Abstract
Cancer-associated thrombosis often lacks a clear etiology. However, it is linked to a poor prognosis and represents the second-leading cause of death in cancer patients. Recent studies have shown that chromatin released into blood, through the generation of neutrophil extracellular traps (NETs), is procoagulant and prothrombotic. Using a murine model of chronic myelogenous leukemia, we show that malignant and nonmalignant neutrophils are more prone to NET formation. This increased sensitivity toward NET generation is also observed in mammary and lung carcinoma models, suggesting that cancers, through a systemic effect on the host, can induce an increase in peripheral blood neutrophils, which are predisposed to NET formation. In addition, in the late stages of the breast carcinoma model, NETosis occurs concomitant with the appearance of venous thrombi in the lung. Moreover, simulation of a minor systemic infection in tumor-bearing, but not control, mice results in the release of large quantities of chromatin and a prothrombotic state. The increase in neutrophil count and their priming is mediated by granulocyte colony-stimulating factor (G-CSF), which accumulates in the blood of tumor-bearing mice. The prothrombotic state in cancer can be reproduced by treating mice with G-CSF combined with low-dose LPS and leads to thrombocytopenia and microthrombosis. Taken together, our results identify extracellular chromatin released through NET formation as a cause for cancer-associated thrombosis and unveil a target in the effort to decrease the incidence of thrombosis in cancer patients.
Thrombosis is the second most common cause of death in cancer patients. Even in the absence of obvious thrombosis, cancer patients commonly have a hypercoagulable condition without a clear etiology (1). Cancer frequently induces a systemic effect similar to infection and/or inflammatory disease, including changes in cell numbers in peripheral blood and levels of inflammatory cytokines (2). A feature of chronic myelogenous leukemia (CML) is the excess of granulocytic myeloid cells of varying maturation stages (3). In murine models of solid tumors and a variety of human cancers, an increase in myeloid cells is observed (4, 5). Granulocyte colony-stimulating factor (G-CSF) is a cytokine produced by leukocytes and endothelium and is often associated with leukocytosis and neutrophilia. G-CSF is also produced by various tumors and cancer cells (6), including leukemic cells of CML patients in chronic phase (7). Its concentration can be elevated in the blood of cancer patients and has been associated with poor clinical outcome (8–10). G-CSF activates neutrophils, stimulates oxidative metabolism (11), and increases agonist-induced platelet aggregation ex vivo (12). Despite these effects of G-CSF, only a few cases of thrombotic events have been associated with G-CSF treatment in healthy donors (13).
The release of neutrophils extracellular traps (NETs) has been identified as a mechanism of bacterial killing (14). Recently, NETs were found to promote thrombosis (15, 16) and coagulation (17). Upon contact with bacteria, neutrophils become activated, and their primary response is the engulfment of pathogens into phagosomes. At later time points, in vitro experiments suggest that NET-mediated entrapment and/or killing becomes predominant (18). Furthermore, in vitro activation of human neutrophils with a strong stimulus such as phorbol-12-myristate-13-acetate or hydrogen peroxide leads to NET generation (18). The same effect is observed with a combination of weaker stimuli such as GM-CSF and LPS or C5a (19). This suggests that priming of neutrophils predisposes them to NET formation upon secondary stimulation. Because an increase in neutrophils is a hallmark of CML, we hypothesized that malignant neutrophils may be more prone to NET formation. To our surprise, not only the transformed neutrophils but also normal neutrophils from mice with CML-like myeloproliferative neoplasia (MPN) were primed to generate extracellular DNA traps. In addition, using solid tumor models, we show that cancers can induce an increase in peripheral blood neutrophils that are sensitized toward NET formation and that spontaneous thrombosis is associated with NET generation in vivo. We also show that cancer-associated G-CSF predisposes the host to an exacerbated innate immune response that results in a prothrombotic state. Our findings may further explain the association of cancer with thrombosis.
Results
Peripheral Blood Neutrophils from Mice with CML-Like MPN Are Prone to Generate Extracellular DNA Traps.
