cells (Fig. S5 C and D). As mentioned earlier, the hepatomas compressed the adjacent hepatic parenchyma and often contained small areas of necrosis.
Fig. 4.
Treatment with Asp/Clo diminishes the incidence/mass of HCCs. (A) Contrast-enhanced liver MRI scans of representative tumor-bearing mice from the indicated groups at day 450 after ST are shown. Red arrows indicate hypointense regions identifying HCCs after i.v. administration of gadoxetic acid (Primovist; SI Materials and Methods). (Scale bar = 500 μm.) (B) Three-dimensional liver reconstructions from the same animals illustrate lesions (in orange-red) that were diagnosed (by MRI and histology) as bona fide HCCs. (C) Percentage of mice from the indicated groups bearing one or more HCCs at the indicated time points (a total of 20 mice per group per time point were analyzed) is shown. nd, not detectable. The percentage of mice bearing one or more HCCs at days 270 and 450 was 30% and 65%, respectively, in Vehicle-treated mice and 5% and 20%, respectively, in Asp/Clo-treated mice. (D) Mean percentage of the total HCC mass (calculated as % of the total liver mass ± SD) in all the Vehicle- or Asp/Clo-treated mice that bore one or more HCCs at day 270 (6 mice and 1 mouse, respectively) or day 450 (13 and 4 mice, respectively) after ST is shown. On average, the total HCC mass detected in Asp/Clo-treated mice was 3.8-fold and 5.4-fold smaller than that detected in Vehicle-treated mice on days 270 and 540, respectively. The statistical significance of differences between Vehicle- and Asp/Clo-treated mice in C and D is indicated (**P < 0.001; ***P < 0.0001). nd, not detectable.
None of the Ctrl or Sham-treated mice displayed evidence of HCC at days 270 and 450. In contrast, at the same time points, as many as 30% and 65% of Vehicle-treated mice had one or more hepatomas, which were detectable in only 5% and 20% of Asp/Clo-treated mice, respectively (Fig. 4C), indicating that hepatomas developed in significantly fewer antiplatelet-treated mice than Vehicle-treated mice. We also compared the overall mass of the hepatomas (a parameter encompassing both the number and the size of tumors per liver) in Vehicle- and Asp/Clo-treated mice with cancerous lesions. By calculating the volume of lesions diagnosed histologically as bona fide HCCs at days 270 and 450, we found that the tumor mass in Asp/Clo-treated mice (expressed as percentage of total liver mass) was significantly lower than in Vehicle-treated mice (Fig. 4D), suggesting that tumor development was delayed in those animals. An example of 3D liver reconstructions depicting number and size of HCCs in representative 450-d Vehicle- or Asp/Clo-treated mice is provided in Fig. 4B and Movies S3 and S4.
Treatment with Asp/Clo Improves Survival.
Within 510 d of ST, 75% of Vehicle-treated mice (15 of 20) were found dead (Fig. 5 and Table 1). In 7 of those animals, liver tissue was harvested before advanced autolysis occurred. Postmortem examination revealed that 100% of these animals showed the presence of numerous large HCCs (Fig. S6 A–D and Table 1). The same was observed in the remaining 5 live animals that were euthanized at day 520 for ethical reasons, because they all showed signs of cachexia. At this time point, only 20% of the Asp/Clo-treated mice (4 of 20) had died (Fig. 5 and Table 1) and none of the survivors appeared cachectic. With the exception of a single Asp/Clo-treated mouse that died by day 550 (Table 1), the remaining 15 Asp/Clo-treated animals survived until day 600 (Fig. 5 and Table 1), when they were euthanized. Two-thirds of these animals (10 of 15) were free of HCC (Table 1), and the remaining mice (5 of 15) had relatively few small HCCs (Fig. S6 C and D and Table 1).
