Abstract
We used structural MRI and EEG to examine brain structure and function in typically developing children in Romania (n = 20), children exposed to institutional rearing (n = 29), and children previously exposed to institutional rearing but then randomized to a high-quality foster care intervention (n = 25). In so doing, we provide a unique evaluation of whether placement in an improved environment mitigates the effects of institutional rearing on neural structure, using data from the only existing randomized controlled trial of foster care for institutionalized children. Children enrolled in the Bucharest Early Intervention Project underwent a T1-weighted MRI protocol. Children with histories of institutional rearing had significantly smaller cortical gray matter volume than never-institutionalized children. Cortical white matter was no different for children placed in foster care than never-institutionalized children but was significantly smaller for children not randomized to foster care. We were also able to explain previously reported reductions in EEG α-power among institutionally reared children compared with children raised in families using these MRI data. As hypothesized, the association between institutionalization and EEG α-power was partially mediated by cortical white matter volume for children not randomized to foster care. The increase in white matter among children randomized to an improved rearing environment relative to children who remained in institutional care suggests the potential for developmental “catch up” in white matter growth, even following extreme environmental deprivation.
Keywords: neglect, brain development, early adversity, brain volume, early experience
A common societal response to orphaned or abandoned children is to rear such children in institutions (1, 2). UNICEF estimates that there are at least 8 million children who live in institutional settings. Institutional rearing of young children represents a severe form of early psychological and physical neglect, and as such, serves as a model system for understanding how early experience—or the lack of thereof—impacts brain and behavioral development.
In most forms of institutional rearing, the ratio of caregivers-to-children is low (e.g., in our sample ∼1:12), care is highly regimented, and caregiver investment in children is low (3). Children raised in institutions are more likely than children raised in families to have deficits in cognitive function (4, 5) and in language production and comprehension (6, 7). Relative to noninstitutionalized children, children reared in institutional settings experience a wide range of developmental problems including markedly elevated rates of attention-deficit/hyperactivity disorder and other forms of psychopathology (8–10) and difficulties with social functioning (11–13). These developmental difficulties are not unique among children exposed to institutionalization. Indeed, exposure to a wide range of adverse early environments—including physical and sexual abuse, neglect, domestic violence, and chronic poverty—also increases a child’s risk for psychopathology (14, 15), language delays (16), and reduced academic achievement (17). Thus, elucidating how institutionalization results in developmental deviations is important both for informing the care of children raised in these environments and for improving our understanding of typical and atypical child development.
One of the most likely explanations for the wide range of developmental problems observed among children exposed to institutional rearing is that the deprived environment of an institution does not provide adequate experience onto which to scaffold normal brain development (5, 18, 19). If true, we would expect to see differences in neural structure and function among children reared in institutions relative to those raised in the community. We examine this possibility in this article.
Effect of Institutionalization on Neural Structure.
Previous research has identified associations between early exposure to institutionalization and neural structure/function. In the English and Romanian Adoptees study, gray and white matter volumes were measured using structural MRI (9, 20). Significantly smaller white and gray matter volume was observed for 14 adolescents adopted from Romania to the United Kingdom (previously institutionalized) vs. 11 never-institutionalized adoptees from the United Kingdom. Both groups were ∼16 y of age at the time of imaging. This study additionally reported smaller uncorrected (for total brain volume) volume of the left hippocampus and larger volume in the right amygdala among previously institutionalized children (20). A second study (21) of 34 institutionalized children adopted into the United States (average age 8.4 y old) and 28 nonadopted children living in the United States with their birth families (average age 9.4 y old), did not replicate the finding of smaller hippocampal or larger amygdala volume across groups. However, the amygdala, relative to total brain volume, was larger for children adopted after 15 mo of age (21).
A third study (22) used diffusion tensor imaging to examine structural connectivity in seven children adopted from Romania and seven children born to families in to North America (22). Here, smaller whole brain, white, and gray matter volumes were observed among previously institutionalized children compared with controls (22). In addition, reduced apparent diffusion coefficients and fractional anisotropy was observed across all white mater tracts in previously institutionalized compared with never-institutionalized children, and most significantly in the left uncinate fasciculus, indicating a general compromise of white matter tract integrity.
In sum, across three samples of internationally adopted, previously institutionalized children, institutionalization was associated with differences in neural structure. However, the findings are not entirely consistent across studies regarding the specific structures or regions affected. This variation in findings across studies may be because of differences in sample composition and age of participants (adolescent versus middle childhood).
Effect of Institutionalization on Neural Function.