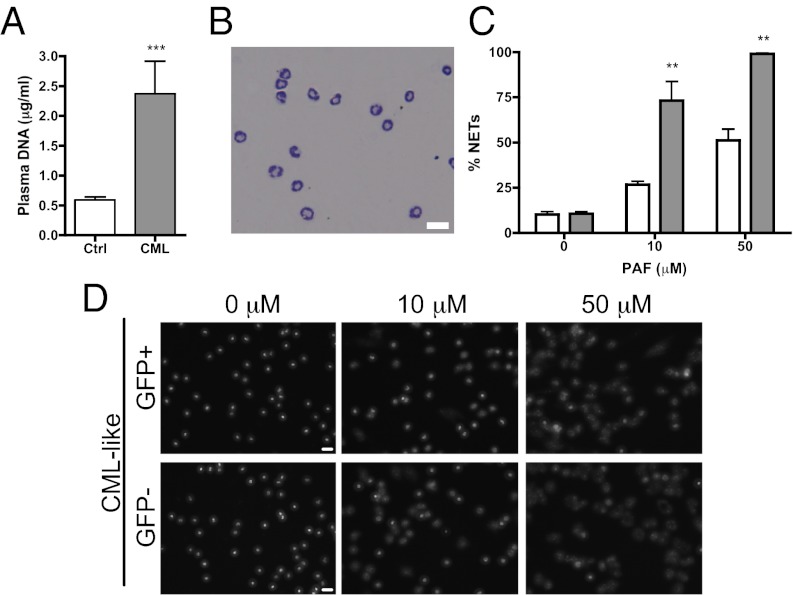
To determine whether malignant transformation promotes NET formation, we first assessed the ability of neutrophils from mice with CML-like MPN to form NETs. In this model, engraftment of bone marrow cells coexpressing breakpoint cluster region–Abelson (BCR-ABL1) and green fluorescent protein (GFP) occurs around 14 d after bone marrow transplant (20) and produces an increase in BCR-ABL1+ peripheral blood neutrophils without a significant increase in platelet count compared with control mice (Fig. S1). Coexistence of normal BCR-ABL1− (GFP−) and BCR-ABL1+ (GFP+) myeloid cells was observed at day 20 posttransplant when 29.49% ± 10.39% of cells were GFP+ (n = 8). Plasma analysis revealed a greater level of plasma DNA in the CML-like mice compared with controls, suggesting a possible generation of NETs in vivo (Fig. 1A). Isolation of peripheral blood neutrophils from control or leukemic mice routinely yielded a purity of greater than 90% (Fig. 1B). Platelet-activating factor (PAF) stimulation of isolated neutrophils from mice with CML-like MPN induced a significant increase in NET formation in a dose-dependent manner compared with neutrophils from control bone marrow recipients (Fig. 1C and Fig. S2A). Interestingly, at a high dose of PAF, the majority of isolated neutrophils from CML-like mice generated NETs, whereas only about 30% of them were BCR-ABL1+. This suggested that it was not only the BCR-ABL1+ neutrophils that were more sensitive to NET formation but rather the entire population. To address this more rigorously, we used FACS sorting to separate the GFP+ BCR-ABL1+ neutrophils from the GFP− neutrophils and evaluated their NETosis potential. Again, the majority of both GFP+ and GFP− neutrophils from CML-like mice generated extracellular DNA traps (Fig. 1D). Normal C57BL/6 neutrophils, which had been sorted by flow cytometry, acted as controls and were shown to make NETs far less efficiently, indicating that the isolation of neutrophils through FACS sorting did not stimulate NET formation (Fig. S2B). In accordance with these results, in vitro pretreatment with imatinib or dasatinib, two abl-specific tyrosine kinase inhibitors (21), had no effect on NET formation (Fig. S2C). Although the leukemic cells underwent apoptosis in response to treatment, normal neutrophils were not affected. Thus, NETs were still being made more efficiently by the nonmalignant neutrophils from CML-like animals than by neutrophils from control mice. These results show that CML predisposes BCR-ABL1+ and also BCR-ABL1− neutrophils to generate extracellular DNA traps, suggesting that a systemically acting factor may be stimulating NET formation.
Fig. 1.
Neutrophils from mice with chronic myelogenous leukemia are more prone to generate extracellular DNA traps. (A) Plasma analysis showed a higher level of DNA in CML mice compared with control mice (n = 6; ***P < 0.001). (B) Wright–Giemsa staining showing the purity of the neutrophil isolation from C57BL/6 mice. (Scale bar: 20 μm.) (C) Quantification of NETs after PAF stimulation of isolated neutrophils from CML mice (gray) shows a significant increase compared with control vector–transduced bone marrow recipients (white) (n = 6; **P < 0.01). (D) Fluorescent images of NET formation after PAF stimulation and Hoechst staining of fluorescence-activated cell–sorted GFP+ leukemic cells or control GFP− cells showed no difference in the numbers of NETs (n = 4). (Scale bar: 20 μm.) Graph presents means ± SEM.
Solid Tumors Generate a Leukemoid Reaction and Predispose Neutrophils to Extracellular DNA Trap Formation.
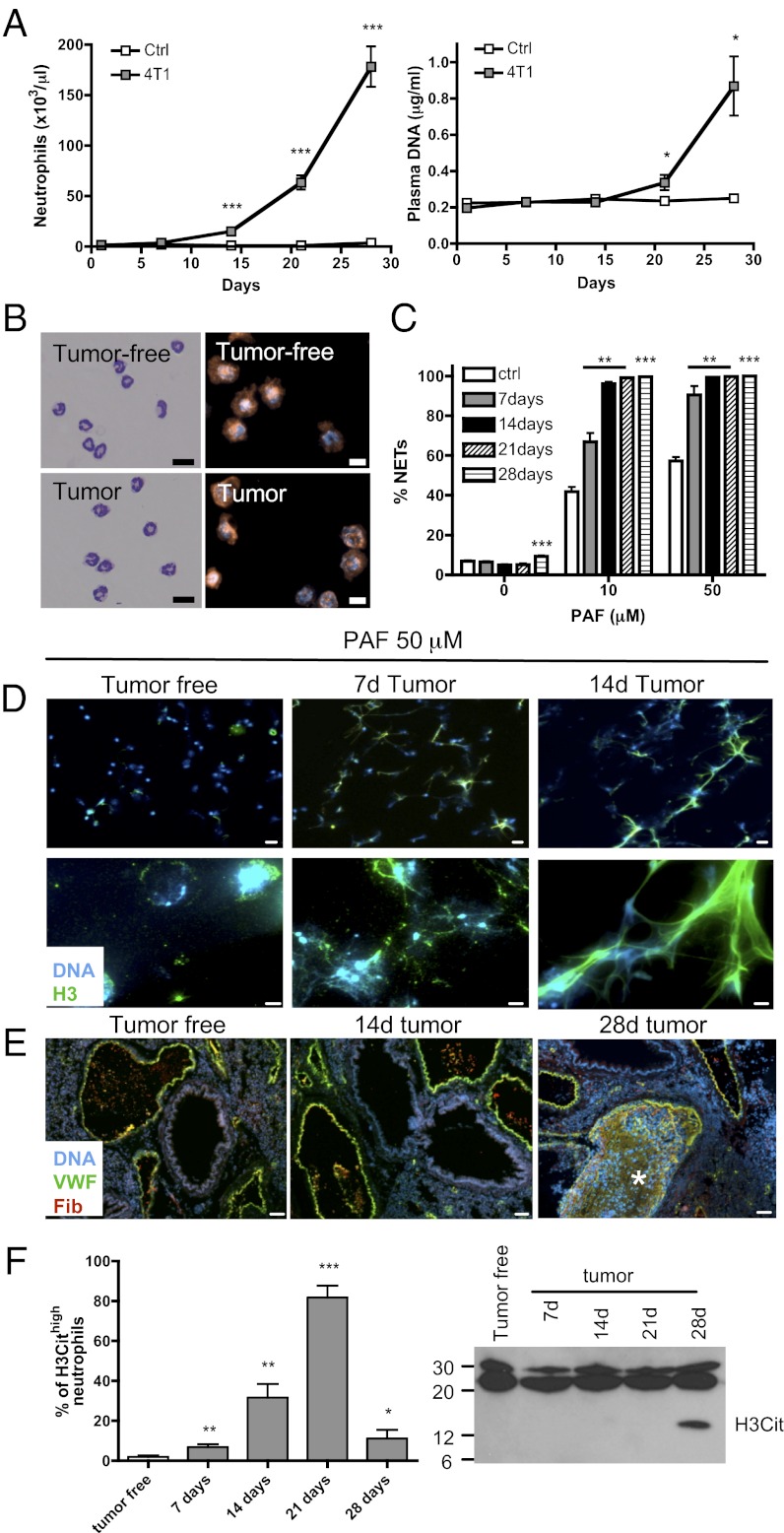
Because all neutrophils from mice with CML-like MPN are more prone to generating NETs, we asked whether neutrophils from mice bearing solid tumors would also be more sensitive to NET formation. As described previously by DuPré et al. (22), injection of the 4T1 mammary carcinoma cell line into BALB/c mice induced a large increase in peripheral blood neutrophils (Fig. 2A) that correlated with tumor growth (Fig. S3A). Plasma analysis also revealed a significant increase in plasma DNA at the later stages of the disease, day 21 postinjection, which drastically increased by day 28 (Fig. 2A). Regression analysis performed with plasma DNA (μg/mL) as the dependent variable suggests that the increase in plasma DNA could be better determined by the number of circulating neutrophils than by tumor size (Fig. S3B). This suggests that NETs could be the source of the plasma DNA. Isolation of peripheral blood Gr-1+ neutrophils