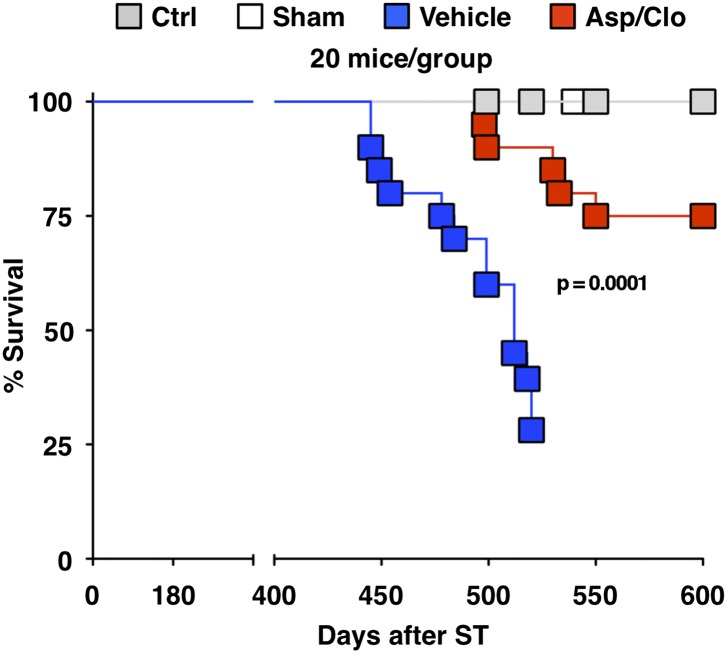
Fig. 5.
Treatment with Asp/Clo improves survival. Kaplan–Meier survival curves of the indicated groups of mice (20 mice per group) are shown. The statistical significance of differences between Vehicle- and Asp/Clo-treated mice is indicated. At day 520, five surviving Vehicle-treated mice showing severe cachexia and lethargy were killed for humanitarian reasons. At day 600, Asp/Clo-treated mice were euthanized to avoid age-related deaths (by this time, the animals were about 2 y old).
Table 1.
Incidence, number, and histology of liver tumors in mice followed for survival
| Group | Mouse ID no. | Days after ST | Liver tumor | No. of tumors | Largest tumor, mm | Tumor histology |
| Ctrl | 1–20 | 600 | − | |||
| Sham | 1–20 | 600 | − | |||
| Vehicle | 1–8 | 445–499* | Unev. | |||
| 9 | 510* | + | >10 | 8 | HCC | |
| 10 | 510* | + | >10 | 11 | HCC | |
| 11 | 510* | + | >10 | 21 | HCC | |
| 12 | 510* | + | >10 | 18 | HCC | |
| 13 | 510* | + | >10 | 14 | HCC | |
| 14 | 510* | + | >10 | 23 | HCC | |
| 15 | 510* | + | >10 | 11 | HCC | |
| 16 | 520 | + | >10 | 9 | HCC | |
| 17 | 520 | + | >10 | 14 | HCC | |
| 18 | 520 | + | >10 | 8 | HCC | |
| 19 | 520 | + | >10 | 19 | HCC | |
| 20 | 520 | + | >10 | 10 | HCC | |
| Asp/Clo | 1 | 499* | Unev. | |||
| 2 | 498* | Unev. | ||||
| 3 | 498* | Unev. | ||||
| 4 | 499* | Unev. | ||||
| 5 | 550* | Unev. | ||||
| 6 | 600 | − | ||||
| 7 | 600 | − | ||||
| 8 | 600 | − | ||||
| 9 | 600 | − | ||||
| 10 | 600 | + | 2 | 3 | HCC | |
| 11 | 600 | − | ||||
| 12 | 600 | − | ||||
| 13 | 600 | + | 3 | 4 | HCC | |
| 14 | 600 | − | ||||
| 15 | 600 | + | 1 | 3 | HCC | |
| 16 | 600 | − | ||||
| 17 | 600 | − | ||||
| 18 | 600 | + | 3 | 2 | HCC | |
| 19 | 600 | + | 1 | 2 | HCC | |
| 20 | 600 | − |
Unev., unevaluable.
*Found dead.
Treatment with Asp/Clo Does Not Induce Bleeding Complications.
Blood and fecal samples were collected monthly from Ctrl mice as well as from Sham-, Vehicle-, and Asp/Clo-treated mice. Hematocrit values and platelet counts remained within the respective normal range in all groups at all time points, consistent with the absence of fecal occult blood (FOB) (Fig. 6 A and B). In addition, there was no evidence of cutaneous petechiae (throughout the body) or hemorrhagic lesions (throughout the head, neck, and thoracic or abdominal cavities) in Asp/Clo-treated mice examined at autopsy, indicating that antiplatelet treatment did not lead to bleeding side effects in these animals.
