Abstract
In rodent sensory neurons, acid-sensing ion channel 3 (ASIC3) has recently emerged as a particularly important sensor of nonadaptive pain associated with tissue acidosis. However, little is known about the human ASIC3 channel, which includes three splice variants differing in their C-terminal domain (hASIC3a, hASIC3b, and hASIC3c). hASIC3a transcripts represent the main mRNAs expressed in both peripheral and central neuronal tissues (dorsal root ganglia [DRG], spinal cord, and brain), where a small proportion of hASIC3c transcripts is also detected. We show that hASIC3 channels (hASIC3a, hASIC3b, or hASIC3c) are able to directly sense extracellular pH changes not only during acidification (up to pH 5.0), but also during alkalization (up to pH 8.0), an original and inducible property yet unknown. When the external pH decreases, hASIC3 display a transient acid mode with brief activation that is relevant to the classical ASIC currents, as previously described. On the other hand, an external pH increase activates a sustained alkaline mode leading to a constitutive activity at resting pH. Both modes are inhibited by the APETx2 toxin, an ASIC3-type channel inhibitor. The alkaline sensitivity of hASIC3 is an intrinsic property of the channel, which is supported by the extracellular loop and involves two arginines (R68 and R83) only present in the human clone. hASIC3 is thus able to sense the extracellular pH in both directions and therefore to dynamically adapt its activity between pH 5.0 and 8.0, a property likely to participate in the fine tuning of neuronal membrane potential and to neuron sensitization in various pH environments.
Keywords: sodium channels, nociception
Acid-sensing ion channels (ASICs) are depolarizing cationic channels gated by extracellular protons (1–3). Four genes encoding at least six subunits (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) have been identified so far in rodents. Functional channels have been proposed to result from the trimeric association of subunits (4), leading to homomeric or heteromeric channels. ASICs are largely expressed in neurons, both in central and peripheral nervous systems. Whereas ASIC1a and ASIC2 are widely present in the rodent nervous system, the expression of ASIC1b and ASIC3 is primarily restricted to sensory neurons (5–7). The ASIC3 subunit is highly expressed in rat nociceptive neurons (8, 9). The expression pattern of ASIC subunits is less well documented in humans, where ASIC3 (10–12) has three variants differing in their C-termini (2). The physiological relevance and properties of these human variants have so far never been studied.
Several physiological and/or physiopathological conditions, such as synaptic transmission, bone resorption, ischemia, inflammation, tumor development, or tissue incisions, are accompanied by extracellular acidifications. Moreover, tissue acidosis is well known to be painful (13) and inhibition of ASICs in healthy human volunteers (14, 15) has revealed the important role of these channels in sensing acid-induced pain provoked by cutaneous acidification. Among ASICs, ASIC3-containing channels are particularly interesting candidates for sensing the nonadaptive pain caused by protons. ASIC3 channels have the property to generate a sustained depolarizing current in response to moderate acidifications, and are able to integrate different inflammatory or ischemic stimuli such as protons, ATP, lactic and arachidonic acid, and hypertonicity (16–19). All these properties have been proposed to be important for the role of ASIC3 in pain (16, 20, 21). Peripheral ASIC3-containing channels have been shown (i) to participate to acidic, inflammatory, and postoperative pain (16, 22, 23); (ii) to contribute to primary and/or secondary mechanical hyperalgesia in muscles and joints after inflammation or injury (3, 24, 25); (iii) to be involved in cutaneous and visceral mechano-sensation and mechano-nociception (24, 26, 27, 28); and (iv) to support acid sensing in gastroesophageal afferents (29).
Most of the studies on ASIC3 were performed in rodents and used rodent cDNAs. The detailed biophysical properties and tissue distribution of human ASIC3 remain poorly characterized. We report here that human ASIC3 (hASIC3) not only sense extracellular acidification but also extracellular alkalization. This intrinsic capacity to behave as an acido-basic sensor brings, together with its wide distribution within the human nervous system, an additional dimension to the role of hASIC3 channel in pain and other neurophysiological processes in humans.
Results
Distribution of Human ASIC3 in Neuronal Tissues.