The Bucharest Early Intervention Project (BEIP) is the first randomized controlled trial (RCT) that compares foster care with continued institutional care. One-hundred and thirty-six children between the ages of 6 and 31 mo of age living in institutions in Bucharest, Romania were randomly assigned to either a foster care intervention (foster care group, FCG) or to remain in the institution (care-as-usual group, CAUG). These children have been followed prospectively and are the subject of the current research (23, 24).
In the BEIP study, neural function was assessed using the EEG, recording resting electrical activity at the scalp at entry to the study (mean age = 22 mo) and again at 30 mo and 42 mo as infants watched an attractive visual stimulus, and at 8 y of age during rest. EEG signal is commonly decomposed into frequency bands and compared across participants. The frequency bands most commonly used are δ, θ, α, β, and γ. Typical maturation has been associated with greater contribution of α-frequencies to the overall EEG signal (25). Because increases in α are observed globally across all scalp electrodes, these changes are likely driven by structural changes, such as increasing cortical white matter across development. In studies of adults, white matter integrity is associated with the contribution of α-power to the EEG signal (26). At entry to the study, children exposed to institutional rearing exhibited decreased α-power compared with never-institutionalized children (27). This pattern was interpreted as signifying developmental delay in neural functioning (25). At 8 y of age, after children who received the foster care intervention had been living with families for 5.5–7.5 y, a significant effect of age at placement emerged for the α-frequency band; that is, children placed younger showed greater improvment (28). These findings suggest developmental catch up in EEG α-power as a function of exposure to foster care.
Current Study.
We are unaware of previous research that has examined neural structure and function in a same sample of previously institutionalized children. More importantly, the extent to which the structural neural sequelae of institutionalization can be mitigated by placement in an improved environment has never been evaluated using a RCT design. We addressed this gap in the literature in the present report using data from the BEIP. We first examined the effect of institutionalization and foster care intervention on cortical and subcortical volume in a subsample of participants (n = 74) from the BEIP who completed an MRI assessment. Based on prior work, we hypothesized that children exposed to institutionalization would have smaller cortical gray and white matter volumes and larger amygdala volumes relative to children never exposed to institutionalization. We hypothesized that the foster care intervention would ameliorate some of these structural differences. Next, we examined whether differences in neural structure explained the effect of institutionalization on power in the α-band. Given previous associations between α-power and white matter integrity (26) and the consistently observed differences in α-power for children with and without exposure to institutionalization (27, 28), we hypothesized that differences in white matter volume explain the association between exposure to institutionalization and EEG α-power. (See Table S1 for characteristics of institutionalized children randomized to the roster care intervention or usual care.)
Results
Effect of Institutionalization on Neural Structures.
All analyses first report the unadjusted associations between institutionalization and neural structure followed by the associations adjusted for covariates. Analyses of the corpus callosum (CC) and subcortical structures additionally adjust for total brain volume (see Materials and Methods and Table S2 for a list of the average volume of all neural structures by group).
Total Cortical Gray Matter Volume.
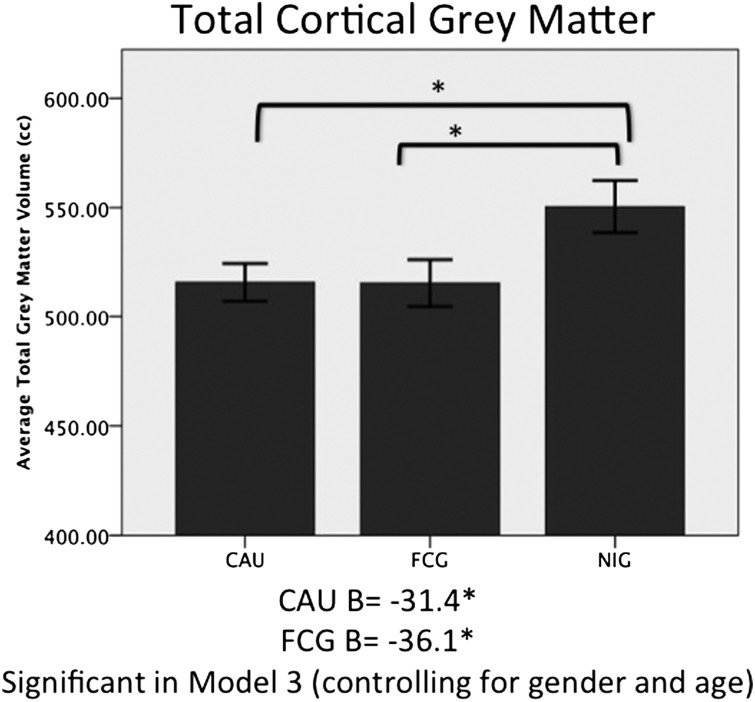
Children in the ever-institutionalized group (EIG, which includes both the CAUG and the FCG), had significantly smaller total cortical gray matter volumes than those in the never-institutionalized group (NIG) (B = −33.98, P = 0.01). When EIG children were separated into FCG and CAUG, both groups had significantly smaller total cortical gray matter volume compared with the NIG (CAUG B = −34.71, P = 0.02; FCG B = −35.05, P = 0.02). After adjustment for age and sex, children in the CAUG and FCG continued to have smaller total cortical gray matter volume than children in the NIG. Total gray matter volume was not different between the two groups of previously institutionalized children (Fig. 1 and Table 1).