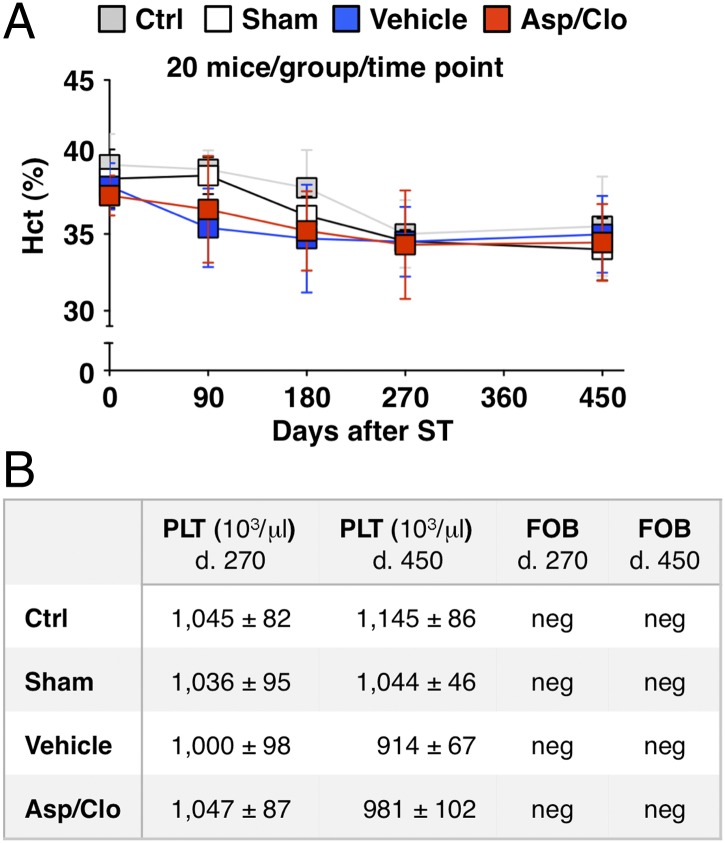
Fig. 6.
Treatment with Asp/Clo does not cause bleeding complications. Hematocrit (Hct) values (mean % ± SD) (A) and platelet counts (mean ± SD) and FOB (B) were assessed individually in the indicated groups of mice at the indicated time points (20 mice per group per time point). d., day; neg, negative.
Treatment with Asp/Clo Does Not Inhibit Carbon Tetrachloride-Induced Cancer Progression in Immunologically Unmanipulated Lineage 107-5 HBsAg Transgenic Mice.
To evaluate whether Asp/Clo treatment might have antitumor effects unrelated to its impact on CD8+ T cell-induced hepatic immunopathology, we studied its impact in a model of chemical hepatocarcinogenesis (15, 16). Starting when they were 8 wk old, male mice from lineage 107-5 were treated with vehicle or Asp/Clo as described above and gavaged (twice weekly for 16 wk) with the hepatotoxic agent carbon tetrachloride (CCl4) (SI Materials and Methods). By day 330 (∼30 wk after CCl4 cessation), the incidence and number of HCCs and the extent of fibrosis were comparable between the two groups of mice (Fig. 7), indicating that antiplatelet therapy was not protective in this setting.