In the rodent nervous system, ASIC3 is mostly expressed in sensory neurons (7), and functional studies have shown that native ASIC currents in central neurons are carried by ASIC1a and ASIC2 channels (30, 31). We performed quantitative RT-PCR experiments on different human neuronal tissues to assess the relative abundance of messenger RNAs for the three human isoforms hASIC3a, hASIC3b, and hASIC3c. We found that the hASIC3a mRNA is the main ASIC3 isoform expressed in human neuronal tissues, although hASIC3c is also significantly detected (Fig. 1, see also Fig. S1). The hASIC3b mRNA expression appears negligible in all tested tissues. We therefore focused on hASIC3a in this study. We found a high level of expression of hASIC3 mRNAs in spinal cord and brain, comparable to the expression level in dorsal root ganglia (DRG), which is different from rodent where the ASIC3 mRNA is mainly restricted to DRG (Fig. 1, Inset). ASIC1 mRNAs remain, however, the predominant ASIC transcripts in human brain (Fig. 1).
Fig. 1.
Relative expression of ASIC mRNAs in human neuronal tissues. Quantitative RT-PCR experiments performed on total RNA from human DRG, brain, and spinal cord. (Inset) relative expression of ASIC3 in rat DRG and brain as a comparison. Expression is normalized to the expression of ASIC3 in DRG.
Nonconventional Gating of hASIC3a.
When heterologously expressed in F-11 cells, rat ASIC3 channel presented a typical biphasic inward current following moderate acidification (pH 8.0 to 7.0), with a transient phase followed by a noninactivating window current (see Fig. S2A). In similar conditions, human ASIC3a showed two different types of currents (Fig. 2). We observed a “classic” ASIC3-type current in 46% of hASIC3-expressing cells (70 out of 153 cells, see Materials and Methods) (Fig. 2, inset), which was related to the typical current associated with ASIC3 (i.e., a transient phase followed by a sustained plateau), and a “nonconventional” current, in 54% of cells (83 out of 153 cells) (Fig. 2), with an inward transient phase followed by an outward-going plateau phase more positive than the current level at pH 8.0. Membrane capacitances of the cells showing classic and nonconventional currents were identical (30.2 ± 2.4 pF and 29.5 ± 1.6 pF, respectively; P = 0.81, unpaired t test). As previously described (11), the classic hASIC3a current (Fig. 2, Inset) displayed a smaller plateau than its rat equivalent (Fig. S2A), because of a shift of the pH-dependent inactivation of hASIC3a toward more alkaline values, which led to a smaller window current. The transient phase of the classic and the nonconventional hASIC3 currents displayed the same activation curve in response to extracellular acidification (pH1/2 = 6.56 and 6.59 with Hill slopes of 3.0 and 2.8, respectively; see Fig. S2B), and were both sensitive to the ASIC3 inhibitory toxin (APETx2) (see Fig. S2B, Inset), which is known to inhibit ASIC3-type channels (32).
Fig. 2.
Macroscopic properties of the human ASIC3 currents. Whole-cell recordings of hASIC3a currents performed at −80 mV from F-11 transfected cells. Currents were activated by rapid changes of the external pH from pH 8.0 to 7.0, as indicated above each current trace. Dashed lines represent the zero current level. The nonconventional current (main trace) and the classic current (Inset) are represented.
Human ASIC3a Channel Is a Sensor of Extracellular Alkalization.
The amplitude and direction of both the classic and the nonconventional sustained hASIC3a current, measured as the difference between the plateau current obtained at pH ranging from 7.7 to 5.0 and the current level at the holding pH of 8.0 (Fig. 3A, Inset), were significantly different for pH values down to 6.6 (Fig. 3A). The nonconventional plateau currents were larger in amplitude and of opposite (i.e., positive) direction. This suggested that the nonconventional hASIC3a current was constitutively activated at pH 8.0; i.e., upon extracellular alkalization. Indeed, the basal current level at pH 8.0 in cells presenting a nonconventional hASIC3a sustained current was significantly larger than in cells exhibiting the classic current (Fig. 3B). Moreover, APETx2 largely reduced the pH 8.0–induced resting current (Fig. 3B, Inset), strongly suggesting the participation of hASIC3a in this activity. We next measured the membrane conductance (G) at different external pH values (Fig. 3C) by applying 10-mV depolarizations to cells expressing the nonconventional sustained current (Fig. 3C, Inset). Whole-cell membrane conductance significantly increased as the external pH was shifted from 6.6 to 8.0, supporting the idea of an alkaline pH-gated hASIC3a sustained current. Furthermore, the pH 8.0–activated membrane conductance was fully abolished by APETx2 (Fig. 3C), demonstrating that hASIC3a carries an alkaline-activated constitutive current in these cells. The hASIC3a alkaline-induced sustained current is highly dependent on external Na+ ions (Fig. 3D). Indeed, the sustained current that developed at pH 8.0 under normal sodium condition (145 Na) was almost suppressed when external sodium was replaced by N-Methyl d-Glucamine (0 Na). This pH 8.0–induced Na+-dependent current was inhibited by APETx2 (Fig. 3D). The I/V curve of the current sensitive to APETx2 (Fig. 3E, IAPETx2), obtained from a voltage ramp protocol on the different sustained current levels recorded at pH 8.0 (Fig. 3D, Lower), showed that the pH 8.0–induced hASIC3a current reversed at ∼33 mV (n = 10). A decrease in the external Na+ concentration from 145 to 45 mM led to a 30-mV shift (n = 4) of this reversal potential, which perfectly corresponded to the shift of the Na+ ions reversal potential (theoretical ENa varying from ∼85 to ∼55 mV at 20 °C, according to the Nernst equation). Although not as high as the classical acid-induced transient current (11), the human alkaline-sensitive current thus exhibits a significant Na+ selectivity with a relative permeability of Na+ to K+ (PNa/PK) of 3.4.