from tumor-bearing mice and control mice showed similar purity and morphology (Fig. 2B). As in the leukemia model, PAF-mediated induction of NET formation by neutrophils from tumor-bearing mice revealed a tumor-age dependence in the susceptibility, reaching almost 100% NET formation 14-d after tumor implantation (Fig. 2C and Fig. S3C). Interestingly, a significant increase in NET formation was observed in isolated neutrophils from 28-d tumor-bearing mice without any additional stimulus, again suggesting that NET formation is occurring in these mice. An increase in peripheral blood neutrophils and enhanced NET formation was also observed after s.c. inoculation of Lewis lung carcinoma (LLC) cells in C57BL/6 mice (Fig. S4 A and B). The susceptibility of mice bearing different types of tumors to produce NETs suggests that priming or activation of the neutrophils occurred in these animals. Immunofluorescence staining for DNA and histone H3 of neutrophils treated with PAF from tumor-free mice showed slight decondensation of the chromatin, whereas neutrophils from 7- and 14-d tumor-bearing mice showed complete destruction of the nuclear shape and, ultimately, a spider web-like pattern, with only a few distinguishable nuclei (Fig. 2D). Moreover, extracellular histone H3 staining was observed along with DNA. Together, these results suggest that in murine models of CML, breast, and lung cancer, a systemic environment is created that sensitizes neutrophils to generate extracellular DNA traps.
Fig. 2.
Neutrophils from mammary tumor–bearing mice are more prone to NET formation and signs of spontaneous NETosis are associated with thrombosis at late stages of the disease. Tumor cells were injected in the mammary fat pad of BALB/c mice. (A) Neutrophil counts and plasma DNA were evaluated every 7 d (n = 6–10; *P < 0.05; ***P < 0.001). (B) Wright–Giemsa staining (scale bar: 20 μm) and Gr-1 (red) immunostaining with Hoechst (blue) counterstaining (scale bar: 10 μm) showing the purity of the neutrophil isolation of tumor-free and 14-d 4T1 tumor–bearing BALB/c mice. (C) Quantification of NETs after PAF stimulation of isolated neutrophils from tumor-bearing mice at different times after tumor cell injections shows a significant increase in NET production compared with tumor-free mice (n = 6–7; **P < 0.01; ***P < 0.001). (D) Histone H3 (green) combined with Hoechst staining (blue) of neutrophils stimulated with 50 μM PAF for 1 h at low (Upper) and high (Lower) magnification. [Scale bars: 20 μm (Upper); 5 μm (Lower).] (E) VWF (green) and fibrinogen/fibrin (red) immunostaining with Hoechst staining (blue) of lungs of tumor-bearing mice and tumor-free mice. VWF- and fibrin-rich thrombi (asterisk) were detected only 28 d after tumor injection (n = 4). (Scale bar: 50 μm.) (F) Percentage of hypercitrullinated neutrophils obtained following H3Cit immunostaining of isolated neutrophils from tumor-bearing-mice. At day 21 after tumor injection, most of the neutrophils are hypercitrullinated. At day 28, only some hypercitrullinated neutrophils remain. A minimum of 10 fields (at least 300 cells) were evaluated for hypercitrullination of histone H3 in the nucleus. Similar observations were made in four different animals. Western blot analysis of H3Cit in the plasma of tumor-bearing mice revealed the presence of H3Cit at day 28. A distinct band was observed in four out of seven plasma from 28-d tumor-bearing mice. Data shown in A, C, and F are means ± SEM.
Spontaneous NET Formation in Cancer Is Associated with the Presence of Lung Thrombosis.
We demonstrated previously that NETs are prothrombotic (15, 16). Our mammary carcinoma model showed signs of a prothrombotic state with increasing levels of plasma von Willebrand factor (VWF), soluble P-selectin, and fibrinogen (23–26) during tumor progression (Fig. S3D). Immunostaining of the lungs of tumor-bearing mice showed VWF- and fibrin-rich thrombi in veins of four out of four lungs evaluated at 28-d post implantation, whereas no indication of thrombi was observed in control mice or at earlier stages of the disease (Fig. 2E). Moreover, citrullinated histone H3 (H3Cit), a histone modification necessary for NET production (27, 28), was present in the plasma at a late stage of the disease when plasma DNA is high, consistent with a drop in the number of hypercitrullinated neutrophils in peripheral blood (Fig. 2F). Thus, our results suggest that at 28-d after mammary tumor implantation, NETosis occurs and is associated with thrombosis. Together, these results suggest that NETs are implicated in cancer-associated thrombosis.
