Abstract
A key question in developmental biology is how cellular patterns are created and maintained. During the formation of the Arabidopsis root, the endodermis, middle cortex (MC), and cortex are produced by periclinal cell divisions that occur at different positions and at different times in root development. The endodermis and cortex arise continuously from the periclinal divisions of cells that surround the quiescent center (QC) at the tip of the root. The MC arises between days 7 and 14 from periclinal divisions of the endodermis. The divisions that produce the middle cortex begin in the basal region of the root meristem away from the QC and then spread apically and circumferentially around the root. Although the transcription factor SHORT-ROOT (SHR) is required for both of these divisions, the mechanism that determines where and when SHR acts to promote cell division along the longitudinal axis of the root is unknown; SHR is present along the entire length of the root tip, but only promotes periclinal divisions at specific sites. Here we show that the abundance of the SHR protein changes dynamically as the root develops, and that the pattern of cell division within the endodermis is sensitive to the dose of this protein: high levels of SHR prevent the formation of the MC, whereas intermediate levels of SHR promote MC formation. These results provide a mechanism for the longitudinal patterning of the endodermis, and represent the first example in plants of a mobile transcription factor whose function (activator or repressor) depends upon concentration.
Keywords: SCARECROW, intercellular protein movement, plasmodesmata, gibberellin
Upon germination, the Arabidopsis root is composed of concentric layers of epidermis, cortex, and endodermis that surround the stele (the vascular tissues and pericycle; Fig. S1) (1). The endodermis and cortex are clonally related cell types that collectively comprise the ground tissue (2, 3). The formation of separate endodermal and cortical cell layers is mediated by the activity of two related GRAS family transcription factors, SHORT-ROOT and SCARECROW (SCR) (4–6). SHR is expressed in the stele cells of the Arabidopsis root and moves into the neighboring cells, which include the quiescent center (QC) cells, the cortical endodermal initials (CEIs), the cortical endodermal daughters (CEDs), and the endodermis. In all of these cells, SHR up-regulates the expression of the SCR transcription factor (4–7). In the CED both SHR and SCR activate the expression of a D-type cyclin, CYCD6;1, which promotes the periclinal divisions that maintain the separate endodermis and cortex cell layers (8). Although SHR and SCR are present in all cells that immediately surround the stele, during the first week of root growth, CYCD6;1 expression is largely confined to the CEI and the CED cells. Later in root development, CYCD6;1 is up-regulated in the endodermis where it promotes the periclinal cell divisions that generate middle cortex (MC) “M” in Fig. S1). MC formation is significantly reduced in cycd6;1 mutants and is lost entirely in shr-2 (nulls) (8, 9). In contrast, SCR, which promotes expression of CYCD6;1 in the CEI and CED cells, inhibits the development of MC; in scr-4 (null) mutants MC forms days earlier than in wild type (9). As all endodermal cells in a wild-type root express both SHR and SCR from the time of germination it is unclear what initiates the formation of MC. It is known however that gibberellin (GA) inhibits the formation of MC, and that GA function decreases in the meristem starting at 5 d postgermination (9–11).
As SHR is a key regulator of root patterning, much work has been done to determine how SHR movement and function are controlled. Structure–function analysis of SHR showed that the movement of SHR is a regulated process that is dependent upon sequences within the SHR protein and upon subcellular localization. Fully nuclear localized SHR does not move, presumably because the protein is trapped in this domain (12, 13). Cui et al. showed that SHR-dependent up-regulation of SCR in the endodermis regulates nuclear localization of SHR and prevents its movement into the cortex (14). Likewise Welch et al. identified the JACKDAW (JKD) transcription factor, which is expressed in the endodermis and limits movement of SHR into the cortex (15). A reduction in SCR or loss of JKD leads to reduced nuclear localization of SHR and movement of SHR outside of the endodermis. As a consequence, these roots show ectopic cell divisions in the ground tissue that lead to an increased number of cell layers in the root (14, 15). In contrast, roots that entirely lack SHR function or movement fail to initiate periclinal cell divisions in the ground tissue and consequently lack an endodermis and MC (4, 9). These results suggest that the regulation of SHR movement is critical to the normal patterning of the Arabidopsis root. They also suggest potential mechanisms by which roots that produce variable numbers of cortex cell layers may regulate circumferential patterning (16).
Recently we identified an essential protein, SHR INTERACTING EMBRYONIC LETHAL (SIEL), which promotes the movement of SHR out of the stele. The effects of weak siel mutations on SHR movement and root growth suggest that SHR both promotes and inhibits cell divisions in the endodermis. Contrary to what was seen in plants with no SHR activity, plants with a moderate decrease in SHR movement or SHR activity showed precocious periclinal cell divisions in the endodermis. This phenotype was observed both in shr-2 hetrozygotes and in siel hypomorphs. These results suggest that when SHR is maintained at high levels in the endodermis, periclinal cell divisions are inhibited, whereas a decrease in SHR stimulates cell division. Interestingly SIEL expression is up-regulated by both SHR and SCR, suggesting that SHR may promote its own movement. These results along with SCR’s role in the nuclear localization of SHR may therefore be a mechanism for concentrating SHR in the endodermis and regulating cell division (17).
Consistent with our proposal, researchers looking at SHR function in poplar showed that plants with a moderate reduction in the level of PtSHR activity actually grew larger than wild type. This affect could be phenocopied in Arabidopsis by reducing SHR levels. They suggest that SHR acts in a concentration-dependent manner to regulate growth (18). Here we show dose-dependent effects of SHR on the regulation of cell divisions in the endodermis and show that one of the mechanisms for regulating the level of SHR in the endodermis and therefore its function (either activator or repressor of cell division) is the modulated movement of SHR.