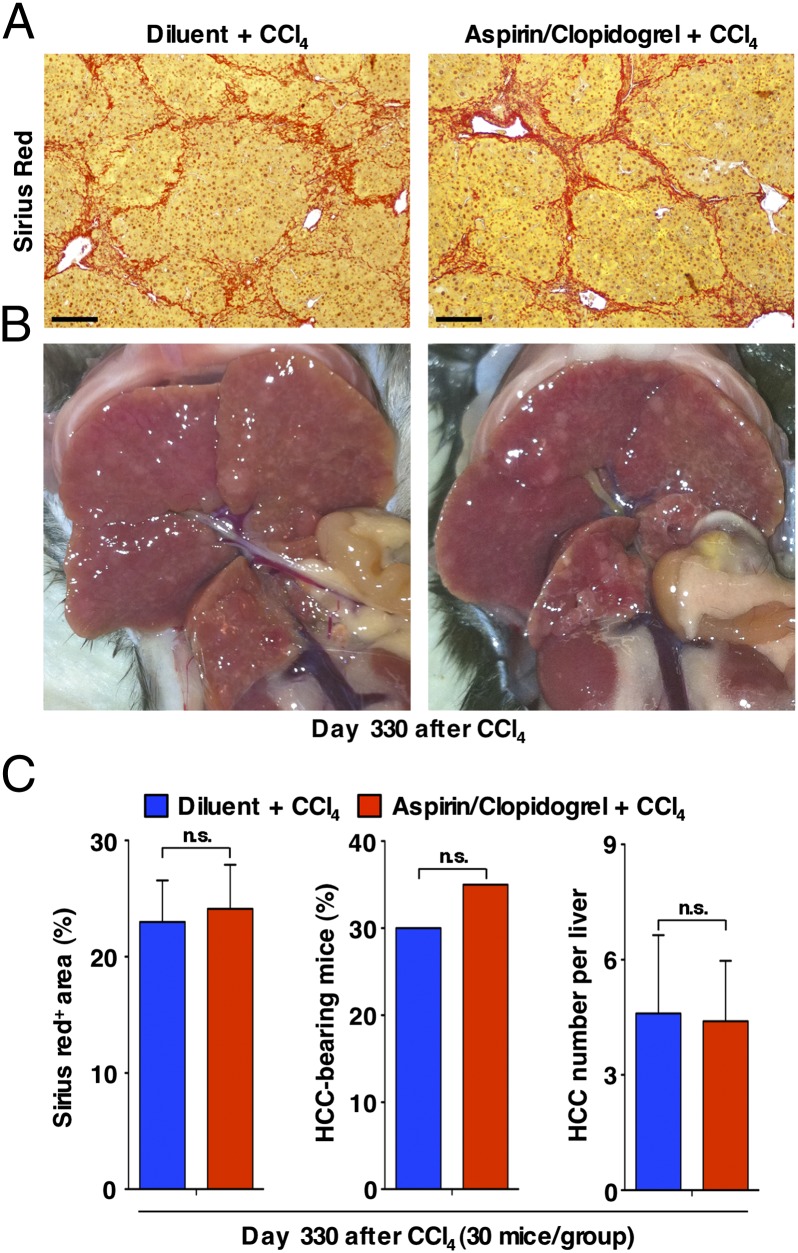
Fig. 7.
Treatment with Asp/Clo does not inhibit cancer progression in immunotolerant 107-5 mice exposed to CCl4. (A) Micrographs of representative livers from the indicated groups of mice that were killed at day 330 after CCl4 treatment initiation (SI Materials and Methods) are shown. Sirius Red staining is shown in red. (Scale bar = 150 μm.) (B) Photographs of representative livers (visceral view) from the same groups of mice described in A are shown. (C) Quantification of liver Sirius Red staining (Left; mean % of positive area ± SD), percentage of HCC-bearing mice (Center), and number of HCCs per liver (Right; mean number ± SD) in the same groups of mice described above (30 mice per group). The statistical significance of differences between Vehicle- and Asp/Clo-treated mice is indicated. ns, not significant.
Discussion
We demonstrate here a hitherto unrecognized role of platelets in the immunopathogenesis of HCC, a life-threatening complication of chronic HBV infection (1, 2). The pathogenesis of chronic HBV infection involves functionally inefficient HBV-specific CD8+ T cells that do not eradicate the infection but sustain repetitive cycles of immune-mediated hepatocellular necrosis, hepatocellular regeneration, and inflammation that are thought to precipitate random genetic damage and promote HCC development (3). Direct evidence linking CD8+ T cell-mediated liver immunopathology to hepatocellular transformation is provided by previous studies in which HBV transgenic mice that do not spontaneously develop liver injury develop HCC after many months of HBV-specific CD8+ T cell-induced chronic hepatitis (10, 11).