Fig. 3.
Properties of the nonconventional hASIC3a sustained current in F11 cells. (A) Amplitudes of the hASIC3a sustained current component (Isst) for the classic (white bars) and nonconventional (black bars) currents recorded at different external pH (HP -80 mV). The amplitudes were measured as the difference between the current level reached at the test pH (7.7, 7.4, 7.0, 6.6, and 5.0) and the current level at the holding pH 8.0 (see Inset). The number of experiment is indicated above each bar (**P < 0.01 and ***P < 0.001, Mann–Whitney test). (B) Comparison of the amplitude of the basal current measured at the holding pH 8.0 in cells expressing the classic and the nonconventional hASIC3a currents (HP -80 mV, ***P < 0.001, Kruskal–Wallis test followed by a Dunn’s post hoc test). As illustrated in the Inset, the APETx2 toxin at 1 μM inhibited the basal current in cells expressing the nonconventional hASIC3a current. (C) The macroscopic membrane conductances G, calculated from 10-mV pulses applied to cells expressing the nonconventional hASIC3a current (Inset), are represented as a function of external pH both in control conditions (black bars) and in the presence of the APETx2 toxin at 1 μM (gray bar; *P < 0.05, **P < 0.01, and ***P < 0.001, repeated measures ANOVA test). (D) Sensitivity of the pH 8.0–induced hASIC3a current to extracellular Na+ ions and APETx2 toxin (3 μM). The 0-Na condition was obtained by replacing external Na+ by N-Methyl d-Glucamine (NMDG+) ions. A voltage ramp protocol was applied to the current levels obtained in the presence (1) and in the absence (2) of APETx2 (3 μM). E, I/V curve of the pH 8.0–induced APETx2-sensitive hASIC3a current.
Thus, the human ASIC3a channel has the original property to sense external pH in both directions (decrease and increase). It can generate a transient activation upon acidification, and a sustained, mainly Na+ selective, current in response to alkalization.
Alkaline Sensitivity Is an Intrinsic Property of Human ASIC3.
The alkaline-gated hASIC3a current was also observed in transfected COS-7 cells (Fig. 4A), injected Xenopus oocytes (Fig. 4B), as well as in transiently transfected mouse cortical neurons (Fig. 4C). The characteristics of the alkaline-induced sustained current were similar in the different expression systems (see SI Results). The alkaline sensitivity of hASIC3a was steep, with maximal current at pH 8.0, zero current level estimated around pH 6.5, and a Hill slope of 1.4 (Fig. 4D); i.e., smaller than that observed for the transient current activated by acidic pH (i.e., ∼3.0, Fig. S2B). hASIC3b and hASIC3c, which generate classical acid-induced currents with properties similar to hASIC3a when expressed in Xenopus oocytes, were also activated when the external pH is shifted from 6.5 to 9 (see Fig. S3). The alkaline-induced current was blocked by amiloride (see Fig. S5A) and was not observed when rat ASIC3 or human ASIC1a were expressed in F-11 cells (Fig. 4E) or in Xenopus oocytes (see Fig. S3F), strongly suggesting that this property was tightly associated with hASIC3. ASIC3 can form heteromeric channels with ASIC1a in rodents (32), and hASIC3 mRNAs are often coexpressed with hASIC1 transcripts in human tissues (Fig. 1 and Fig. S1). We thus tested whether the heteromeric channel also displayed the alkaline-sensitivity. All of the oocytes expressing a heteromeric hASIC1a/hASIC3c channel (n = 7; see SI Materials and Methods) showed an alkaline-induced sustained current in addition to the classical transient inward current (see Fig. S3 E and F). Both the sustained and the transient currents were blocked by APETx2 (500 nM) with a potency (38%, P < 0.01 and 28%, P < 0.05, respectively) that is in good agreement with the effects of the toxin already described on rat ASIC3-containing heteromeric channels (32).