Results
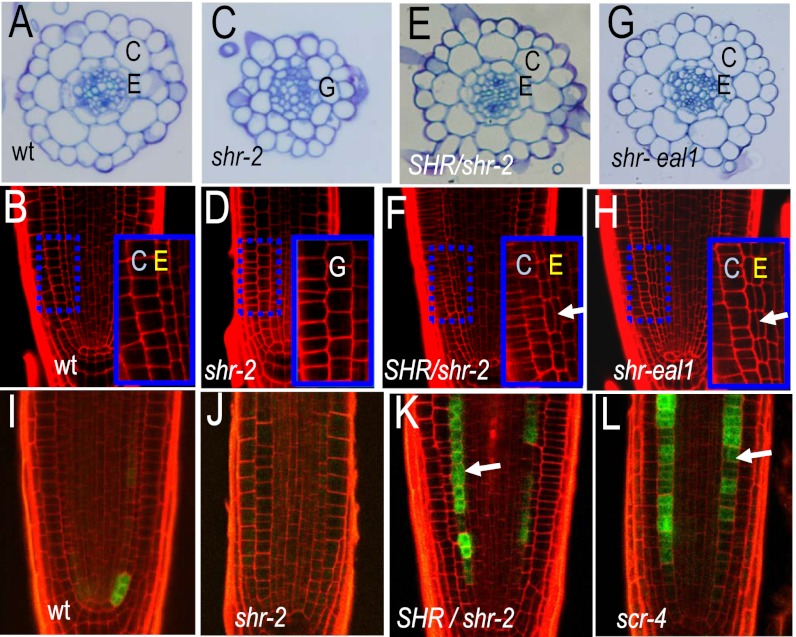
At the time of germination, wild-type Arabidopsis roots have two ground tissue layers (Fig. S1 and Fig. 1 A and B), which are maintained by periclinal divisions of the CED cells (Fig. S1) (1). In contrast, in the shr-2 mutants there is only one ground tissue layer between the stele and epidermis (Fig. 1 C and D) and no periclinal divisions in the ground tissue (4, 9). Recently we showed that roots that are heterozygous for the shr-2 allele have a third ground tissue layer at a stage in development when the wild-type root has only two (Fig. 1 E and F) (17). With respect to root length and patterning of the meristem, the shr-2 heterozygotes are otherwise largely normal. As the expression of CYCD6;1 both correlates with and promotes the formation of MC, we examined expression of pCYCD6;1:GFP-GUS in the endodermis of shr-2 heterozygotes and for comparison in the roots of wild-type plants, shr-2 homozygotes (which form no MC) and scr-4 homozygotes (which form MC precociously). Consistent with their respective phenotypes, in 5-d-old roots we saw expression of pCYCD6;1:GFP-GUS predominately in the CEI and CED cells of the wild type (Fig. 1I), in the ground tissue of shr-2 heterozygotes (Fig. 1K), and no expression of pCYCD6;1:GFP-GUS in the meristem of shr-2 mutants (Fig. 1J). However, the pCYCD6;1:GFP-GUS transgene was strongly expressed in lateral root primordia in shr-2 roots, indicating that the transgene was present and functional in these roots (Fig. S2A). In the scr-4 nulls, which make precocious MC, there was moderate to strong expression of pCYCD6;1:GFP-GUS in the ground tissue that correlated with periclinal cell divisions (Fig. 1L). In none of the lines examined did we see expression of pCYCD6;1:GFP-GUS in the QC or an increase in the expression in the initial cells.
Fig. 1.
Roots with reduced SHR activity show ectopic periclinal cell divisions in the endodermis. (A, C, E, and G) Toluidine blue stained transverse cross-sections through 5-d-old roots; genotypes as indicated. (B, D, F, and H) Longitudinal confocal cross-sections through the root meristems of wild-type and mutant individuals; genotypes as indicated. Arrows point to precocious divisions. Region in the blue dashed box is magnified in the Inset. E, endodermis; C, cortex; and G, ground tissue. (I–L) Wild-type and mutant roots (as indicated) expressing pCYCD6;1:GFP-GUS. Arrows point to precocious periclinal cell divisions.
To test whether the precocious ground tissue phenotype we observed in the shr-2 heterozygotes was peculiar to the shr-2 allele, we obtained a hypomorphic allele of SHR, eal1 (endodermal-amyloplast less 1; to avoid confusion this allele is designated as shr-eal1 throughout the rest of the study) that has a single amino acid deletion (E230) and is reported as having normal root growth, but no gravitropic response in inflorescence stems and a reduced gravitropic response in the hypocotyl (19). The shr-eal1 roots were the same length as wild type and overall the seedlings looked normal; however, they were always slightly larger than the same stage wild type (Fig. S2B). Examination of the cellular patterning of roots homozygous for the shr-eal1 allele revealed sporadic increases in the number of ground tissue layers, similar, but at a higher frequency to what was seen in the shr-2 heterozygotes, (Fig. 1 G and H) and expression of pCYCD6;1:GFP-GUS in the endodermis at day 5 (Fig. S2C). In addition, the shr-eal1 roots showed some abnormally oriented cell divisions in the QC and surrounding cells similar to what is seen in shr-2 homozygotes. These results are consistent with the hypothesis that a decrease in SHR promotes periclinal cell divisions in the endodermis and therefore MC formation.