Fig. 1.
Average total cortical gray matter volume in cubic centimeters (cm3) for the CAUG, FCG, and NIG; error bars are ± 1 SEM.
Table 1.
Association between institutionalization and total cortical volume
| Model 1 |
Model 2 |
Model 3 (adjusted for covariates)* |
|
| MRI total cortical volume | β (SE) | β (SE) | β (SE) |
| Gray matter | |||
| EIG | −33.9† (13.2) | ||
| CAU | −34.7† (14.8) | −31.4† (12.4) | |
| FCG | −35.1† (15.2) | −36.1† (12.9) | |
| White matter | |||
| EIG | −20.2 (11.6) | ||
| CAU | −27.7† (12.8) | −25.7† (11.4) | |
| FCG | −13.3 (13.3) | −17.1 (11.8) | |
*Covariates are age and sex.
†Significant at the 0.05 level, two-sided test.
Total White Matter Volume.
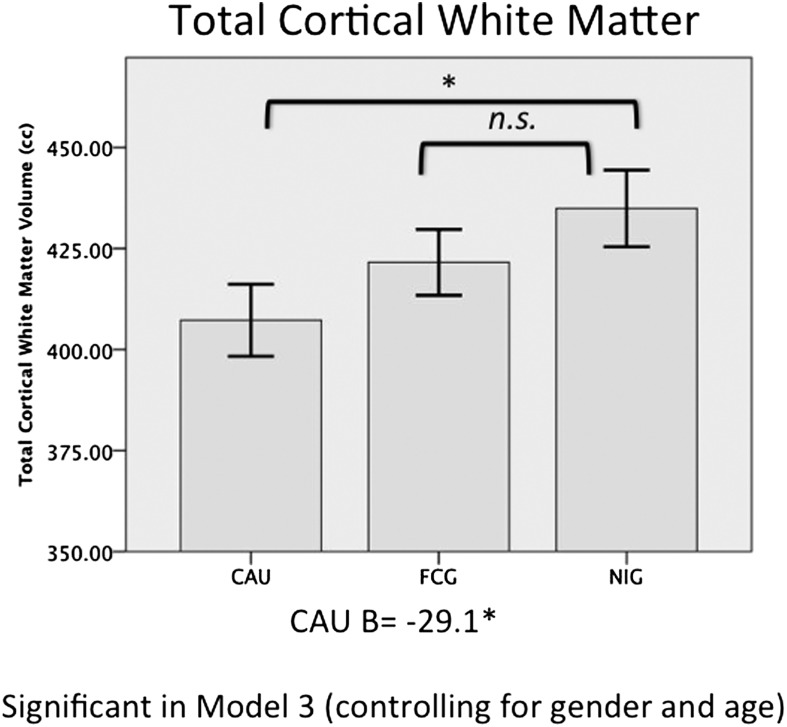
Children in the EIG had marginally significantly smaller total cortical white matter volumes than those in the NIG (B = −20.19, P = 0.08). When EIG children were separated into FCG and CAUG, only the CAUG had significantly smaller total cortical white matter volume compared with the NIG (CAUG B = −27.67, P = 0.04; FCG B = −13.3, P = 0.32). After adjustment for age and sex, membership in the CAUG significantly predicted smaller total cortical white matter volume compared with NIG (B = −25.74, P = 0.03). Children in the FCG, however, did not have smaller total cortical white matter volume compared with children in the NIG group (B = −17.1, P = 0.15; Fig. 2 and Table 1), The CAUG and FCG did not differ from each other in total cortical white matter volume.
Fig. 2.
Average total cortical white matter volume in cubic centimeters (cm3) for the CAUG, FCG, and NIG; error bars are ± 1 SEM.
Corpus Callosum.