Fig. 4.
The alkaline-induced sustained current is independent of the expression system. (A) pH 6.6–evoked current recorded at −80 mV from COS cells transfected with hASIC3a. The dotted line represents the zero current level, and the typical alkaline-induced sustained current is magnified. (B) Xenopus oocytes injected with hASIC3a also display a typical alkaline-induced sustained current. The two current traces were recorded at −80 mV from the same oocyte. (C) pH 7.0–evoked current recorded at −80 mV from a mouse cortical neuron transfected with hASIC3a. The current displays typical alkaline-induced sustained activity. (D) pH-dependent activation of the hASIC3a nonconventional sustained currents recorded in F-11 cells (data from five different cells). Amplitudes of the sustained currents are normalized to the current measured at pH 8.0 (I/IpH8.0). (E) Typical effects of extracellular alkalization to pH 8.0 (from the physiological pH 7.4) on whole-cell current recorded at −80 mV from F-11 cells expressing either hASIC3a, rASIC3, or hASIC1a channels. The alkaline-induced current was not observed in rASIC3- or hASIC1a-expressing cells. rASIC3 showed a small widow current at pH 7.4 that was absent at pH 8.0.
All together, these results show that the alkaline-sensitivity is an intrinsic and specific property of human ASIC3 channels. This property is probably not associated with the C-terminal domain that differs between the three hASIC3 isoforms.
Two Arginine Residues in the Extracellular Loop Are Involved in the Alkaline Sensitivity of Human ASIC3a Channel.
Our data strongly suggested that the sensitivity of hASIC3a to alkaline pH was directly supported by the channel itself. Therefore, we looked at the structural elements involved in the alkaline sensitivity of the channel using a site-directed mutagenesis approach (Fig. 5). An ASIC3 chimeric channel containing rat ASIC3 transmembrane and intracellular domains and human ASIC3a extracellular loop showed alkaline sensitivity (Fig. 5A, Upper); whereas, a human ASIC3a chimera containing the rat ASIC3 extracellular loop did not (Fig. 5A, Lower). The human and rat ASIC3 extracellular loops share a high percentage of homology (∼90%), and we focused our attention on two arginine residues (R68 and R83) that are only present in the extracellular loop of the human isoforms (Fig. 5B). The arginine 68 is located at the junction between the transmembrane domain 1 (TM1) and the extracellular loop, a critical hydrophobic location that suggested an unusual pKa for this arginine, which could be involved in alkaline pH sensing, similarly to what has been shown for two-pore domain alkali-activated K+ channel (33). Arginine 83 is located at the junction between β1 and β2 linkers of the palm domain, a region that has recently been shown to control the sustained opening of ASIC1 (34). Single (R68G or R83Q) or dual (RR68,83GQ) mutations of those two arginine residues significantly reduced the amplitude of the alkaline-induced sustained current, compared with the wild-type hASIC3a current (Fig. 5 C and D). The transient current amplitudes at pH5.0 were not different between the mutants and the wild-type channels (Fig. 5E), showing a specific effect of the two arginine residues on the alkaline-induced current. Introducing these two arginine residues within the extracellular loop of the rat channel (GQ69,84RR mutant) induced a small alkaline sensitivity in 11 out of the 26 oocytes tested, although this mutant did not fully reconstitute the human properties (see Fig. S4A). These results demonstrate that the sustained activation of hASIC3a in response to extracellular alkalization is fully supported by the extracellular loop where the R68 and R83 residues are playing an important role.
Fig. 5.
Mapping of the structural elements involved in the alkaline sensitivity of hASIC3. (A) Effect of external alkalization (from pH 6.6 to 8.0) on oocytes expressing the rat ASIC3 chimera containing the extracellular loop of hASIC3a; (Upper) rASIC3-hLoop3 or the human ASIC3a chimera containing the extracellular loop of rASIC3; (Lower) hASIC3-rLoop3. (B) Schematic representation of two ASIC subunits in a functional channel. The two arginines only present in human ASIC3, and not found in rat, are indicated. Adapted by permission from ref. 4 (Copyright 2007, Macmillan Publishers Ltd). (C) Effect of external alkalization on hASIC3a wild type and mutants. (D) Bar graph representing the effects observe in (C) (*P < 0.05 and ***P < 0.001, one-way ANOVA followed by Tukey’s post hoc test). (E) Bar graph of the amplitudes of the pH 5.0–induced transient currents measured from oocytes expressing the wild-type or the arginine mutants described in C and D.