Although virus-specific CD8+ T cells appear to mediate most of the hepatocellular injury associated with HBV infection (17), other cells may participate in the process. The accumulation of virus-nonspecific CD8+ T cells in the liver of chronically infected patients has been linked to signs of active organ damage (6, 18, 19), and inhibiting the hepatic accumulation of virus-nonspecific CD8+ T cells and other virus-nonspecific inflammatory cells [i.e., CD4+ T cells, neutrophils, dendritic cells, monocytes, natural killer (NK) cells, NKT cells] in mouse models of acute viral hepatitis has been shown to reduce liver disease severity (5, 20–23). Of note, the recruitment process of virus-nonspecific inflammatory cells into the liver of these animals is different from that of virus-specific CD8+ T cells. The former largely relies on chemokines and damage-associated molecular pattern molecules whose hepatic expression/release follows hepatocellular antigen recognition by the virus-specific CD8+ T cells (5, 20–23), and the latter significantly depends on platelets. Indeed, using HBV transgenic mice as recipients of HBV-specific CD8+ T cells and mice acutely infected with adenovirus, we recently showed that platelets are detectable within CD8+ T cell-containing hepatic necroinflammatory foci and that their selective depletion profoundly ameliorates the severity of liver disease (7). This reduction in liver disease severity is associated with a proportional reduction in the hepatic accumulation of virus-specific CD8+ T cells, both of which are restored on reconstitution with normal platelets but not with platelets treated with inhibitors of platelet activation (7). The mechanisms by which platelets get recruited into the liver and facilitate the hepatic accumulation of virus-specific CD8+ T cells are poorly understood. One hypothesis is that an initial inflammatory response within the liver may result in changes of the vessel wall that promote platelet adhesion, platelet activation, and activation-dependent events, resulting in interaction with virus-specific CD8+ T cells; this interaction may facilitate virus-specific CD8+ T cells to egress from the bloodstream, enter the liver parenchyma, and perform pathogenic functions. Another nonmutually exclusive hypothesis is that activated platelets may favor CD8+ T-cell division. Along these lines, we have proposed that the activation-dependent expression of platelet CD40 ligand contributes to the expansion phase of virus-specific CD8+ T cells, resulting in their accumulation at sites of infection (24); this effect may reflect direct interaction of activated platelets with CD8+ T cells that express CD40 (25, 26). Others have indicated that platelet CD40 ligand has the potential to enhance virus-specific CD8+ T-cell responses indirectly, mostly by promoting the maturation of dendritic cells (27, 28). That activated platelets contribute to acute hepatocellular injury by enhancing the accumulation of virus-specific CD8+ T cells and, secondarily, virus-nonspecific inflammatory cells into the liver is further supported by experimental evidence indicating that these events are attenuated by the administration of two specific inhibitors of platelet activation (Asp and Clo) (9). Based on these observations, we reasoned that platelets might also contribute to liver immunopathology under conditions of sustained hepatocellular injury, and we took advantage of the previously mentioned HBV transgenic mouse model of chronic hepatitis that progresses to HCC to evaluate the long-term consequences of Asp and Clo administration (10, 11).
Our results demonstrate that clinically achievable doses of these drugs (8) administered continuously after the onset of chronic immune-mediated hepatitis could prevent or delay hepatocarcinogenesis and greatly improved overall survival in HBV transgenic mice. These outcomes were preceded by and associated with reduced hepatic accumulation of virus-specific CD8+ T cells and virus-nonspecific inflammatory cells, reduced hepatocellular injury and hepatocellular proliferation, and reduced severity of liver fibrosis. The results support the notion that a sustained immune-mediated necroinflammatory liver disease involving platelets is responsible for the development of HCC in this HBV transgenic mouse model, and they suggest that similar events may be responsible for the development of HCC in chronically infected patients as well.
The ability of Asp/Clo to inhibit the hepatic accumulation of HBV-specific CD8+ T cells in this model, as it does in models of acute viral hepatitis, may be sufficient to explain the results reported herein. Whether this effect depends on reduced hepatic recruitment of HBV-specific CD8+ T cells from lymphoid tissues and blood or whether it depends on other processes (e.g., T-cell expansion within or outside the liver) potentially affecting the overall accumulation of antigen-specific T cells into the liver is unclear. Of note, HBV-specific CD8+ T cells accumulated at detectable levels only in the liver (where the cognate antigen is easily measurable) and they were not detected in the spleen or in liver-draining lymph nodes (where the cognate antigen is not measurable) of mice receiving either Vehicle or Asp/Clo.
The observation that the liver of Vehicle-, Asp-, Clo-, or Asp/Clo-treated mice showed the presence of small platelet aggregates only within hepatic necroinflammatory foci and the absence of infarction contradicts the possibility that antiplatelet therapy suppresses liver disease by inhibiting the formation of vessel-occluding clots. This is consistent with what is observed in models of acute hepatitis, where vessel-occluding clots do not develop and warfarin-based anticoagulant therapy (preventing fibrin deposition) does not affect the capacity of virus-specific CD8+ T cells to cause liver damage (7).