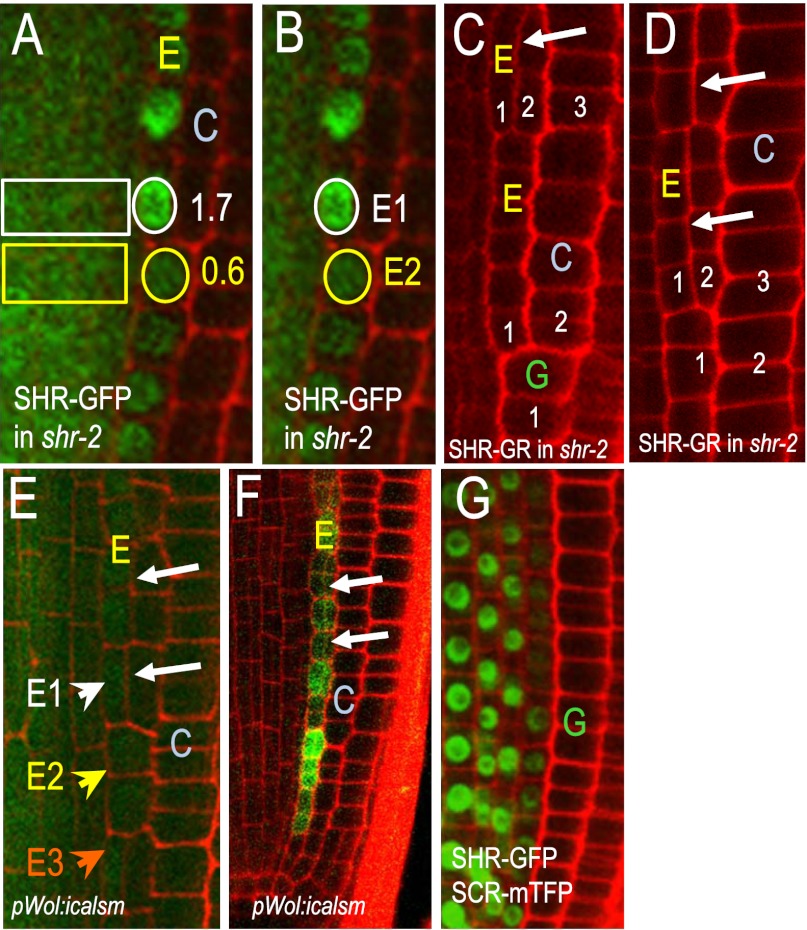
If high levels of SHR inhibit periclinal cell divisions in the endodermis, then one would expect to see a reduction in the levels of SHR before the formation of MC. When roots were grown on solid Murashige and Skoog (MS) growth medium with agar, MC began forming between day 7 and day 14 (9). Periclinal cell divisions began in endodermal cells that were in the basal region of the meristem (away from the QC; Fig. S1) and then progressed apically toward the QC (in a rootward direction) until all of the cells in the endodermis had divided (9, 20). To test whether SHR levels changed during this process, we examined 5 and 14-d-old roots expressing the pSHR:SHR-GFP transgene and measured the level of GFP fluorescence in the endodermis and expressed this as a ratio of stele fluorescence, as the stele is the source of the SHR protein in the endodermis. In 5 d-old plants that are not forming MC, the average ratio of endodermal-to-stele fluorescence was 1.2. A similar ratio (1.1) was found in regions of 14-d-old roots that were not in the process of forming MC. In comparison we found striking differences in the endodermal-to-stele ratios of SHR-GFP in cells that had recently divided to form MC and those that were next in line to divide (Fig. 2A). In cells that were in-line to divide (but still had an intact nucleus) the endodermal-to-stele ratio was 0.6. In cells that had recently divided, the ratio was 1.7. The difference in the ratios between these two regions was not the result of changes in the levels of SHR in the stele as this was fairly stable during the first 2 wk of root growth. In addition, to eliminate the stele signal entirely from the calculation, we measured the level of fluorescence in the first divided endodermal cell (Fig. 2B; white circle, E1) as well as the cell immediately apical to it (Fig. 2B; yellow circle, E2). We found that the average fluorescent intensity in the recently divided cell was 2.4 times that of the undivided cell (Fig. 2B; E1/E2), indicating that a reduction in SHR-GFP levels correlates with induction of MC formation.
Fig. 2.
A reduction in SHR precedes cell division. (A and B) SHR-GFP in 14-d-old roots. Numbers in A indicate the endodermal-to-stele ratios of SHR-GFP fluorescence. White circles outline nuclei of recently divided endodermal cells, whereas yellow encircles those that are next in line to divide. (B) To eliminate the stele from the calculation of SHR-GFP levels in the endodermis, the fluorescence intensity in recently divided endodermal cells like E1 were compared with those that are in line to divide, E2. This comparison yielded an average ratio of 2.4. (C and D) Transfer of the shr-2 roots expressing pSHR:SHR-GR from dex-containing medium to standard MS medium lacking dex resulted in periclinal cell divisions in the endodermis (arrows). Small white numbers indicate the ground tissue layers relative to the stele. (E) Reduction in the movement of SHR-GFP in the pWOL:icalsm line resulted in periclinal cell divisions (arrows) and (F) activation of expression of pCYCD6;1:GFP-GUS in the endodermis. In E the white arrowhead E1 points to the nucleus of a divided endodermal cell, whereas the yellow E2 and orange E3 arrowheads point to undivided cells. (G) Expression of pSHR:SCR-mTFP in wild-type roots inhibits the movement of SHR-GFP and the formation of separate endodermis and cortex. E, endodermis; C, cortex; and G, ground tissue throughout.