The CC was subdivided into anterior, central, and posterior sections. It is common to divide the CC into anterior and posterior sections to reflect the fact that crossing fibers in the front of the brain reflect functionally different interhemispheric connectivity than crossing fibers in the back of the brain. EIG membership predicted smaller anterior CC volume (B = −0.09, P = 0.05). When EIG children were separated into FCG and CAUG, only the CAUG had significantly smaller anterior CC volume compared with the NIG (CAUG B = −0.115, P = 0.03; FCG B = −0.06, P = 0.22). After controlling for age, sex, and total brain volume, neither CAUG nor FCG remained significant predictors of anterior CC volume (Table 2). Central CC was not related to institutionalization in any model (Table 2).
Table 2.
Association between institutionalization and CC volume
| Model 1 |
Model 2 |
Model 3 (adjusted for covariates)* |
|
| MRI volume | β (SE) | β (SE) | β (SE) |
| Anterior CC | |||
| EIG | −0.09† (0.05) | ||
| CAU | −0.12† (0.05) | −0.07 (0.05) | |
| FCG | −0.07 (0.05) | −0.02 (0.05) | |
| Central CC | |||
| EIG | −0.04 (0.02) | ||
| CAU | −0.04 (0.03) | −0.04 (0.03) | |
| FCG | −0.04 (0.03) | −0.03 (0.03) | |
| Posterior CC | |||
| EIG | −0.09 (0.04) † | ||
| CAU | −0.14† (0.05) | −0.11† (0.05) | |
| FCG | −0.03 (0.05) | −0.01 (0.05) | |
*Covariates are age, sex, and total brain volume.
†Significant at the 0.05 level, two-sided test.
EIG membership significantly predicted smaller posterior CC volume (B = −0.09, P = 0.05). When EIG children were separated into FCG and CAUG, only the CAUG had significantly smaller posterior CC volume compared with the NIG (CAUG B = −0.14, P = 0.004; FCG B = −0.03, P = 0.56). After controlling for age, sex, and total brain volume, CAUG membership continued to predict smaller posterior CC volume compared with the NIG (CAUG B = −0.11, P = 0.02), whereas the FCG did not differ from either the NIG or the CAUG (FCG B = −0.01, P = 0.81) (Table 2 and Fig. S1).
Subcortical Structures.
In contrast to differences in total cortical gray matter and white matter volume, or white matter volume in the CC, institutionalization had little impact on subcortical structures after controlling for age, sex, and total brain volume (Table S3). See SI Materials and Methods for an analysis of the impact of institutionalization on amygdala volume (Fig. S2).
Effect of Institutionalization on α-Power.
EIG membership predicted lower α-power (B = −10.06, P = 0.02) compared with NIG children. When EIG children were separated into FCG and CAUG, membership in the both groups predicted lower α-power (CAUG B = −9.9, P = 0.03; FCG B = −10.3, P = 0.03). After controlling for age and sex, the CAUG and FCG continued to predict lower α-power than the NIG (Table 3).
Table 3.
Association between institutionalization and α-power before and after adjustment for white and gray matter
| Model 1 |
Model 2 |
Model 3 (adjusted for covariates)* |
Model 4 (adjusted for covariates and white matter)* |
Model 5 (adjusted for covariates and gray matter)* |
|
| Eyes open | β (SE) | β (SE) | β (SE) | β (CI) | β (CI) |
| EIG | −10.1† (4.0) | ||||
| CAUG | −9.9† (4.4) | −9.6† (4.3) | −2.28 (−6.9, −0.22)† | −0.32 (− 2.99, 0.63) | |
| FCG | −10.3† (4.5) | −8.7† (4.4) | −2.37 (− 6.9, −0.01)† | −1.38 (− 5.0, 0.025) |
CI, confidence interval.
*Covariates are sex and age.
†Significant at the 0.10 level, two-sided test.
Mediation Analysis.
To provide evidence for mediation, four criteria must be met. First, we reported significant associations between predictors (here FCG and CAUG membership) and the outcome of interest (EEG α-power). Second, we reported that FCG and CAUG membership were associated with smaller total cortical gray matter volume, and that membership in CAUG was associated with significantly smaller total cortical white matter volume. Third, we tested the association between the potential mediators and the outcome. To evaluate this criterion, we examined the association between total gray and white matter volume with α-power, controlling for sex and age. Total gray matter volume significantly and positively predicted α-power (B = 0.08, P = 0.04). Similarly, total white matter volume significantly and positively predicted α-power (B = 0.12, P = 0.007). The fourth criterion for mediation is that the association between the predictor (CAUG and FCG) and outcome (α-power) is significantly attenuated when the mediator (total gray or white matter cortical volume) is included in the model. In the final mediation models, associations of CAUG with α-power decreased when total cortical white matter was included in the model (Table 3). To test the significance of this indirect effect, bootstrap resampling was used (90% confidence intervals are reported; significant confidence intervals do not include 0). These analyses revealed that total cortical white matter volume was a significant mediator of the association between CAUG membership and power in the α-frequency band (−6.9, −0.22). Total cortical gray matter was not a significant mediator of the association between CAUG membership and EEG power in the α-frequency band (−2.99, 0.63).