Exacerbated Effect of Low-Dose LPS in Mammary Tumor–Bearing Mice on NET Formation and Induction of a Prothrombotic State.
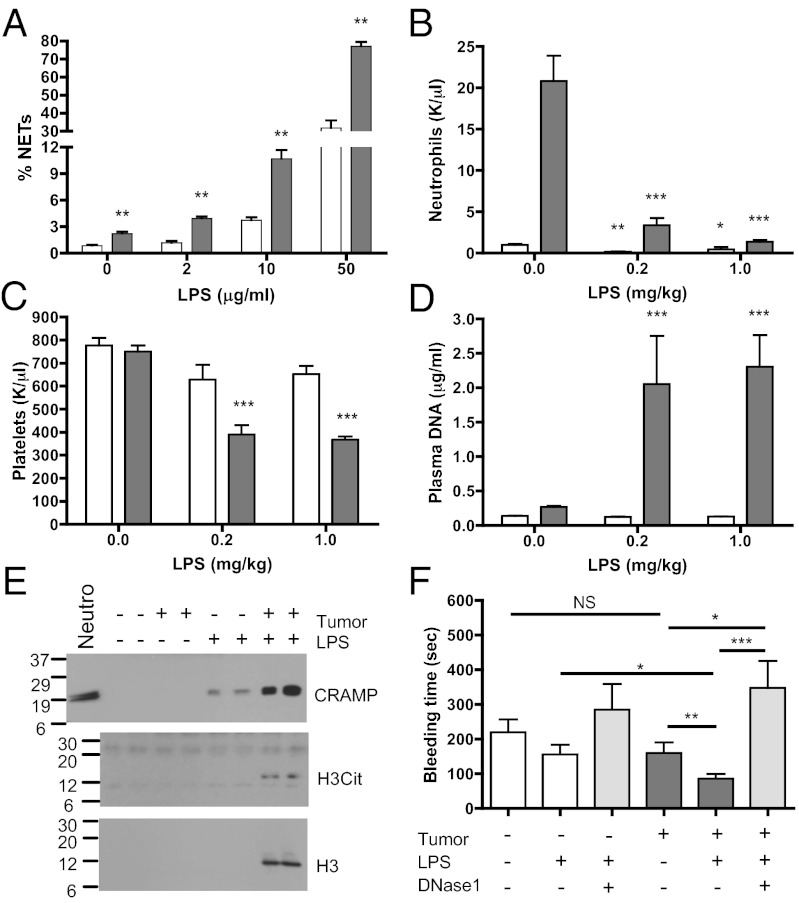
NETosis has been defined previously as part of the innate immune defense against infection (14, 18). We, thus, evaluated the effect of LPS in vitro on neutrophils isolated from tumor-free and 14-d mammary tumor–bearing mice. As observed with PAF stimulation, LPS-treated neutrophils from tumor-bearing mice showed a significant increase in NET formation (Fig. 3A), suggesting that a minor infection in a tumor-bearing host would generate a larger quantity of NETs than in tumor-free mice. To test this, we injected tumor-free and tumor-bearing mice with low doses of LPS and assessed whether the predisposition of the neutrophils to form NETs would materialize in vivo and generate a procoagulant state. Two hours after injection of LPS, the neutrophil count in the blood was reduced by more than 15,000 neutrophils/μL in tumor-bearing mice and by only about 1,000 in tumor-free mice (Fig. 3B). This large decrease in neutrophil count in tumor-bearing LPS-treated mice was associated with a significant reduction in platelet count that was not observed in mice free of tumors (Fig. 3C). To determine if these effects could be related to NET formation in vivo, we evaluated NET biomarkers in blood. Plasma analysis revealed an increase in DNA and in histone H3 only in the tumor-bearing LPS-treated mice (Fig. 3 D and E). No increase was observed in the tumor-bearing mice treated with vehicle or in the tumor-free mice treated with LPS, suggesting a stronger effect of LPS on chromatin release in the tumor-bearing mice. To assess whether DNA originated from NETs, we evaluated the presence of cathelicidin-related antimicrobial peptide (CRAMP), the murine homolog of human cathelicidin, a protein highly expressed in neutrophils and shown to be associated with NETs (29), and H3Cit in the plasma. Whereas a small increase in CRAMP was observed in the plasma of LPS-treated tumor-free mice, a higher level was observed in the plasma of LPS-treated tumor-bearing mice (Fig. 3E), likely indicating greater formation of NETs. H3Cit was detected only in the plasma of tumor-bearing mice treated with LPS. These results suggest that although low-dose LPS injection activates neutrophils and probably generates small quantities of NETs in the vasculature of tumor-free mice, as observed by the presence of CRAMP in plasma, NET formation was strongly enhanced only in the presence of cancer. Moreover, low-dose LPS treatment reduced the bleeding time of tumor-bearing mice without affecting that of tumor-free mice, a sign of a powerful effect of LPS on hemostasis in tumor-bearing mice (Fig. 3F). Administration of DNase1, which digests NETs, to tumor-bearing mice prevented the reduction of bleeding time associated with LPS injection. This suggests that the presence of undigested circulating extracellular DNA may promote platelet plug formation. DNase1 pretreatment did not affect neutrophil counts or prevent the reduction in the number of platelets (Fig. S5). These results show that mice with cancer develop a systemic environment that increases the ability of neutrophils to generate NETs, which contribute to the prothrombotic state of the host.
Fig. 3.