We next asked whether we could directly affect cell divisions in the endodermis by controlling SHR activity. If the amount of active SHR in the endodermis affects SHR function, then direct modification of SHR levels should affect ground tissue patterning. As a way of directly affecting SHR activity, we examined shr-2 roots expressing the pSHR:SHR-GR transgene. As previously shown by Levesque et al., germination of shr-2 plants expressing the pSHR:SHR-GR transgene on media containing 10 μM dexamethasone (dex) rescued both the root length and root patterning defects associated with the shr-2 mutation (21). We reasoned that if SHR acts in a concentration-dependent manner, then removal of the shr-2 roots expressing pSHR:SHR-GR from dex treatment should decrease SHR activity and cause a transient increase in periclinal cell divisions in the endodermis. To test this possibility, we grew the shr-2 pSHR:SHR-GR roots on dex-containing medium for 3 d and then transferred them overnight to fresh medium without dex. Upon removal of dex treatment, the effective levels of SHR should gradually decrease as dex is degraded and the active SHR in the nucleus turns over. When we examined root patterning 18–24 h after removal from dex, ∼50% of the shr-2 pSHR:SHR-GR roots had reverted (in the region examined) back to one ground tissue layer (Fig. 2C labeled “G”) instead of two, consistent with a loss of active SHR. In these roots, we saw regions of the endodermis that had undergone expansion to three ground tissue layers before the transition to a single ground tissue layer. In addition, in roots that had not yet transitioned back to one ground tissue layer there were often three ground tissue layers, which is indicative of periclinal cell divisions in the endodermis (Fig. 2D). In 4-d-old wild-type roots, we never observed periclinal cell divisions in the endodermis. These results are consistent with intermediate levels of SHR inducing periclinal cell divisions in the endodermis.
Multiple groups have shown that GA inhibits the periclinal cell divisions in the endodermis that form the MC (9, 10). Therefore, one can induce precocious MC formation by treating roots with paclobutrazol (PAC), an inhibitor of GA biosynthesis. Previous results showed that when plants were treated with 2.0 μM PAC for 7 d, there was a significant increase in periclinal cell divisions in all cells in the endodermis and that these cells adopted a MC cell fate (9). Consistent with published results, we found that wild-type, but not shr-2 roots responded to PAC treatment with an induction of periclinal cell divisions in the endodermis (Fig. S3A). The formation of MC in the presence of PAC was not the same as it was when it occurred naturally. In the presence of PAC, periclinal cell divisions occurred sporadically throughout the endodermis instead of in a basal-to-apical (a rootward) progression. The increase in cell divisions in the presence of PAC was supported by the expression of pCYCD6;1:GFP-GUS in the endodermis (Fig. S2 D and E). This was also true in the shr-2 heterozygotes, which were more sensitive to PAC treatment than wild type (Fig. S3A), suggesting that wild-type levels of SHR partially buffer the effects of PAC.
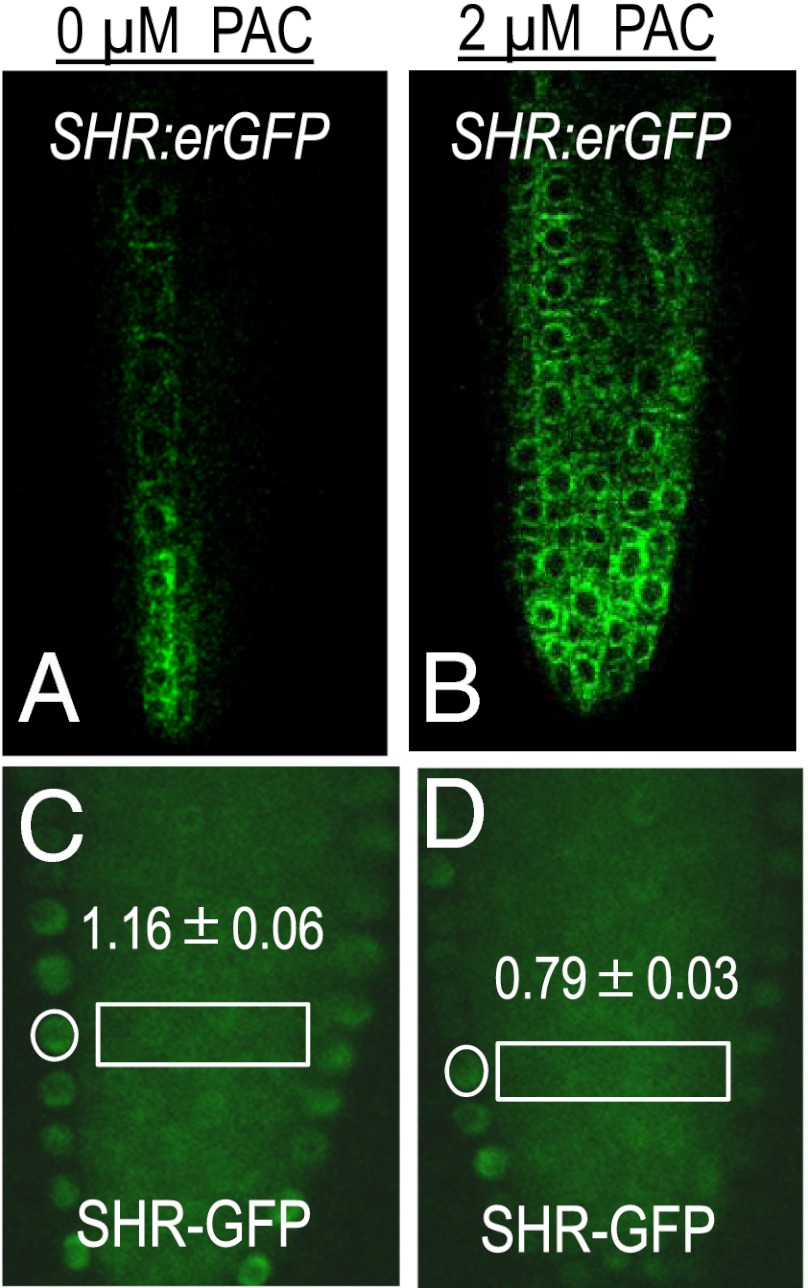
To test whether PAC-mediated induction of MC formation occurred through the regulation of SHR levels, we measured SHR mRNA in 5-d-old whole roots that were treated short-term with PAC. Surprisingly we found a 2.5-fold increase in SHR mRNA (Fig. S3B) in PAC-treated roots relative to the controls. As these results represent an overall increase in SHR mRNA throughout the root, including incipient and emerging lateral roots, which are inhibited by GA the bulk of this increase could represent lateral root production (22). To eliminate lateral root effects, we examined GFP levels in the meristems of roots expressing the pSHR:erGFP construct. By this method, we also found an increase in pSHR expression in the presence of PAC. However, in the meristem the increase was only 1.5-fold of the untreated controls (Fig. 3 A and B and Fig. S3C). To look at overall SHR protein levels, we measured the SHR-GFP signal in stele cells of the control and PAC-treated roots (stele-to-stele ratios) and found that the SHR-GFP levels were unchanged by PAC treatment (Fig. S3E). However, when we examined the endodermal-to-stele ratio, we saw a significant difference between the untreated and the PAC-treated roots (Fig. 3 C and D). Compared with the untreated control plants whose endodermal-to-stele ratio of SHR-GFP was 1.2, the ratio in the PAC-treated roots was 0.8 (Fig. 3D), very similar to what we observed in the 14-d-old plants that were undergoing normal MC formation (Fig. 2A). Collectively these results show that, whereas PAC moderately increased the activity of pSHR in the meristem, the overall SHR protein levels in the stele were unchanged. There was however a significant reduction in SHR in the endodermis of PAC-treated roots, which is consistent with the reduction in SHR that preceded normal formation of MC (Fig. 2 A and B). These results suggest that PAC acts via SHR to induce MC and that PAC reduces SHR in the endodermis at the level of the protein, not at the level of transcription.