Finally, it was possible to strongly reduce the acid sensitivity of the hASIC3 channel without affecting the alkaline-induced current (see Fig. S4B) by mutating two amino acids in the post-TM1 region that are crucial for H+ gating (35) (hASIC3-HH71,72NN). Thus, our data suggest that it is possible to partially dissociate both functioning modes by mutating either arginines 68 and 83 or histidines 71 and 72.
Alkaline Current of hASIC3a Is Modulated by Calcium, Lactic Acid, and Arachidonic Acid.
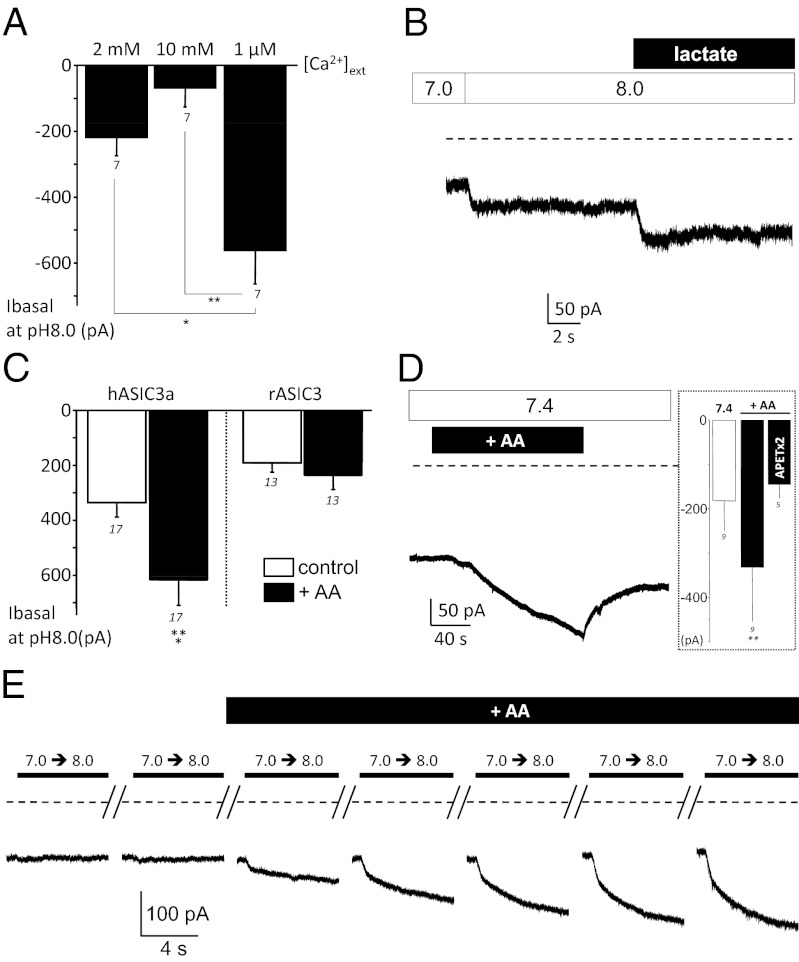
The alkaline sensitivity of hASIC3a is only observed in about half of the cells, suggesting the existence of regulatory mechanisms. We have evaluated the effect of several factors previously described that modulate rat ASIC3, including extracellular calcium, lactic acid, and arachidonic acid, on the alkaline sensitivity of hASIC3a. Extracellular calcium ions have been involved in the gating mechanism of ASIC3 by protons (36). We thus tested whether the alkaline sensitivity of hASIC3a was dependent on external calcium concentration. Changing the extracellular calcium concentration, from normal (2 mM) to high (10 mM) or low (1 μM) levels, did not induce any alkaline sensitivity in cells only displaying the conventional hASIC3a current; i.e., not sensitive to external alkaline pH (n = 5). However, the basal hASIC3a current recorded at pH 8.0 from cells displaying the alkaline sensitivity was modulated by changes of external calcium concentration (Fig. 6A). It was increased in low calcium conditions whereas it was reduced at high calcium concentrations, similarly to what was previously described for acid-induced rat ASIC3 current (36). Lactic acid, a potentiator of the rat ASIC3 current, which acts by lowering the concentration of Ca2+ and Mg2+ ions (17), also increased the alkaline-induced hASIC3a current (Fig. 6B).