Low-dose LPS induces extracellular DNA trap formation in mammary tumor–bearing mice. (A) Quantification of NET formation by isolated neutrophils from tumor-free (white) or 14-d 4T1 tumor–bearing (gray) mice after stimulation with low doses (as indicated) of LPS (n = 6; **P < 0.01). (B–F) Fourteen-day tumor-bearing mice (gray) or tumor-free mice (white) were injected with low doses of LPS. Significant decreases in peripheral blood neutrophil counts (B) and platelet counts (C) were observed in tumor-bearing mice. Plasma analysis of DNA (D) and Western blot analysis for histone H3, histone H3Cit and CRAMP (E) showed much higher levels of these NET biomarkers in tumor-bearing mice than in tumor-free mice when treated with LPS (1 mg/kg). (F) Tail-bleeding time 1 h after LPS injections was significantly reduced in tumor-bearing mice, indicating increased prothrombotic activity. Pretreatment with DNase1 before LPS injections prevent the reduction in tail bleeding (n = 9–17; *P < 0.05; **P < 0.01; ***P < 0.001). Data shown are means ± SEM.
G-CSF Potentiates Neutrophils to Generate NETs.
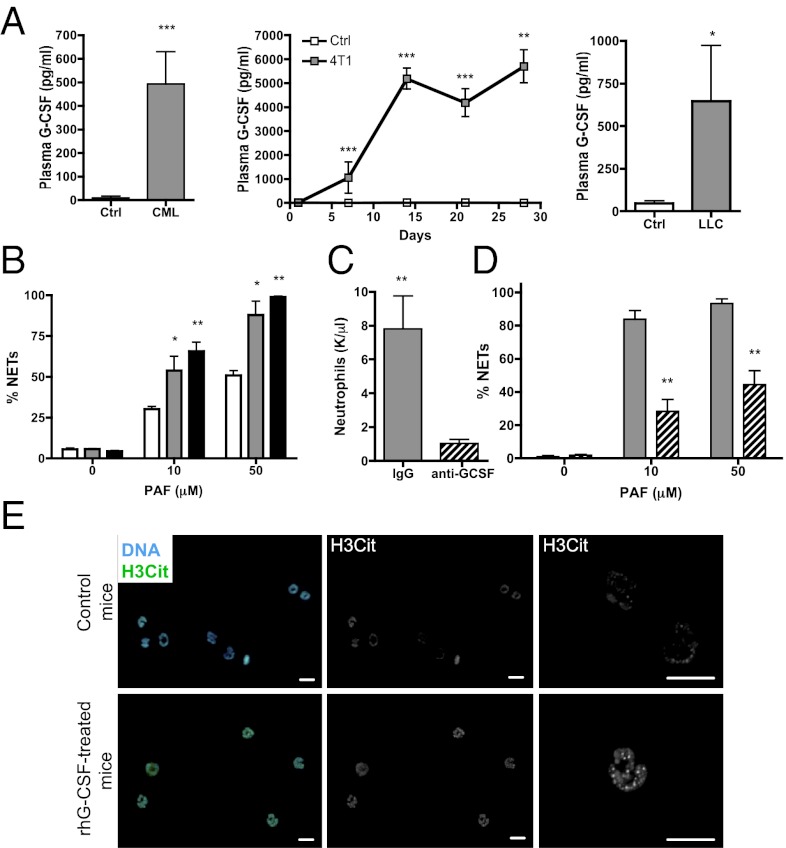
G-CSF increases neutrophil numbers in the circulation and activates them. The 4T1 tumor cells produce G-CSF, and its presence in the serum of tumor-bearing mice is associated with a leukemoid-like reaction (22). Elevated G-CSF levels were observed in the plasma of the CML-like mice and both the mammary and lung carcinoma models compared with control mice (Fig. 4A). To assess whether G-CSF could be responsible for the increased predisposition of peripheral blood neutrophils to form NETs, we treated healthy mice with recombinant human (rh)G-CSF. A 4-d treatment led to an increase in neutrophil count and a decrease in platelet count (Fig. S6A). PAF stimulation resulted in a dose-dependent increase in NET formation in isolated neutrophils similar to what we observed with the three cancer models (Fig. 4B). Moreover, treatment of 4T1 tumor–bearing mice with a G-CSF–neutralizing antibody prevented accumulation of neutrophils in the blood (6) and reduced their sensitization toward NET generation in vitro (Fig. 4 C and D). Similar to the mammary carcinoma model, immunostaining of isolated neutrophils revealed hypercitrullination of histone H3, corroborating their predisposition to form NETs (Fig. 4E).
Fig. 4.
Tumors produce G-CSF, which elevates blood neutrophil count and predisposes neutrophils to generate NETs. (A) Increased quantities of G-CSF were observed in the plasma of CML (Left), mammary carcinoma (Center), and lung carcinoma (Right) tumor–bearing mice compared with tumor-free mice (white) (n = 5–9; *P < 0.05). (B) BALB/c mice were treated in vivo with 2.5 μg (gray) or 10 μg (black) rhG-CSF, neutrophils were isolated, and PAF-mediated induction of NETs was evaluated and compared with neutrophils from control mice (white). rhG-CSF significantly increased the percentage of cells forming NETs (n = 4–5; *P < 0.05; **P < 0.01). (C) Mice bearing 4T1 tumors were treated daily with neutralizing anti–G-CSF antibody starting 2 d after tumor cell injection. The anti–G-CSF treatment reduced the number of peripheral blood neutrophils in 4T1 tumor–bearing mice (hashed) (n = 5). (D) The anti–G-CSF treatment significantly diminished the ability of neutrophils to form NETs ex vivo upon PAF stimulation compared with control isotype–treated 4T1 tumor–bearing mice (gray) (n = 5). **P < 0.05. (E) Citrullinated histone H3 (green) immunostaining with Hoechst (blue) counterstain revealed an increase in citrullination in isolated neutrophils from rhG-CSF–treated mice (Left). (Center and Right) H3Cit alone (Center) and higher magnification (Right). (Scale bar: 10μm). Data shown in A–D represent means ± SEM.