Fig. 3.
Reduction in GA signaling results in a decrease in SHR-GFP in the endodermis. (A and B) Short-term treatment of 5-d-old roots with PAC (B) resulted in a significant increase in the expression of pSHR-erGFP (quantified in Fig. S3C) that did not translate into an increase in the amount of SHR-GFP protein as indicated by similar levels of SHR-GFP in the stele of untreated and PAC-treated roots. (C and D, also see Fig. S3D) However, there was a significant decrease in the endodermal-to-stele ratio of SHR-GFP after PAC treatment (D), indicating an overall decrease in the levels of SHR-GFP in the endodermis.
As SHR movement is required for its function (12, 13), one way in which the activity of SHR can be controlled is through the regulation of its movement. If reduced SHR levels in the endodermis are what induce periclinal cell divisions in this tissue, then reducing SHR movement should promote cell division. To test this possibility, we turned to an estradiol-inducible system that takes advantage of a dominant, synthetic allele of CALLOSE SYNTHASE 3 (icalsm) that is expressed from the stele-specific WOODEN-LEG promoter (pWOL). Previously we showed that expression of the pWOL:icalsm transgene caused an increase in the deposition of callose at plasmodesmata and a uniform decrease in the plasmodesmatal aperture that correlated with a reduction in SHR movement between the stele and endodermis (23). For the experiments examining SHR movement, plants carrying both the pWOL:icalsm construct and the pSHR:SHR-GFP transgene were grown on normal growth medium for 4 d before being treated with 10 μM estradiol for 12–20 h. Roots that showed a moderate reduction in SHR movement had a dramatic increase in the number of periclinal cell divisions in the endodermis (Fig. 2E) and a dramatic increase in the expression of pCYCD6;1:GFP-GUS (Fig. 2F). Periclinal cell divisions were not observed in the endodermis of wild-type plants of the same age. Nor were periclinal cell divisions observed in the ground tissue of plants in which SHR movement was fully blocked by the expression in the stele of the SCR protein as a fusion to mTFP (in the endodermis SCR normally blocks movement of SHR into the cortex or back into the stele) (Fig. 2G). Plants expressing pSHR:SCR-mTFP failed to produce MC or separate cortex and endodermal cell layers. These results indicate that a complete block to SHR movement results in the loss of a ground tissue layer. In contrast, a decrease in SHR movement induces periclinal cell divisions in the endodermis and increases the number of ground tissue layers. These results are consistent with the finding that SHR levels decreased in the endodermis before formation of the MC (Fig. 2 A and B) and that SHR is required for these divisions.
The difference in SHR levels between divided (E1) and soon-to-divide endodermal cells (E2) observed in Fig. 2B could result from inherent differences in the stability of the SHR protein or the regulation of SHR movement into the different cells in the endodermis. For example, loss of SHR may be accelerated in the endodermal cells that are in line to form MC (E2 in Fig. 2B) or SHR movement into these cells (E2 in Fig. 2B) may be reduced. To distinguish between these two possibilities, we examined the levels of SHR-GFP in the endodermal cells of pWOL:icalsm roots that were induced to form MC through a reduction in SHR movement (Fig. 2E). If the difference in the E1:E2 ratio of SHR-GFP in Fig. 3B was entirely the result of differential stability of the SHR protein in these two endodermal cell types (divided versus soon to divide), the elevated E1:E2 ratio should not be diminished by an overall decrease in SHR movement. Conversely if the disparity in protein levels is the result of unequal movement, a block to movement should normalize these differences. This is largely what we observed. The level of SHR-GFP in the pWOL:icalsm lines tended to be marginally higher in the divided endodermal cells relative to the undivided cells (E1/E2 = 1.2 ± 0.08); nonetheless this ratio was significantly different from what we observed in roots where SHR movement had not been modified (Fig. 2B E1/E2 = 2.4). Likewise there was no difference in the ratio of fluorescence between the undivided endodermal cells (Fig. 2E the E2:E3 ratio = 0.95 ± 0.19). These results suggest that, whereas there may be inherent differences in SHR stability in the different endodermal cells, this cannot explain all of the variation observed.