Discussion
In the present study, we examined the effect of institutionalization on neural structure and function, capitalizing on our RCT design in which some children were randomized to foster care intervention, to evaluate whether removal from institutional care ameliorated the neural effects of early-life deprivation. Using structural MRI, we demonstrated that children who were assigned to care as usual had smaller total white matter volume and smaller posterior CC volume than children who were never institutionalized. For children who were randomized into foster care, neither total white matter volume nor posterior CC volume was significantly different from those of children who had never been institutionalized. White mater was not significantly different between the CAUG and the FCG. In contrast, total cortical gray matter was significantly smaller among children who were ever-institutionalized—regardless of placement into foster care—compared with children who had never been institutionalized. These findings replicate previous studies that have observed decreased total cortical white matter and gray matter volume in children exposed to institutionalization (20, 21). We extend these previous findings by demonstrating that among institutionalized children, randomization to an improved environment resulted in smaller decreases in total cortical white matter and posterior CC volume. This pattern suggests neuroplasticity of white matter following severe environmental deprivation.
Studies of typical development have demonstrated that white matter volume increases across development, but gray matter volume decreases (29–31). One possible explanation of our findings of institutionalization-related reductions in total cortical white matter volume is that this relative decrease reflects a developmental delay. In this study, white matter volume in the CAUG may be increasing at a slower pace than in the FCG or NIG. If that result were true, white matter could continue to increase, resulting in eventual catch up in adolescence or adulthood. In contrast, decreased total cortical gray matter volume for children who experienced institutionalization likely reflects either a deficit or an acceleration in brain development, where children who were previously institutionalized reach maturity earlier than their never-institutionalized peers. This last interpretation is not, however, consistent with the numerous developmental deficits observed in children exposed to institutionalization (8, 9, 23). Given that studies of typical development have shown that gray matter decreases with increasing age across childhood, we would expect that the difference in gray matter volume of ever-institutionalized children versus never-institutionalized children will only grow with time. Future studies should examine subdivisions of gray and white mater cortical volume to determine whether regional specificity in the effects of institutionalization exists and should attempt to link these changes in cortical volume with the cognitive outcomes associated with institutionalization.
Previous studies have identified associations between volume of the amygdala and exposure to institutionalization (20, 21); these studies sometimes used the volume of the amygdala relative to total brain volume as the dependent measure of interest (amygdala volume divided by total brain volume). They reported institutionalization to be associated with larger relative amygdala volume. In our study we first controlled for total brain volume statistically (results presented above) when examining amygdala volume. This difference in statistical approach is addressed in the SI Materials and Methods and Fig. S2. When we controlled for total brain volume by dividing total amygdala volume by total brain volume, we found no effect of institutionalization on relative amygdala volume. There may be several reasons why our findings on amygdala volume in postinstitutionalized children were dissimilar to previous studies. First, there are differences in analysis. We considered the entire amygdala, instead of examining differences in laterality, as was done in a previous study (20). Additionally, we lacked statistical power to examine timing effects (i.e., age of placement in foster care) on amygdala volume, as were observed in a previous study (21). Second, there are differences in sample; our sample was more homogenous compared with previous studies, as our participants were all from institutions in the same city in Romania and had been previously screened for physical and neurological disorders. Finally, although children in our study were placed into foster care families within Bucharest, previous studies have observed children who were adopted into predominantly upper-middle class homes in wealthy countries. Some of the differences between our observations and others may be a result of differences in enrichment because of these differing patterns of adoption.
In addition to using MRI to examine neural structure in this sample, resting EEG was collected to assess neural function. In our previous reports we demonstrated that children who were exposed to institutionalization had decreased power in α-power compared with those who were never institutionalized (28). We also observed this result in the subsample of children who completed MRI assessments. Consistent with our hypothesis, we observed that total cortical white matter was a significant mediator of the association between group membership and α-power for children in both the CAUG and FCG. These findings suggest that reduced α-power observed in the EEG of children exposed to institutional rearing may be the result of delay in the development of white matter in the cerebral cortex. As white matter increases across development, signal conduction becomes faster and more efficient, allowing increasingly higher frequency contributions to the overall signal. More efficient conduction because of increases in myelination may be one reason why α-power increases with white matter volume in our sample of children exposed to institutionalization, with white matter integrity in adults, and with age in all children. Consistent with this idea are findings from adults that indicate that the structural integrity of white matter tracts is responsible for modulations of α-frequency among neuro-typical adults (26) and in patients with mild cognitive impairment who evidence a decline in structural connectivity and decreased contribution of α-frequencies to the EEG signal (32–34).