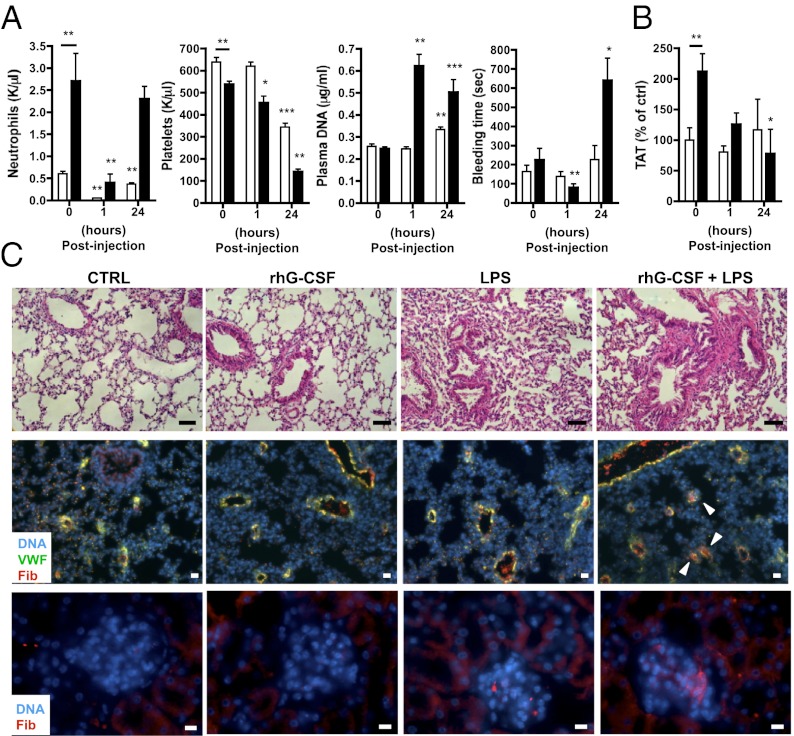
Combination of G-CSF and Low-Dose LPS Induces NETs, and This Leads to Thrombocytopenia and Microthrombosis.
As with the tumor-bearing mice, injection of low-dose LPS in the rhG-CSF–treated mice decreased neutrophil and platelet counts and increased plasma DNA (Fig. 5A). Similarly, reduction in tail-bleeding time was observed in the rhG-CSF–treated mice that received low-dose LPS for 1 h (Fig. 5A). Interestingly, 24 h after LPS challenge, DNA was still present in the plasma of rhG-CSF–treated mice, but an increase in tail-bleeding time was observed. This correlated with marked thrombocytopenia, suggesting platelet consumption possibly by microthrombosis. In control mice, the 24 h LPS challenge did not affect tail-bleeding time; however, it increased plasma DNA and decreased platelet numbers to a lesser extent than in rhG-CSF–treated mice. This suggests that NET formation occurs in control mice but is strongly enhanced by rhG-CSF treatment. After 24 h, in both groups, the number of blood neutrophils rebounded, likely because of the up-regulation of G-CSF after LPS injection (30). To determine whether the rhG-CSF–treated mice challenged with LPS for 24 h showed signs of thrombosis, we measured the level of thrombin–anti-thrombin (TAT) complexes, a marker of thrombin generation, in the plasma and analyzed the lungs for signs of fibrosis. As has been reported in healthy donors receiving rhG-CSF (31), a higher level of TAT was observed in mice treated with rhG-CSF compared with control mice at baseline (Fig. 5B). Whereas no significant change was observed in control mice 24 h after LPS challenge, TAT levels were significantly reduced in rhG-CSF–treated mice, suggesting a consumption of coagulation factors. Histology and immunofluorescence analysis revealed signs of fibrosis and fibrinogen-/fibrin-rich microthrombi in the lungs of both rhG-CSF–treated and untreated mice after LPS challenge. However, the effect was greatly enhanced in rhG-CSF–treated mice (Fig. 5C). No signs of fibrosis were observed in mice that had not received LPS. A greater accumulation of fibrinogen/fibrin deposits was also found in the renal glomeruli of the rhG-CSF–treated group after LPS challenge (Fig. 5C). These results suggest that in rhG-CSF–treated mice, NETs are formed early after LPS injection and induce a prothrombotic state. Such a state could lead, in the extreme, to the consumption of platelets and coagulation factors and microthrombosis. Taken together, our results indicate that increased levels of G-CSF, which are generated in different types of cancers, can produce a systemic environment that primes peripheral blood neutrophils to generate NETs more readily. This effect may contribute, at least in part, to the prothrombotic state observed in cancer.
Fig. 5.
Low-dose LPS injection induces a prothrombotic state in rhG-CSF–treated mice. Mice were treated with vehicle (control; white) or rhG-CSF (black) and challenged with low-dose LPS (1 mg/kg) for 1 or 24 h. (A) One hour after LPS injection, the blood counts showed a significant reduction in neutrophils and platelets, which corresponded to an increase in plasma DNA and a reduction in tail-bleeding time only in rhG-CSF–treated mice. Only the neutrophil count was reduced in control mice. Twenty-four hours after LPS treatment, decreased platelet counts and increased DNA levels were also observed in control mice without modulation of tail-bleeding time. In contrast, 24 h after LPS injection the tail-bleeding time was prolonged in rhG-CSF–treated mice (n = 5–9; *P < 0.05; **P < 0.01; ***P < 0.001 compared with no LPS treatment). (B) Twenty-four hours after LPS challenge, a decrease in TAT complexes was observed in rhG-CSF–treated compared with control mice (n = 5–9; *P < 0.05; **P < 0.01). (C) Hematoxylin and eosin staining (top images) of the lungs of mice 24 h after LPS challenge showed some signs of fibrosis in mice treated with LPS, but fibrosis was strongly enhanced in rhG-CSF–treated mice. (Scale bar: 50 μm.) Anti-fibrinogen staining (red) revealed an enhanced presence of fibrinogen-/fibrin-rich microthrombi (arrows) in the lungs (middle images) and the glomeruli of the kidneys (bottom images) of rhG-CSF–treated mice challenged with LPS for 24 h. [Scale bar: 20 μm (Middle) and 10 μm (Lower)]. Hoechst, blue. Data in A and B represent means ± SEM.