Fig. 6.
Modulation of the hASIC3a alkaline-sensitive current. Whole-cell patch-clamp experiments performed on transfected F-11 cells clamped at −80 mV. (A) Effect of different external calcium concentrations on the basal sustained current recorded at pH8.0 in cells transfected with hASIC3a. The concentration of 1 μM free calcium was obtained by combining 5 mM EGTA and 4.97 mM CaCl2 at pH 8.0, as calculated with the maxchelator software (*P < 0.05 and **P < 0.01, Friedman test followed by a Dunn’s post hoc test). (B) Effect of lactate (20 mM) on the pH 8.0–induced hASIC3a sustained current. (C) Bar graph showing the potentiating effect of AA (10–20 μM) on the basal hASIC3a current recorded at pH 8.0 in cells displaying the alkaline sensitivity (Left; ***P < 0.001, Wilcoxon test). For comparison, the effect of AA of the basal current recorded at pH 8.0 from cells expressing rASIC3 is also represented (Right). (D) External application of AA (20 μM) was sufficient to trigger a hASIC3a current at physiological pH 7.4 in cells displaying the alkaline sensitivity. This AA-induced current was inhibited by APETx2 (5 μM, Inset). (E) Representative current trace showing the effect of arachidonic acid (10 μM) on a cell that initially displayed no pH 8.0–induced sustained current.
Arachidonic acid (AA) is a well-known mediator of inflammation, which potentiates rat ASIC3 current (16, 19, 36). Over all of the hASIC3a-expressing F-11 cells tested (n = 37), 14 initially displayed an alkaline-induced sustained current when external pH was switched from 7.0 to 8.0 (see Materials and Methods). This alkaline-sensitive current was always potentiated by AA, as indicated by the effect on the basal current recorded at pH 8.0 (Fig. 6C, Left). The effect of AA was also observed in Xenopus oocytes injected with hASIC3a (Fig. S5B), but was absent in F-11 cells transfected with rat ASIC3 (Fig. 6C, Right). It is interesting to note that AA alone was sufficient to trigger a current at physiological pH 7.4 from cells displaying the alkaline sensitivity (Fig. 6D), and this AA-induced hASIC3a current was inhibited by APETx2 (Fig.6D, Inset). Moreover, AA was also able to induce, or unmask, the alkaline sensitivity in 10 of the 23 remaining cells (43%) that initially did not display an alkaline-induced current in response to a pH switch from 7.0 to 8.0 (Fig. 6E). Arachidonic acid, calcium, and lactic acid are thus modulators of the hASIC3a alkaline-sensitive current, with a particularly potent effect of AA.
Discussion
In humans, ASIC3 has three splice variants and little is known about the molecular and biophysical properties of these channels. We found here that the ASIC3a variant represents the main ASIC3 mRNA in the human nervous system. The ASIC3c transcript is also present in nervous tissues but at a much lower level, and ASIC3b is barely detected. Our data show the presence of ASIC3 transcripts in human brain and spinal cord, which reinforces the idea that, conversely to the rat, the expression of human ASIC3 is not restricted to peripheral sensory neurons (10). These results suggest that hASIC3 may play a role in central neurophysiological functions although ASIC1 transcripts remain largely predominant in human brain. It is difficult to know to what extent hASIC channels are functionally expressed in central neurons. The only study of ASIC currents performed in cultured human cortical neurons exclusively reported the presence of ASIC1a-type currents (37). This does not preclude an expression of ASIC3 in other brain regions or neuronal subpopulations.
We demonstrate that all of the hASIC3 variants (i.e., hASIC3a, hASIC3b, and hASIC3c) have an original biophysical property that makes them capable to sense extracellular pH variations in both directions; i.e., acidification and alkalization. This original property never described for ASICs is supported by two distinct functioning modes of hASIC3, a classical transient mode in response to pH decrease, combined with a sustained mode in response to pH increase (see Fig. S6). The alkaline sensitivity appears to be a specific and intrinsic property of hASIC3 channels, and seems to be different, for instance, from the NH4+-induced ASIC currents described by Pidoplichko and colleagues (38). This property can be potentiated and /or induced by one or more “molecular switches,” such as arachidonic acid, which is able to induce an hASIC3 current at physiological resting pH, and it may be associated with particular physiological or pathological conditions in the peripheral and central nervous system.