Institutional rearing is associated with a variety of cognitive and emotional functions. Previously institutionalized children have lower IQ, deficits in language use, and executive function (5, 9). In addition, these children exhibit impairments and delays in a variety of social-emotional domains and a very high prevalence of mental health problems. In this study we observe that white but not gray matter appears to respond moderately to foster care intervention, and to mediate previously observed associations between institutionalization and neural function. This observation may reflect the fact that it is networks of areas, not single structures acting alone, which subserve the complex functions that are influenced by institutionalization and foster care intervention. On the other hand, because white matter does not develop as a unit but instead is organized into tracts, it may be that what we observe here as a global difference in white matter is, in fact, driven by a minority of tracts or even a single tract.
White matter volume has a linear developmental trajectory (30, 35) in comparison with gray matter volume, which generally reflects a nonlinear trajectory with peaks of developmental change, potentially indicative of sensitive periods occurring from early to late development (31, 36–38). It may be that the difference in these patterns of development makes white matter more malleable under foster care intervention. These findings point to the importance of examining structural and functional connectivity in future investigations. It is possible that white matter damage and associated “disconnections” are one important neural underpinning of the pervasive deficits that accompany exposure to adversity. The current findings are consistent with recent work identifying similar deficits associated with other forms of exposure to adversity and white matter integrity (35), and with the general role that white matter appears to play in neurodevelopmental plasticity (39). Until recently, examination of the effect of environment on neural structure was often the examination of specific gray matter structures. Here we highlight the importance of understanding the impact of the environment on the network of areas that give rise to cognition, and on the structure of those networks.
Materials and Methods
Participants.
We acquired structural MRI scans for 79 participants in the BEIP between the ages of 8 and 11 in Bucharest, Romania. Participants included children randomized out of institutions into foster care (FCG), children randomly selected to remain in institutional care (CAUG), and children who had never been in institutional care (NIG). The BEIP was initiated at the request of the Secretary of State for Child Protection in Romania. All study procedures were approved by the local commissions on child protection in Bucharest, the Romanian ministry of health, and the institutional review boards of the home institutions of the three principal investigators. A more complete description of procedures used to ensure ethical integrity has been published previously and commented on by the scientific community (40, 41). Information about the randomization procedures and foster care intervention can be found in SI Materials and Methods.
Of the 79 children who completed MRI assessments, 74 children were included in all analyses: 29 CAUG children (16 F, mean age = 9.68, SD = 0.79), 25 FCG children (11 F, mean age = 9.92, SD = 0.62), and 20 NIG children (10 F, mean age = 9.63, SD = 0.83). Four participants were excluded from all analyses because poor scan quality prevented proper segmentation of cortical and subcortical gray/white matter boundaries (two CAUG, one FCG, and one NIG), and one child was excluded from all analyses because of frank neurological abnormality (FCG). No differences were observed across the three groups in age at MRI assessment (F = 0.348, P = 0.707), and boys and girls were approximately the same age [t(72) = −1.18, P = 0.24). A final four subjects are not included in the analyses of EEG data only, one CAUG and three NIG, because these data were unavailable for these subjects. These four subjects are included in all other analyses.
MRI Acquisition and Processing.
Structural magnetic resonance images were acquired at Regina Maria Health Center (Bucharest, Romania) on a Siemens Magnetom Avanto 1.5T syngo system. Images were obtained using a transverse magnetization-prepared rapid gradient echo 3D sequence (TE = 2.98 ms, TI = 1,000 ms, flip angle = 8°, 176 slices with 1 × 1 × 1 mm isometric voxels) with a 16-channel head coil. The TR for this sequence varied between 1,650 and 1,910 ms. Five subjects were acquired in the sagittal plane; one was acquired in the coronal plane. Acquisition parameters did not differ by group membership nor were they associated with scan quality, thus all scans are considered together.
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). The technical details of these procedures are described in prior publications (42, 43) and in SI Materials and Methods. FreeSurfer morphometric procedures have demonstrated good test-retest reliability across scanner manufacturers and field strengths (44, 45). In addition, they have been successfully used in studies of children between the ages of 8 and 11 y (21, 29).
EEG Acquisition and Processing.