Discussion
Cancers are known to elevate the risk of thrombosis. Leukocytosis, thrombocytosis, microparticles, cytokines, tissue factor, soluble P-selectin, and elevation in coagulation factors could all be partially responsible for the prothrombotic state (32). Here, we report that cancer induces a systemic environment that primes neutrophils to release NETs, thereby further promoting a prothrombotic state. Our results show that (i) peripheral blood neutrophils from leukemic mice and solid tumor–bearing mice are more prone to NET formation ex vivo; (ii) NETosis is associated with lung thrombosis in the breast carcinoma model; (iii) injection of a low dose of LPS in tumor-bearing mice increases plasma NET biomarkers and induces a prothrombotic state; and (iv) the increased predisposition of neutrophils to NET formation could be attributable to elevated G-CSF in the plasma of mice with cancer. Thus, by generating G-CSF, cancers prime neutrophils to undergo NETosis.
NETs were originally described as a defense mechanism against infection (14). Recently, our group showed that NETs activate platelets and trigger thrombosis (15) and are implicated in the pathogenesis of deep vein thrombosis (DVT) in mice (16). An increased risk of thrombosis is associated with many cancers, and such cancers may even be diagnosed only following a thrombotic event such as DVT. Therefore, one may hypothesize that the predisposition to generate extracellular DNA traps in cancer patients could increase the risk of thrombosis. DNA, histones, and neutrophil granular proteins have been shown to promote coagulation and to be injurious to tissues (17, 33–35). NETs' products and histones also induce platelet activation and aggregation, red blood cell accumulation, and VWF release, hallmarks of venous thrombus formation (15–17, 36).
Although CML is not associated with a high risk of thrombosis (37), our results show that the neutrophils from mice with CML-like MPN are primed to NET formation, and plasma DNA is observed. It is conceivable that neutrophil activation and NET generation are important players in cancer-associated thrombosis but are not sufficient. Production of tissue factor by various tumors (38), for example, could further potentiate the prothrombotic state. In addition, our results indicate that a further activation of the innate immune system in a cancer patient could precipitate a thrombotic event and organ damage through NET-/histone-induced injury. Given the close interaction of platelets and neutrophils during infection and the implication of platelet activation in the generation of NETs (39, 40), their potential contribution in the cancer models should be addressed.
NET induction by LPS in the solid tumor model rapidly generates a large quantity of injurious products in the bloodstream with the onset of a prothrombotic state, leading to pulmonary microthrombosis. This latter effect has also been observed in mice after an injection of large quantities of histones, leading to sepsis-like disease (34). Moreover, our laboratory showed that injection of a sublethal dose of histones in healthy mice results in thrombocytopenia (36). In mice with late-stage cancer, we observed thrombi in the lung, even in the absence of additional stimulation. This correlated with the presence of a high quantity of plasma DNA. Interestingly, our laboratory in collaboration with that of Bernhard Lämmle recently reported increased levels of DNA and neutrophil markers in plasma from cancer patients with acute thrombotic microangiopathies (41).
Similar to the solid cancer mouse models, in humans, elevated serum G-CSF levels (8–10, 42) and extreme leukocytosis (>40,000/μL) related to a paraneoplastic leukemoid reaction have been reported for a variety of solid tumor types (43). Although initially clinically stable, the vast majority of patients with a neutrophilic predominance have poor clinical outcomes, with 76% dying within 12 wk of development of extreme leukocytosis (43). It is, thus, possible that NETs are generated in late-stage cancer patients and play a role in the critical outcome. Determination of DNA levels in the plasma of these patients in relation to leukocytosis would assess this further.
G-CSF is broadly used to treat neutropenia or for hematopoietic stem cell mobilization in patients and healthy donors. Studies have reported endothelial cell dysfunction, clotting activation, an increase in blood oxidative status, platelet aggregation, and neutrophil activation in healthy donors during treatment with G-CSF (31, 44). Despite this, most of the G-CSF–treated healthy subjects do not experience thrombotic events (13, 44, 45), and G-CSF is considered a safe mobilizing agent. The prothrombotic effects that have been associated with G-CSF have been linked to its use in the treatment of inflammatory or already-prothrombotic states, such as acute myocardial infarction, through mobilization of autologous stem cells (45, 46). This is in accordance with our results suggesting that, in the presence of G-CSF, neutrophils may be more sensitive to NET formation, in particular, upon encountering a “second hit,” such as low-grade infection.
In conclusion, we have uncovered an important role for extracellular chromatin that is generated in animals with cancer, predisposing them to thrombosis. Release of large quantities of DNA in the blood occurs at late stages of the disease or upon a “second hit,” such as a minor infection, and could be detrimental to the host. It will be important to determine whether agents neutralizing G-CSF and/or NETs can decrease the incidence of thrombosis in cancer patients.
Materials and Methods
For a full description of all methods, see SI Materials and Methods.
Animals.
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Immune Disease Institute and Massachusetts General Hospital. Experiments are described in SI Materials and Methods.
Stainings and Plasma Analysis.
Neutrophils/NETs were stained with anti–Gr-1 and anti–histone H3 antibodies. Lung sections were stained with hematoxylin and eosin or anti-fibrinogen antibody and anti-VWF. Hoechst-33342 was used as a counterstain. ELISAs are described in SI Materials and Methods. DNA was quantified with a Quant-iT Picogreen assay (Invitrogen). For Western blot analysis, equal amounts of plasma were analyzed using anti-CRAMP, anti–histone H3 or anti–histone H3 (citrulline 2, 8, 17) antibodies.
Peripheral Blood Neutrophil Isolation and NET Induction.