Sensitivity to alkalization is shared by other excitatory ion channels expressed in the sensory pathway, such as transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) (39, 40). TRPV1, like hASIC3, is activated by acidic pH in addition to alkaline pH (39). However, contrary to hASIC3, neither TRPV1 nor TRPA1 are directly activated by extracellular alkalization. They are activated by the secondary intracellular alkalization that follows extracellular application of high-pH solution. Accordingly, human ASIC3 directly senses extracellular alkalization via two arginine residues in its extracellular domain; R68, which is only present in human and primates, and R83, also found in a few other species, while TRPV1 and TRPA1 need residues in the N-terminal cytoplasmic domain (a histidine and two cysteines, respectively). This makes hASIC3 very sensitive to extracellular alkalization, with a maximal sustained depolarizing current reached around pH 8.0 and a Hill slope of 1.4. This channel appears therefore as a unique and very efficient sensor of extracellular acidification or alkalinization near the resting physiological pH.
Although it remains to be established whether acidic- and alkaline-pH activations of hASIC3 produce action potentials in human neurons, it is however possible to speculate, based on the experiments done in rodent neurons, that the fast and large depolarization generated by a rapid drop in extracellular pH will most probably lead to the generation of action potentials. On the other hand, the alkaline-activated current, which has slower kinetics and smaller amplitudes, will generate a long-lasting depolarization that would significantly modulate neuron excitability in response to other stimuli (sensitization). How neurons expressing hASIC3 can distinguish and integrate the different signals associated with external acidification or alkalization? This clearly depends on the presence or absence of the alkaline sensitivity, and on the amplitude, direction, and kinetics of the pH change within the tissue (see SI Discussion). It is also important to consider that the hASIC3 channel could fulfill different roles in different neuron populations and/or in different physiological conditions. This is further supported by the fact that the alkaline sensitivity is not always expressed but can be induced by factors such as AA and probably others that remain to be identified.
In sensory neurons, the human ASIC3 channels could therefore contribute to pain perception associated with both tissue acidosis, as in rodents, and tissue alkalization. Tissue alkalization, although less frequent than acidification, occurs in several physiological or pathophysiological situations including for instance hyperventilation, which produces peripheral nerves hyperexcitability as pC02 decline, and leads to paraesthesia (41), or the effusion of alkaline pancreatic fluids in patients having a pancreatic-pleural fistula that often causes chest pain (42).
Human ASIC3 can thus constitute a particularly efficient molecular sensor for alkalization and/or recovery from acidification in neurons and other cell types where it may be expressed. Unveiling the biological conditions that control the alkaline sensitivity of this channel will certainly help to better understand the physiological consequences of this remarkable property.
Materials and Methods
F-11 and COS Cells Culture and Transfection.
The F-11 cell line was grown as described previously (16). One day after plating, cells were transfected with either pIRES2-hASIC3a-HcRed, pIRES2-hASIC3a-EGFP, pIRES2-hASIC3b-EGFP, pIRES2-hASIC3c-EGFP, pIRES2-hASIC1-EGFP, or pIRES2-rASIC3-EGFP vectors using the JetPEI reagent according to the supplier’s protocol (Polyplus Transfection SA). Fluorescent cells were used for patch-clamp recordings 2–4 d after transfection, and cells were considered as “hASIC3-positive” only when the transient current amplitude was at least 400 pA at pH 6.6 and/or 1 nA at pH 5.0 (i.e., IpH5.0 > 400 pA and/or IpH5.0 > 1 nA). Among these cells, we considered that an alkaline sensitivity was present only when the amplitude of the sustained current induced upon alkalization (pH 8.0) was of at least 10 pA (i.e., IpH8.0 > 10 pA).
Patch-Clamp Experiments.
We used the whole-cell configuration of the patch-clamp technique to measure membrane currents (voltage clamp). Recordings were made at room temperature using an axopatch 200B amplifier (Axon Instruments) with a 3-kHz low-pass filter (Krohn-Hite). Data were sampled at 10 kHz, digitized by a Digidata 1440 A-D/D-A converter (Axon Instruments) and recorded on a hard disc using pClamp software (version 10; Axon Instruments). Patch pipettes (1–4 MΩ) contained (in millimolars): 135 KCl, 2.5 Na2-ATP, 2 MgCl2, 2.1 CaCl2, 5 EGTA, 10 Hepes (pH 7.25 with KOH). The control bath solution contained (in millimolars): 145 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 Hepes, 10 glucose (pH 7.4 with NaOH). Mes was used instead of Hepes to buffer the solution pH, which ranged from 6 to 5 and ASIC currents were induced by shifting one out of eight outlets of the microperfusion system from a holding control solution (i.e., pH 7.4 or 8.0) to an acidic (pH < 7.4) or an alkaline (pH > 7.4) test solution. Glucose (10 mM) was added to the control bath solution.