Before acquisition of MRI data all participants in the present study had participated in an EEG recording session. EEG was recorded when children were between 8 and 11 y of age (see SI Materials and Methods for details) (28). EEG was recorded while the children sat quietly in a chair, alternating 1-min epochs of eyes open and closed for a total of 6 min. We examine eyes-open segments only, as these segments best represent awake-behaving EEG signal. EEG was recorded using a lycra stretchable cap (Electro-Cap International) that had 12 tin electrodes sewn into it. Electrodes were distributed over the head and labeled using the 10-20 system. EEG was referenced to the vertex (Cz) during recording, and a midanterior electrode served as the ground (AFz).
EEG was processed using the EEG Analysis System from the James Long Company. The EEG was rereferenced through software to an average mastoids reference. Epochs containing blink artifact were regressed from the EEG signals and any epochs in which the EEG signal exceeded ± 200 μV were excluded. Following preprocessing, the signal was decomposed into frequency bands using a discrete Fourier transform with a 1-s Hamming window and 50% overlap. There were no group differences in number of artifact-free windows used in this analysis [F(60) = 1.296, P = 0.28]. We examined absolute EEG power in the α (7–12 Hz) frequency band, because power in this frequency is impacted by institutionalization and foster care (28).
Data Analysis.
We used ordinary least squares (OLS) linear regression to examine differences in neural development resulting from institutionalization. We first estimated a model that directly compared children in the EIG to those in the NIG (model 1). Next, we examined the effect of foster care on brain volume by comparing children in the CAUG to those in the NIG and comparing children in the FCG to those in the NIG (model 2). Finally, we assessed the effect of covariates on model 2 by controlling for sex and age, or in the case of the CC and subcortical structures, age, sex, and total brain volume (model 3). These models were considered significant if P < 0.006. This value represents a Bonferonni-corrected P value to adjust for multiple comparisons (eight structural volumes: anterior CC, central CC, posterior CC, amygdala, hippocampus, basal ganglia, total cortical gray matter, and total cortical white matter). See Fig. S3 for a scatter plot of each area of the brain that we studied, showing each subject’s data by group. Sex and age were included as control covariates because the ratios of boys to girls were different across group and because sex and age are strongly associated with total brain volume (35). For the three subcortical structures considered here and the three subdivisions of the CC, we additionally controlled for total brain volume. Subcortical structures change in size with total brain volume, and we were interested in variance in subcortical structures not predicted by total brain volume in this analysis (29).
Effect of Institutionalization on α-Power.
After identifying the effect of institutionalization on brain volume we replicated previous findings of the effect of institutionalization on α- (7–12 Hz) power in this subsample of subjects. We used an identical OLS regression approach as described above in this analysis. Models were run for α-power in eyes-open blocks. Absolute power in the α-frequency band was averaged across all electrode sites.
Mediation Analysis.
In the final analysis, a mediation model was used to test the hypothesis that the effect of institutionalization on α-power was correctly accounted for by changes in neural structure. Given the small sample size available to us in this analysis, we set the criteria for significance at P < 0.10 for the mediation analysis only. Two hypotheses were tested. First, as previously observed, differences in α-power would be accounted for by differences in white matter volume and, second, a more exploratory hypothesis that differences in α-power would be accounted for by differences in cortical gray matter volume. To test mediation, we assessed the four criteria for mediation described by Baron and Kenny (46), and we tested the significance of the mediation model using a nonparametric bootstrapping approach (45). A more complete description of this method can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Adina Chirita, MD, PhD, who oversaw all MRI scanning; our dedicated Bucharest-based laboratory members; Elizabeth Furtado for US-based administrative support; and the many families and children who participated in this project. This work was supported by the Robert Wood Johnson Health and Society Scholars Program, Harvard School of Public Health (M.A.S. and K.A.M.); the John D. and Catherine T. MacArthur Foundation (Research Network on Early Experience and Brain Development); the Binder Family Foundation; the Help the Children of Romania, Inc. Foundation; and National Institute of Mental Health Grant MH091363 (to C.A.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200041109/-/DCSupplemental.
References
- 1.Jacobs S. The Congressional Coalition on Adoption Institute (CCAI) 2011. The Way Forward: A Special Report from the CCAI and the US Department of State. Available at: http://adoption.state.gov/about_us/video/national_adoptions_month_extra.php.