Discussion
How developmental patterns are created and maintained is a fundamental question of biology. In animals, secreted proteins like Wnt, FGF, and TGF-β have been shown to act as morphogens (24–30). Their function depends upon their concentration and is generally inversely proportional to the distance from the source. During the development of Drosophila melanogaster, transcription factors like Bicoid and Hunchback are able to function as mobile signals (31, 32) due to the syncytial nature of the early Drosophila embryo. As nearly all plant cells are connected via plasmodesmata, plants are essentially syncytial throughout most, if not all of their development. Surprisingly, however there are no proteins or peptides with the exception of the phytohormones that move between cells and act in a concentration-dependent manner. The PLETHORA proteins have been shown to act in a concentration-dependent manner, but their concentration is dependent upon transcription and perhaps protein stability, but not cell-to-cell movement (33, 34). In contrast, miRNA165/66 is a mobile RNA that moves through plasmodesmata and acts in a dose-dependent manner (23, 35). Here we show that the function of the SHR transcription factor varies with dosage. In the root, the SHR protein is required to maintain the meristem and for the formation of endodermis (4, 7). Later in the development of the root, SHR is necessary for the periclinal cell divisions that form the MC (9). Examination of SHR-GFP showed that a decrease in SHR levels precedes the periclinal cell divisions that form MC and that following the formation of the MC, SHR levels are considerably higher in the divided endodermal cells than they were before MC formation began. These results are consistent with a model in which high levels of SHR inhibit periclinal cell division and moderate levels promote it.
It is known that the GA pathway regulates MC formation. Mutations that inhibit GA production or sensing promote formation of MC; whereas mutations that enhance GA signaling delay this process. Therefore, one can speed the appearance of periclinal cell divisions in the endodermis through the application of PAC, an inhibitor of GA biosynthesis (9, 10). The MC that forms after PAC induction does not follow the regular stepwise pattern that is seen in 7- to 14-d-old roots; instead periclinal cell divisions are induced throughout the endodermis (9). Consistent with a role for SHR in this process, the amount of SHR in the endodermis decreased uniformly in the endodermis upon PAC treatment. One explanation for this decrease is that GA regulates SHR turnover in the endodermis. GAs promote the degradation of other GRAS proteins, specifically the DELLA class proteins (36, 37). As GA inhibits the formation of MC, one would predict an opposite effect on SHR function (i.e., that GA would decrease SHR degradation). An alternative explanation is that GA regulates SHR movement. It is known that GAs can induce the expression of 1,3 β-glucanases to regulate plasmodesmata (PD). The 1,3 β-glucanases break down callose and therefore increase transport through PD particularly in perennial plants, which restrict symplastic signaling during dormancy (38–41). In these plants, GAs open PD and allow regrowth. Recent results by Moubayidin suggest that GA activity decreases in the root meristem at 5 d past germination (11), which in our experiments would correspond to a day 7 seedling, the time at which we first see the start of MC formation. Therefore, a local reduction in GA during MC formation may be the driving force upstream of SHR that precipitates the periclinal cell divisions in the endodermis.
Although we found an overall decrease in the levels of SHR-GFP in endodermal cells after PAC treatment, there was actually a 1.5-fold increase in the expression of pSHR:SHR-GFP after PAC treatment. This increase in SHR transcription was not borne out as an increase in SHR protein, as there was no significant difference in SHR-GFP levels in the stele cells of the PAC-treated and the untreated control roots. This may indicate either an overall increase in the breakdown of SHR in the stele after translation or an uncoupling of SHR transcription and translation. In support of the second hypothesis, Nicolai et al. found a significant number of genes whose transcripts increased in the absence of sucrose, without a concomitant increase in the levels of polysomal RNA (42). Likewise Ribeiro et al. showed that for several of the genes in the GA pathway there was a significant increase in mRNA expression upon PAC treatment, but not an associated increase in translation (43). These results suggest that a considerable level of translational buffering is possible. This may explain the difference between the SHR mRNA and protein levels in the presence of PAC.
To test whether a reduction in SHR can directly trigger the asymmetric divisions that generate MC, we reduced SHR activity by either inhibiting its ability to effectively enter the nucleus or by decreasing the movement of SHR from the stele to the endodermis. In shr-2 plants that were dependent upon SHR-GR for normal radial patterning, removal of the roots from dex resulted in ectopic perclinal cell divisions in the endodermis and a transient increase in the number of ground tissue layers before reversion to one ground tissue layer. Similarly a partial blockage of SHR movement into the endodermis through activation of pWOL:icalsm (23) increased the number of ground tissue layers from two to three. In these roots, the reduction in SHR movement largely diminished the difference in SHR-GFP levels between the divided cells (E1 in Fig. 2B) and those in line to divide (E2 cells Fig. 2B), suggesting that differential movement of SHR plays a larger role in creating the E1:E2 ratio (shown in Fig. 2B) than differential degradation of the SHR protein. However, we cannot rule out the possibility that the amount of SHR entering the endodermis affects the rate at which SHR is degraded. Therefore, we cannot conclusively assert that the reduction in SHR in the endodermis primarily reflects a decrease in movement rather than an increase in protein degradation. Independent of the precise mechanism (i.e., reduced movement of SHR, increased protein turnover or both) our results clearly show that a moderate reduction in SHR promotes periclinal cell divisions in the endodermis and that this reduction precedes formation of MC. In light of these results it will be interesting to see how SHR homologs, which are found throughout the plant kingdom (44) function in roots that undergo multiple rounds of ground tissue expansion, and whether MC formation plays a significant role in the generation of a multilayered cortex. For example in rice, OsSHR1 is expressed in the stele and retains the ability to interact with OsSCR1 (and AtSCR), indicating a conservation of function. However, in rice, the series of repetitive periclinal divisions that results in a radial expansion of the ground tissue occurs very early in root growth and at the level of the initials, suggesting a minor role for MC in the creation of a multilayered cortex in rice (16).