Although the suppression of CD8+ T cell-induced hepatic immunopathology provides strong mechanistic evidence for the antitumor effect of antiplatelet therapy, other Asp/Clo-dependent processes might have been operative in our system, and they include the inhibition of platelet-derived factors that could support tumor growth independent of CD8+ T cells. Platelet factors, as such, are contained in both α-granules [e.g., FGF, EFG, hepatocyte growth factor, insulin-like growth factor, VEGF, PDGF (29)] and dense granules [e.g., serotonin (30)]. Dissecting the effect of Asp/Clo on all these factors in our system is beyond the scope of this report. It is worth mentioning, however, that PDGF and serotonin have been reported to support HCC development in mice exposed to diethylnitrosamine (31) or to CCl4 (32), respectively, two situations in which carcinogenesis is independent of adaptive immune responses and, as shown here for CCl4-treated mice, might not be influenced by antiplatelet therapy. Although indirectly, this may argue against the hypothesis that Asp/Clo-dependent amelioration of HCC in mice with immune-mediated chronic hepatitis reflects inhibition of PDGF- and serotonin-dependent pathways. The lack of efficacy of antiplatelet therapy in CCl4-treated mice is also an argument against the possibility that other platelet-derived factors potentially involved in HCC development may be targets of Asp/Clo treatment. These considerations should be taken cautiously, however, because the process of hepatocarcinogenesis in mice undergoing immune-mediated liver disease and in mice treated with CCl4 differs substantially, possibly beyond the role of adaptive immune responses.
As per the possibility that antiplatelet therapy might have prevented/delayed HCC development by inhibiting inflammatory events that are independent of platelets, it is relevant to note that although Clo is highly specific for platelets [i.e., it inactivates the ADP-receptor P2Y12, which is present on these cells and megakaryocytes (8)], Asp is not [i.e., it inhibits TX production by altering the activity of both COX-1 and COX-2 (8), with the latter enzyme being absent in platelets but present and inducible in a variety of cell types (33)]. When administered at the low dose used herein, however, Asp has almost exclusively platelet-specific effects with minimal antiinflammatory or analgesic/antipyretic properties (8); in keeping with this, we found that hepatic COX-2 expression and its relative activity were not altered in mice treated with dual antiplatelet therapy.
It is also relevant to note that Asp alone, at a dosage known to inhibit the release of serotonin and other small molecules markedly from dense granules, has no effect on the release of α-granule content, where proteins and peptides are stored (34). This is relevant in relation to the potential role of platelet-derived proteins in the functional cross-talk with lymphocytes; for example, CD40 ligand translocates to the platelet membrane surface in association with the activation-induced α-granule release reaction (35). Unlike Asp, Clo has been found by different investigators to down-regulate the expression of markers of inflammation, including CD40 ligand, on the surface of activated platelets (36), an effect through which it inhibits heterotypic platelet-leukocyte interactions linking vascular injury to inflammation (37). Therefore, the beneficial effect of dual antiplatelet therapy in ameliorating the course of immune-mediated chronic hepatitis may involve distinct pharmacological effects of the two administered drugs (8). In synergy, they may reduce platelet contribution to a complex pathogenic mechanism in which multiple pathways modulate virus-specific CD8+ T-cell accumulation in the liver.
In conclusion, we show here that the antiplatelet drugs Asp and Clo effectively prevent or delay HCC and improve survival in a mouse model of chronic immune-mediated hepatitis B. These results identify platelets as potentially key players in the pathogenesis of HBV-associated liver cancer, and they reinforce the hypothesis that the cellular immune response is sufficient to induce liver cancer during chronic viral hepatitis. With the limitation that our study could not assess the impact of antiplatelet therapy on viral replication, the results also suggest that drugs targeting platelet function may be a therapeutic option in patients with chronic HBV infection. A concern about such a treatment could be increasing bleeding risk in individuals with compromised coagulation associated with impaired liver function. However, as pointed out in a recent review (38), excessive bleeding that is prevalent in the advanced stage of liver cirrhosis could be a lesser risk than thrombosis in some patients with chronic liver disease. Of note, at present, no relevant information is available on the possibility that cardiovascular patients chronically infected with HBV and receiving long-term antiplatelet therapy (either Asp monotherapy or dual therapy with Asp and Clo) are protected against HCC development. Future epidemiological studies aimed at assessing whether antiplatelet therapy prevents HCC are certainly warranted.