- 2.Walker SP, et al. Inequality in early childhood: Risk and protective factors for early child development. Lancet. 2011;378:1325–1338. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 3.McCall R, Van Ijzendoorn MH, Juffer H, Groark CJ, Groza VK, editors. Hoboken, NJ: Wiley-Blackwell; 2012. Children Without Permanent Parents: Research, Practice, and Policy. [Google Scholar]
- 4.O’Connor TG, Rutter M, Beckett C, Keaveney L, Kreppner JM. English and Romanian Adoptees Study Team The effects of global severe privation on cognitive competence: Extension and longitudinal follow-up. Child Dev. 2000;71:376–390. doi: 10.1111/1467-8624.00151. [DOI] [PubMed] [Google Scholar]
- 5.Nelson CA, 3rd, et al. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 6.Albers LH, Johnson DE, Hostetter MK, Iverson S, Miller LC. Health of children adopted from the former Soviet Union and Eastern Europe. Comparison with preadoptive medical records. JAMA. 1997;278:922–924. [PubMed] [Google Scholar]
- 7.Windsor J, et al. Effect of foster care on young children’s language learning. Child Dev. 2011;82:1040–1046. doi: 10.1111/j.1467-8624.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreppner JM, O’Connor TG, Rutter M. English and Romanian Adoptees Study Team Can inattention/overactivity be an institutional deprivation syndrome? J Abnorm Child Psychol. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- 9.Rutter M, Sonuga-Barke EJX. Conclusions: Overview of findings from the era study, inferences, and research implications. Monogr Soc Res Child Dev. 2010;75:212–229. doi: 10.1111/j.1540-5834.2010.00557.x. [DOI] [PubMed] [Google Scholar]
- 10.Zeanah CH, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 11.Rutter M, et al. English and Romanian Adoptees (ERA) Study Team Quasi-autistic patterns following severe early global privation. J Child Psychol Psychiatry. 1999;40:537–549. [PubMed] [Google Scholar]
- 12.Zeanah CH, Smyke AT, Koga SF, Carlson E. Bucharest Early Intervention Project Core Group Attachment in institutionalized and community children in Romania. Child Dev. 2005;76:1015–1028. doi: 10.1111/j.1467-8624.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- 13.Bos KJ, Zeanah CH, Smyke AT, Fox NA, Nelson CA. Stereotypies in children with a history of early institutional care. Arch Pediatr Adolesc Med. 2010;164:406–411. doi: 10.1001/archpediatrics.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green JG, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin KA, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: Associations with persistence of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:124–132. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black E, Peppé S, Gibbon F. The relationship between socio-economic status and lexical development. Clin Linguist Phon. 2008;22:259–265. doi: 10.1080/02699200801918887. [DOI] [PubMed] [Google Scholar]
- 17.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychol Bull. 2000;126:309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- 18.Nelson CAI, Furtado EA, Fox NA, Zeanah CHJ. The deprived human brain. Am Sci. 2009;97:222. [Google Scholar]
- 19.Nelson CAI, Bos KJ, Gunnar MR, Sonuga-Barke EJ. The neurobiological toll of early human deprivation. In: McCall R, Van Ijzendoorn MH, Juffer H, Groark CJ, Groza VK, editors. Children without Permanent Parental Care: Research, Practice, and Policy. New York: Wiley-Blackwell; 2012. pp. 127–146. [Google Scholar]
- 20.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 21.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eluvathingal TJ, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 23.Nelson C. A neurobiological perspective on early human deprivation. Child Dev Perspect. 2007;1(1):13–18. [Google Scholar]
- 24.Zeanah CH, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev Psychopathol. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- 25.Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- 26.Valdés-Hernández PA, et al. White matter architecture rather than cortical surface area correlates with the EEG alpha rhythm. Neuroimage. 2010;49:2328–2339. doi: 10.1016/j.neuroimage.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Marshall PJ, Fox NA. Bucharest Early Intervention Project Core Group A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- 28.Vanderwert RE, Marshall PJ, Nelson CA, 3rd, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS ONE. 2010;5:e11415. doi: 10.1371/journal.pone.0011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostby Y, et al. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 31.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 32.Babiloni C, et al. Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer’s disease. Clin Neurophysiol. 2006;117:1113–1129. doi: 10.1016/j.clinph.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Rossini PM, et al. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143:793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 34.Wolf H, et al. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- 35.Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 37.Sowell ER, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw P, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 39.Berlucchi G. Brain plasticity and cognitive neurorehabilitation. Neuropsychol Rehabil. 2011;21:560–578. doi: 10.1080/09602011.2011.573255. [DOI] [PubMed] [Google Scholar]
- 40.Zeanah CH, et al. Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Infant Ment Health J. 2006;27:559–576. doi: 10.1002/imhj.20107. [DOI] [PubMed] [Google Scholar]
- 41.Miller FG. The randomized controlled trial as a demonstration project: An ethical perspective. Am J Psychiatry. 2009;166:743–745. doi: 10.1176/appi.ajp.2009.09040538. [DOI] [PubMed] [Google Scholar]
- 42.Fischl B, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 43.Fischl B, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 45.Jovicich J, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.