One of the most striking phenotypes of the shr nulls is the drastic reduction in root length caused by an inability to maintain the QC. Interestingly, we did not see any effects of reduced SHR levels on the regulation of root growth. Roots with largely blocked (those expressing pSHR:SCR-mTFP) or reduced SHR movement (pWOL:icalsm on estradiol), along with the shr-2 heterozygotes and the shr-eal1 homozygotes all had normal root lengths, indicating that even a dramatic reduction in SHR levels does not obviously affect meristem maintenance. These results are consistent with our previously published results showing that cell-autonomous SHR can rescue shr-2 root length.
A recent publication by Wang et al. (18) examining SHR function (PtSHR1) in hybrid poplar showed that reduced PtSHR1 activity (through the introduction of RNAi constructs) resulted in an increase in the size of the plant. Plants with reduced PtSHR1 were taller and showed an increase in girth compared with plants with normal levels of PtSHR activity. In addition they showed that reduction of SHR in Arabidopsis increased the overall size of the plant (18). While under our growth conditions we did not see an overall increase in plant growth in the shr-2 heterozygotes, there was an increase in the size of the shr-eal1 homozygotes compared with wild type. The length of the shr-eal1 roots was similar to wild type; however, the aerial portions were larger and there were generally more leaves (Fig. S2B). This effect was seen at day 7 and was even more pronounced at later stages. Taken together with Wang’s result, our results suggest that SHR may have a dose-dependent effect on overall plant growth through the regulation of cell division.
Conclusions
Together with data from multiple different groups (8–10, 14, 15, 17) we propose an integrated model for SHR function (Fig. S4) in which SHR is maintained at high levels in the endodermis through its targeted movement (directly facilitated by SIEL and indirectly by SCR, which promotes expression of SIEL) (16) followed by its subsequent trapping in the endodermis by SCR and JKD. Under these conditions, SHR levels are sufficient to inhibit the formation of MC. Later in development, SHR levels decrease in the endodermis. This reduction allows the expression of CYCD6;1 and therefore the formation of MC. It remains to be determined what signals precipitate the reduction in SHR in the endodermis, but one likely candidate is GA, as GA is known to inhibit MC formation and GA function gradually decreases in the meristem starting at 5 d postgermination (11).
Materials and Methods
Plant Materials and Growth Conditions.
The sources of the mutant and transgenic lines are referenced throughout the paper. To observe the expression pattern of pCYCD6;1:GFP-GUS in the mutant backgrounds, crosses were conducted and the F1 and F2 individuals genotyped by PCR. Surface sterilized seeds were sown on plates containing 0.5× Murashige and Skoog (MS; Caisson) salt, 0.05% (wt/vol) Mes (pH 5.7), 1% (wt/vol) sucrose, and 0.5% (wt/vol) phytagel (Sigma-Aldrich), and cold treated for 3–5 d at 4 °C in the dark. Plates were then incubated vertically in a growth chamber at 23 °C under 16 h light/8 h dark cycle.
PAC Estradiol and Dex Treatment.
Stock solutions of 100 mM PAC (PhytoTechnology Laboratories), 100 μM β-estradiol (Sigma-Aldrich), and 100 μM dexamethasone (Sigma-Aldrich) were made and stored at −20 °C. For PAC treatment, seeds were germinated for 30 h on regular MS plates and individually transferred to new MS plates supplemented with 2 μM PAC. Roots were then examined at day 7 for the percentage showing periclinal cell divisions in the endodermis. For all treatments a minimum of 17 and maximum of 47 roots were examined. For short-term (6–7 h) PAC treatment, 4- to 5-d-old seedlings were individually transferred to MS plates supplemented with PAC. For analysis of cell division in the pWOL:icalsm line, seedlings were treated with 10 μM β-estradiol for 12–24 h before observation. For SHR-GR, seeds were grown for 3 d on MS plates supplemented with 10 μM dex before being individually transferred to normal MS plates. For all direct comparisons the same transgene insertion lines were used to control for position effects.
Microscopy and Imaging.
Root cross-sections were prepared as described (45). Confocal images were obtained using a Leica TCS SL microscope. Before visualization, the roots were stained in 0.01 μg/mL PI in water. For GFP quantification of pSHR:SHR-GFP and pSHR:erGFP, images were collected on the same day using identical confocal settings. The average GFP intensity was measured in ImageJ software (http://rsbweb.nih.gov/ij/) on unmodified root images. Quantitative analysis of pSHR:SHR-GFP signal intensity in the endodermis relative to the stele was done on medial sections through the meristem in which the endodermal cell nuclei were clearly visible (17). Briefly the average intensity of SHR-GFP in a single endodermal cell nucleus was calculated using ImageJ. In the same longitudinal domain as the measured endodermal cell the average stele intensity was determined (as shown in the boxed region in Figs. 2A and 3C) yielding the endodermal-to-stele ratio. More than 10 paired measurements were made for each root. For quantification of SHR-GFP levels between different roots the average intensity of SHR-GFP in the meristem region of the stele was calculated from medial, longitudinal cross-sections using ImageJ. Statistics were done using Excel (Microsoft Office). A minimum of 8 and maximum of 30 roots were used for each analysis. For all direct comparisons the same transgene insertion lines were used to control for position effects. PCR-based genotyping of SHR/SHR and SHR/shr-2 was carried out using the aerial parts of seedlings.
Supplementary Material
Acknowledgments
We thank Dr. P. Benfey and Dr. R. Sozzani (Duke University), Dr. M. Morita (Nara Institute of Science and Technology), and Dr. Y. Helariutta (University of Helsinki) for providing seeds and R.S. Poethig and D. Wagner for critical suggestions. This work was supported by National Science Foundation Grant 0920327 (to K.L.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205579109/-/DCSupplemental.