Supplementary Material
Acknowledgments
We thank M. Lazdunski for fruitful discussion and comments on the manuscript; A. Baron, S. Diochot, P. Inquimbert, and M. Christin for helpful discussion; and C. Chevance for secretarial assistance. We thank the Fondation pour la Recherche Medicale (FRM); the Association Française contre les Myopathies (AFM); the Agence Nationale de la Recherche (ANR); the Fédération pour la recherche sur le cerveau (FRC); and FIS PI08/0014, FIS PI11/01601, RD07/0062/0006 (Inst. Salud Carlos III, Spain), and 2009SGR869 (Gen. Catalunya) for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120350109/-/DCSupplemental.
References
- 1.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 2.Deval E, et al. Acid-sensing ion channels (ASICs): Pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- 4.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 5.Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 6.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann R, et al. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 8.Molliver DC, et al. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babinski K, Lê KT, Séguéla P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–57. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- 11.de Weille JR, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett. 1998;433:257–260. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- 12.Ishibashi K, Marumo F. Molecular cloning of a DEG/ENaC sodium channel cDNA from human testis. Biochem Biophys Res Commun. 1998;245:589–593. doi: 10.1006/bbrc.1998.8483. [DOI] [PubMed] [Google Scholar]
- 13.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res. 1996;113:143–151. doi: 10.1016/s0079-6123(08)61085-7. [DOI] [PubMed] [Google Scholar]
- 14.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ugawa S, et al. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deval E, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 18.Smith ES, Cadiou H, McNaughton PA. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience. 2007;145:686–698. doi: 10.1016/j.neuroscience.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Birdsong WT, et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68(4):739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 21.Salinas M, Lazdunski M, Lingueglia E. Structural elements for the generation of sustained currents by the acid pain sensor ASIC3. J Biol Chem. 2009;284:31851–31859. doi: 10.1074/jbc.M109.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deval E, et al. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31:6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen YT, et al. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol Pain. 2009;5:1. doi: 10.1186/1744-8069-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price MP, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 25.Sluka KA, et al. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page AJ, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fromy B, Lingueglia E, Sigaudo-Roussel D, Saumet J-L, Lazdunski M. Asic3 is a neuronal mechanosensor for pressure-induced vasodilation that protects against pressure ulcers. Nat Med. 2012 doi: 10.1038/nm.2844. in press. [DOI] [PubMed] [Google Scholar]
- 29.Wultsch T, et al. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain. 2008;134:245–253. doi: 10.1016/j.pain.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wemmie JA, et al. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diochot S, et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niemeyer MI, et al. Gating of two-pore domain K+ channels by extracellular pH. Biochem Soc Trans. 2006;34:899–902. doi: 10.1042/BST0340899. [DOI] [PubMed] [Google Scholar]
- 34.Springauf A, Bresenitz P, Gründer S. The interaction between two extracellular linker regions controls sustained opening of acid-sensing ion channel 1. J Biol Chem. 2011;286:24374–24384. doi: 10.1074/jbc.M111.230797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paukert M, Chen X, Polleichtner G, Schindelin H, Gründer S. Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem. 2008;283:572–581. doi: 10.1074/jbc.M706811200. [DOI] [PubMed] [Google Scholar]
- 36.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 37.Li M, et al. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J Cereb Blood Flow Metab. 2010;30:1247–1260. doi: 10.1038/jcbfm.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci USA. 2006;103:11376–11380. doi: 10.1073/pnas.0600768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhaka A, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita F, et al. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain. 1991;114(Pt 1B):527–540. doi: 10.1093/brain/114.1.527. [DOI] [PubMed] [Google Scholar]
- 42.Greenwald RA, Deluca RF, Raskin JB. Pancreatic-pleural fistula: Demonstration by endoscopic retrograde cholangiopancreatography (ERCP) and successful treatment with radiation therapy. Dig Dis Sci. 1979;24:240–244. doi: 10.1007/BF01308438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.