Peripheral blood neutrophils were isolated on a Percoll gradient, followed by hypotonic lysis and stimulated with PAF or LPS. DNA was stained with Hoechst-33342, and cells were fixed before visualization. NETs were counted from six different fields in triplicate wells and expressed as percentage of NET-forming cells per total number of cells in the field.
Statistical Analysis.
Data are represented as means ± SEM and were analyzed by a two-sided Mann–Whitney test performed between groups. All P values were considered significant at or below 0.05.
Supplementary Material
Acknowledgments
We thank Lesley Cowan for help with manuscript preparation, Myriam Armant for help with G-CSF studies, and Julian I. Borissoff for help with regression analysis and thoughtful discussions. This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health Grant R01 HL102101 (to D.D.W.), Terry Fox Foundation Grant TF-018748 through the Canadian Cancer Society (to M.D.), and National Cancer Institute Grant 5K08CA138916-02 (to D.S.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200419109/-/DCSupplemental.
References
- 1.Rickles FR, Levine M, Edwards RL. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992;11:237–248. doi: 10.1007/BF01307180. [DOI] [PubMed] [Google Scholar]
- 2.Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010;10:2–3. doi: 10.1038/nrc2782. [DOI] [PubMed] [Google Scholar]
- 3.Champlin RE, Golde DW. Chronic myelogenous leukemia: Recent advances. Blood. 1985;65:1039–1047. [PubMed] [Google Scholar]
- 4.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueha S, Shand FH, Matsushima K. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–788. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc Natl Acad Sci USA. 1999;96:12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshita S, et al. Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course. Intern Med. 2009;48:687–691. doi: 10.2169/internalmedicine.48.1900. [DOI] [PubMed] [Google Scholar]
- 9.Kaira K, et al. Lung cancer producing granulocyte colony-stimulating factor and rapid spreading to peritoneal cavity. J Thorac Oncol. 2008;3:1054–1055. doi: 10.1097/JTO.0b013e3181834f7b. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi M, et al. Aggressive recurrence of gastric cancer as a granulocyte-colony-stimulating factor-producing tumor. Int J Clin Oncol. 2010;15:191–195. doi: 10.1007/s10147-010-0023-3. [DOI] [PubMed] [Google Scholar]
- 11.Avalos BR, et al. Human granulocyte colony-stimulating factor: Biologic activities and receptor characterization on hematopoietic cells and small cell lung cancer cell lines. Blood. 1990;75:851–857. [PubMed] [Google Scholar]
- 12.Spiel AO, et al. Increased platelet aggregation and in vivo platelet activation after granulocyte colony-stimulating factor administration. A randomised controlled trial. Thromb Haemost. 2011;105:655–662. doi: 10.1160/TH10-08-0530. [DOI] [PubMed] [Google Scholar]
- 13.Quillen K, Byrne P, Yau YY, Leitman SF. Ten-year follow-up of unrelated volunteer granulocyte donors who have received multiple cycles of granulocyte-colony-stimulating factor and dexamethasone. Transfusion. 2009;49:513–518. doi: 10.1111/j.1537-2995.2008.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs TA, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brill A, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2011;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massberg S, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 22.DuPré SA, Hunter KW., Jr Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp Mol Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23.André P, Hartwell D, Hrachovinová I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97:13835–13840. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 25.van Hylckama Vlieg A, Rosendaal FR. High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thromb Haemost. 2003;1:2677–2678. doi: 10.1111/j.1538-7836.2003.0543b.x. [DOI] [PubMed] [Google Scholar]
- 26.Myers DD, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38:1075–1089. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 27.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jann NJ, et al. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J Leukoc Biol. 2009;86:1159–1169. doi: 10.1189/jlb.0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barsig J, et al. Lipopolysaccharide-induced interleukin-10 in mice: Role of endogenous tumor necrosis factor-alpha. Eur J Immunol. 1995;25:2888–2893. doi: 10.1002/eji.1830251027. [DOI] [PubMed] [Google Scholar]
- 31.Falanga A, et al. Neutrophil activation and hemostatic changes in healthy donors receiving granulocyte colony-stimulating factor. Blood. 1999;93:2506–2514. [PubMed] [Google Scholar]
- 32.Connolly GC, Khorana AA. Emerging risk stratification approaches to cancer-associated thrombosis: Risk factors, biomarkers and a risk score. Thromb Res. 2010;125(Suppl 2):S1–S7. doi: 10.1016/S0049-3848(10)00227-6. [DOI] [PubMed] [Google Scholar]
- 33.Hirahashi J, et al. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation. 2009;120:1255–1265. doi: 10.1161/CIRCULATIONAHA.109.873695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9:2313–2321. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehmeier A, Daum I, Jamin H, Schneider W. Incidence and clinical risk factors for bleeding and thrombotic complications in myeloproliferative disorders. A retrospective analysis of 260 patients. Ann Hematol. 1991;63:101–106. doi: 10.1007/BF01707281. [DOI] [PubMed] [Google Scholar]
- 38.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124(3 Suppl):58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 39.Andonegui G, et al. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 40.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lämmle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012 doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stathopoulos GP, et al. Granulocyte colony-stimulating factor expression as a prognostic biomarker in non-small cell lung cancer. Oncol Rep. 2011;25:1541–1544. doi: 10.3892/or.2011.1226. [DOI] [PubMed] [Google Scholar]
- 43.Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: A retrospective, single-institution study. Cancer. 2009;115:3919–3923. doi: 10.1002/cncr.24480. [DOI] [PubMed] [Google Scholar]
- 44.Cella G, et al. Blood oxidative status and selectins plasma levels in healthy donors receiving granulocyte-colony stimulating factor. Leukemia. 2006;20:1430–1434. doi: 10.1038/sj.leu.2404271. [DOI] [PubMed] [Google Scholar]
- 45.Hill JM, et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1643–1648. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuroiwa M, et al. Effects of granulocyte colony-stimulating factor on the hemostatic system in healthy volunteers. Int J Hematol. 1996;63:311–316. doi: 10.1016/0925-5710(96)00459-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.