References
- 1.Dolan L, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Scheres B, McKhann HI, Van Den Berg C. Roots redefined: Anatomical and genetic analysis of root development. Plant Physiol. 1996;111:959–964. doi: 10.1104/pp.111.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheres B, et al. Embryonic origin of the Arabidopsis primary root and meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- 4.Helariutta Y, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- 5.Di Laurenzio L, et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 6.Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN. Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development. 2000;127:595–603. doi: 10.1242/dev.127.3.595. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 8.Sozzani R, et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466:128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paquette AJ, Benfey PN. Maturation of the ground tissue of the root is regulated by gibberellin and SCARECROW and requires SHORT-ROOT. Plant Physiol. 2005;138:636–640. doi: 10.1104/pp.104.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heo JO, et al. Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc Natl Acad Sci USA. 2011;108:2166–2171. doi: 10.1073/pnas.1012215108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moubayidin L, et al. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr Biol. 2010;20:1138–1143. doi: 10.1016/j.cub.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher KL, Benfey PN. Both the conserved GRAS domain and nuclear localization are required for SHORT-ROOT movement. Plant J. 2009;57:785–797. doi: 10.1111/j.1365-313X.2008.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher KL, Paquette AJ, Nakajima K, Benfey PN. Mechanisms regulating SHORT-ROOT intercellular movement. Curr Biol. 2004;14:1847–1851. doi: 10.1016/j.cub.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 14.Cui H, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316:421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- 15.Welch D, et al. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007;21:2196–2204. doi: 10.1101/gad.440307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauluzzi G, et al. Surfing along the root ground tissue gene network. Dev Biol. 2012;365:14–22. doi: 10.1016/j.ydbio.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Koizumi K, Wu S, MacRae-Crerar A, Gallagher KL. An essential protein that interacts with endosomes and promotes movement of the SHORT-ROOT transcription factor. Curr Biol. 2011;21:1559–1564. doi: 10.1016/j.cub.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, et al. Reduced expression of the SHORT-ROOT gene increases the rates of growth and development in hybrid poplar and Arabidopsis. PLoS ONE. 2011;6:e28878. doi: 10.1371/journal.pone.0028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita MT, Saitob C, Nakanob A, Tasaka M. Endodermal-amyloplast less 1 is a novel allele of SHORT-ROOT. Adv Space Res. 2007;39:1127–1133. [Google Scholar]
- 20.Baum SF, Dubrovsky JG, Rost TL. Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am J Bot. 2002;89:908–920. doi: 10.3732/ajb.89.6.908. [DOI] [PubMed] [Google Scholar]
- 21.Levesque MP, et al. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 2006;4:e143. doi: 10.1371/journal.pbio.0040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Péret B, et al. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Vatén A, et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21:1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 25.Abud HE, Skinner JA, Cohn MJ, Heath JK. Multiple functions of fibroblast growth factors in vertebrate development. Biochem Soc Symp. 1996;62:39–50. [PubMed] [Google Scholar]
- 26.Goldfarb M. Functions of fibroblast growth factors in vertebrate development. Cytokine Growth Factor Rev. 1996;7:311–325. doi: 10.1016/s1359-6101(96)00039-1. [DOI] [PubMed] [Google Scholar]
- 27.Entchev EV, Schwabedissen A, González-Gaitán M. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 28.Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 29.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 30.Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 31.Hülskamp M, Pfeifle C, Tautz D. A morphogenetic gradient of hunchback protein organizes the expression of the gap genes Krüppel and knirps in the early Drosophila embryo. Nature. 1990;346:577–580. doi: 10.1038/346577a0. [DOI] [PubMed] [Google Scholar]
- 32.Gregor T, Wieschaus EF, McGregor AP, Bialek W, Tank DW. Stability and nuclear dynamics of the bicoid morphogen gradient. Cell. 2007;130:141–152. doi: 10.1016/j.cell.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aida M, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Galinha C, et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- 35.Carlsbecker A, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465:316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dill A, Jung HS, Sun TP. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA. 2001;98:14162–14167. doi: 10.1073/pnas.251534098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverstone AL, et al. Repressing a repressor: Gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell. 2001;13:1555–1566. doi: 10.1105/TPC.010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leubner-Metzger G. Functions and regulation of -1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci Res. 2003;13:17–34. [Google Scholar]
- 39.Rinne PL, Kaikuranta PM, van der Schoot C. The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. Plant J. 2001;26:249–264. doi: 10.1046/j.1365-313x.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 40.Rinne PL, et al. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell. 2011;23:130–146. doi: 10.1105/tpc.110.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou HL, et al. OsGLU1, a putative membrane-bound endo-1,4-beta-D-glucanase from rice, affects plant internode elongation. Plant Mol Biol. 2006;60:137–151. doi: 10.1007/s11103-005-2972-x. [DOI] [PubMed] [Google Scholar]
- 42.Nicolaï M, et al. Large-scale analysis of mRNA translation states during sucrose starvation in arabidopsis cells identifies cell proliferation and chromatin structure as targets of translational control. Plant Physiol. 2006;141:663–673. doi: 10.1104/pp.106.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribeiro DM, Araújo WL, Fernie AR, Schippers JH, Mueller-Roeber B. Translatome and metabolome effects triggered by gibberellins during rosette growth in Arabidopsis. J Exp Bot. 2012;63:2769–2786. doi: 10.1093/jxb/err463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirsch S, Oldroyd GE. GRAS-domain transcription factors that regulate plant development. Plant Signal Behav. 2009;4:698–700. doi: 10.4161/psb.4.8.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koizumi K, Sugiyama M, Fukuda H. A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: Calling the auxin signal flow canalization hypothesis into question. Development. 2000;127:3197–3204. doi: 10.1242/dev.127.15.3197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.