Materials and Methods
Disease Models.
The mouse models of immune-mediated or CCl4-induced chronic hepatitis have been reported previously (10, 39), and they are summarized in SI Materials and Methods. Twice a week, all mice from both groups were subjected to clinical examination by a board-certified veterinarian (M.M.); according to the approved Permit No. 358 from the Animal Review Board of the San Raffaele Scientific Institute, they were removed from the study when signs of excessive pain/distress (i.e., cachexia characterized by weigh loss and lethargy) became apparent.
Treatments with Asp, Clo, Asp/Clo, or Diluents.
Mice that were transferred with HBsAg-primed spleen cells and that showed comparable serum ALT profiles during the initial 30-d flare of hepatitis were assigned to receive Asp, Clo, Asp/Clo, or diluents (Vehicle). Asp (Sigma–Aldrich) was administered (alone or in combination with Clo) through food pellets containing 5 mg of the drug per kilogram of food (Mucedola S.R.L.). This dose was chosen based on the actual food intake of our animals (∼6 g/d for a 30-g mouse), such that the cumulative daily dose of Asp was ∼1 mg/kg of body weight. Clo bisulfate (Sanofi–Aventis) was dissolved in 0.003% HCl and administered (alone or in combination with Asp) through drinking water containing 10 μg of the drug per milliliter of water. This dose was chosen based on the actual water intake of our animals (∼3 mL/d for a 30-g mouse), such that the cumulative daily dose of Clo was ∼1 mg/kg of body weight. Vehicle-treated mice received identical food pellets not containing Asp (Mucedola S.R.L.) and drinking water supplemented with identical amounts of the Clo-related diluent (0.003% HCl).
Blood Analyses and Liver Histopathology.
Blood analyses (e.g., serum ALT activity, complete cell counts, platelet aggregation, platelet TX release) and liver histopathology are described in SI Materials and Methods.
Analyses of the Cellular Immune Response.
Analyses of hepatic and splenic cellular immune responses are summarized in SI Materials and Methods.
Liver RNA Analyses.
Total liver RNA was isolated and analyzed by real-time PCR for the expression of COX-2 and GAPDH as described (23).
COX Activity Assays.
COX-1 and COX-2 activities were measured on liver extracts that were prepared and processed according to the instructions of the manufacturer of the COX Fluorescent Activity assay Kit (Cayman Chemical Company).
FOB.
FOB tests were performed using a Hemoccult Kit (Hemoccult SENSA; Beckman Coulter) according to the manufacturer’s instruction.
Statistical Analysis.
In all experiments, values are expressed as mean ± SD. Means between two groups were compared using a two-tailed t test. Means between three or more groups were compared using one-way ANOVA. Categorical variables were compared using a χ2 test. Bonferroni correction was applied to counteract the effect of multiple comparisons. Comparing four groups (Vehicle, Asp, Clo, and Asp/Clo) involved six different assessments; in this case, P values less than 0.0083 (0.05/6) were considered statistically significant. Kaplan–Meier survival curves were compared using the log-rank (Mantel–Cox) test. All statistical analyses were performed with Prism (version 5.0a; GraphPad Software).
Supplementary Material
Acknowledgments
We thank Y. Nakamoto and P. Marra for their help and expertise, as well as their assistance in setting up the surgical procedures and the 3D liver reconstructions, respectively. We also thank M. Isogawa, R. Pardi, and M. E. Bianchi for helpful discussions and B. Fiore, D. Covarello, T. Cataudella, and T. Canu for excellent technical assistance. This work was supported by National Institutes of Health Grants R01-AI40696 (to L.G.G.), R01-AI020001 (to F.V.C.), and R01-HL42846 (to Z.M.R.); Italian Association for Cancer Research Grants 4643 and 6278 (to L.G.G.); European Research Council Grant 250219 (to L.G.G.); Italian Ministry of Health Grant GR08.17 (to G.S.); and a Career Development Award from the Giovanni Armenise–Harvard Foundation (to M.I.).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 12854 (volume 109, number 32).
See Commentary on page 12840.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209182109/-/DCSupplemental.
References
- 1.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: Epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 2.Ganem D, Prince AM. Hepatitis B virus infection—Natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 4.Ando K, et al. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J Immunol. 1994;152:3245–3253. [PubMed] [Google Scholar]
- 5.Kakimi K, et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med. 2001;194:1755–1766. doi: 10.1084/jem.194.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maini MK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannacone M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo M. Aspirin and clopidogrel: Efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24:1980–1987. doi: 10.1161/01.ATV.0000145980.39477.a9. [DOI] [PubMed] [Google Scholar]
- 9.Iannacone M, Sitia G, Narvaiza I, Ruggeri ZM, Guidotti LG. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clin Vaccine Immunol. 2007;14:1532–1535. doi: 10.1128/CVI.00298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamoto Y, Suda T, Momoi T, Kaneko S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 2004;64:3326–3333. doi: 10.1158/0008-5472.can-03-3817. [DOI] [PubMed] [Google Scholar]
- 12.Ishak K, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 13.Barbier L, et al. Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology. 2009;49(Suppl):S61–S71. doi: 10.1002/hep.22930. [DOI] [PubMed] [Google Scholar]
- 15.Castro GD, Díaz Gómez MI, Castro JA. DNA bases attack by reactive metabolites produced during carbon tetrachloride biotransformation and promotion of liver microsomal lipid peroxidation. Res Commun Mol Pathol Pharmacol. 1997;95:253–258. [PubMed] [Google Scholar]
- 16.Farazi PA, et al. Differential impact of telomere dysfunction on initiation and progression of hepatocellular carcinoma. Cancer Res. 2003;63:5021–5027. [PubMed] [Google Scholar]
- 17.Thimme R, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res. 2003;60:61–66. doi: 10.1016/j.antiviral.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Bertoletti A, Maini MK, Ferrari C. The host-pathogen interaction during HBV infection: Immunological controversies. Antivir Ther. 2010;15(Suppl 3):15–24. doi: 10.3851/IMP1620. [DOI] [PubMed] [Google Scholar]
- 20.Sitia G, et al. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2002;99:13717–13722. doi: 10.1073/pnas.172521999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitia G, et al. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest. 2004;113:1158–1167. doi: 10.1172/JCI21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitia G, Iannacone M, Müller S, Bianchi ME, Guidotti LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol. 2007;81:100–107. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- 23.Sitia G, et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 2011;7:e1002061. doi: 10.1371/journal.ppat.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannacone M, et al. Platelets prevent IFN-alpha/beta-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc Natl Acad Sci USA. 2008;105:629–634. doi: 10.1073/pnas.0711200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 26.Meunier S, et al. Synergistic CD40 signaling on APCs and CD8 T cells drives efficient CD8 response and memory differentiation. J Leukoc Biol. 2012;91:859–869. doi: 10.1189/jlb.0611292. [DOI] [PubMed] [Google Scholar]
- 27.Elzey BD, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 28.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 29.Sabrkhany S, Griffioen AW, Oude Egbrink MG. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. 2011;1815:189–196. doi: 10.1016/j.bbcan.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Fanburg BL, Lee SL. A new role for an old molecule: Serotonin as a mitogen. Am J Physiol. 1997;272(Pt 1):L795–L806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- 31.Maass T, et al. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128:1259–1268. doi: 10.1002/ijc.25469. [DOI] [PubMed] [Google Scholar]
- 32.Soll C, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244–1254. doi: 10.1002/hep.23441. [DOI] [PubMed] [Google Scholar]
- 33.Reiter R, Resch U, Sinzinger H. Do human platelets express COX-2? Prostaglandins Leukot Essent Fatty Acids. 2001;64:299–305. doi: 10.1054/plef.2001.0276. [DOI] [PubMed] [Google Scholar]
- 34.Rinder CS, Student LA, Bonan JL, Rinder HM, Smith BR. Aspirin does not inhibit adenosine diphosphate-induced platelet alpha-granule release. Blood. 1993;82:505–512. [PubMed] [Google Scholar]
- 35.Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 36.Graff J, Harder S, Wahl O, Scheuermann EH, Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: Effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther. 2005;78:468–476. doi: 10.1016/j.clpt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Smyth SS, et al. Beta(3)-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: Correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–2507. doi: 10.1161/01.cir.103.20.2501. [DOI] [PubMed] [Google Scholar]
- 38.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 39.Jang JH, et al. Ischemic preconditioning and intermittent clamping confer protection against ischemic injury in the cirrhotic mouse liver. Liver Transpl. 2008;14:980–988. doi: 10.1002/lt.21467. [DOI] [PubMed] [Google Scholar]