Abstract
The complex and coordinated regulation of flowering has high ecological and agricultural significance. The maturity locus E1 has a large impact on flowering time in soybean, but the molecular basis for the E1 locus is largely unknown. Through positional cloning, we delimited the E1 locus to a 17.4-kb region containing an intron-free gene (E1). The E1 protein contains a putative bipartite nuclear localization signal and a region distantly related to B3 domain. In the recessive allele, a nonsynonymous substitution occurred in the putative nuclear localization signal, leading to the loss of localization specificity of the E1 protein and earlier flowering. The early-flowering phenotype was consistently observed in three ethylmethanesulfonate-induced mutants and two natural mutations that harbored a premature stop codon or a deletion of the entire E1 gene. E1 expression was significantly suppressed under short-day conditions and showed a bimodal diurnal pattern under long-day conditions, suggesting its response to photoperiod and its dominant effect induced by long day length. When a functional E1 gene was transformed into the early-flowering cultivar Kariyutaka with low E1 expression, transgenic plants carrying exogenous E1 displayed late flowering. Furthermore, the transcript abundance of E1 was negatively correlated with that of GmFT2a and GmFT5a, homologues of FLOWERING LOCUS T that promote flowering. These findings demonstrated the key role of E1 in repressing flowering and delaying maturity in soybean. The molecular identification of the maturity locus E1 will contribute to our understanding of the molecular mechanisms by which a short-day plant regulates flowering time and maturity.
Keywords: photoperiodism, quantitative trait locus, photoperiodic insensibility
The complex processes that control flowering are critical to how plants maximize their reproductive success, and therefore have high ecological and agricultural importance. Plants use various regulatory networks or pathways to respond to photoperiod and other environmental cues (1, 2). In 1920, Garner and Allard demonstrated that soybean and several other plant species flower in response to changes in day length and described this phenomenon as “photoperiodism” (3). Deciphering genes involved in photoperiodic pathways is the key to understanding the exquisite coordination of various flowering processes. In Arabidopsis thaliana and rice (Oryza sativa), a conserved pathway that confers day length responses through regulation of FLOWERING LOCUS T (FT) transcription by CONSTANS (CO) has been well characterized (4–7). The FT protein is a main component of florigen, which is a mobile flowering-promotion signal produced in leaves and transported through the phloem to the meristems (8–12). In Arabidopsis, CO directly induces FT expression under long-day conditions (4). Despite the conserved functions of FT homologues, the regulation and expression of FT may vary in different plant species (13, 14). Research on morning glory (Pharbitis nil) (14, 15) and tomato (Solanum lycopersicum) (13, 16) showed that the regulatory mechanism for flowering time could be different even in plants that exhibit the same overall response to day length. This is because the day length response can diverge rapidly during evolution (17).
Soybean is a valuable plant species for studying photoperiodic effects on flowering because of its typical short-day flowering, widespread cultivation, and agricultural importance. Flowering time and time to maturity in soybean are important quantitative traits related to photoperiod adaptability, domestication, and productivity. As early as the 1920s, researchers started to identify genetic factors controlling flowering and maturity in soybean. In 1927, Owen detected a major pair of genes controlling maturity and designated them as E and e (18). In 1971, Bernard concluded that these genes were the same as E1 and e1, two alleles of a single major locus affecting maturity in his study (19). The E1 locus is largely responsible for the variation in flowering time among soybean cultivars (19, 20). To date, eight flowering time or maturity loci, designated E1 to E8 (19, 21–27), along with the J locus for “long juvenile period” (28), have been genetically identified. Of these, E1, E3, and E4 are involved in photoperiod responses (20–23, 29, 30).
To date, three of the maturity loci have been molecularly identified. E3 and E4 encode GmPHYA3 (31) and GmPHYA2 (32), respectively, which are homologues of the photoreceptor phytochrome A (PHYA) (33). E2 encodes a homologue of GIGANTEA (34), a nuclear-localized membrane protein that functions upstream of CO and FT in A. thaliana (35). In addition, homologues of many other Arabidopsis flowering-time genes are present in soybean (36, 37). Two functionally coordinated soybean GmFT genes (GmFT2a and GmFT5a), the homologues of the Arabidopsis FT, are responsible for inducing flowering under short-day conditions and are likely to be involved in the phytochrome A signaling pathway (38). Functional phytochrome A genotypes suppressed the expression of GmFT2a and GmFT5a under long-day conditions and delayed flowering, whereas double-recessive PHYA genotypes induced GmFT expression and promoted early flowering regardless of day length (38).
Thakare et al. compared the transcriptional profiles of homologues of Arabidopsis flowering-time genes between soybean near-isogenic lines (NILs) harboring contrasting E1 alleles (39). Although several genes, including the homologues of CO, displayed different expression patterns under long- and short-day conditions, they did not differ noticeably in their expression patterns between the E1 NILs (39, 40), except that the abundance of GmFT transcripts was negatively correlated with flowering time under long-day conditions (40). The regulatory network controlling photoperiod response and flowering time through photoreceptor genes (E3, E4) and GmFT genes in soybean therefore remains largely unknown because of the lack of understanding of the key gene at the E1 locus.
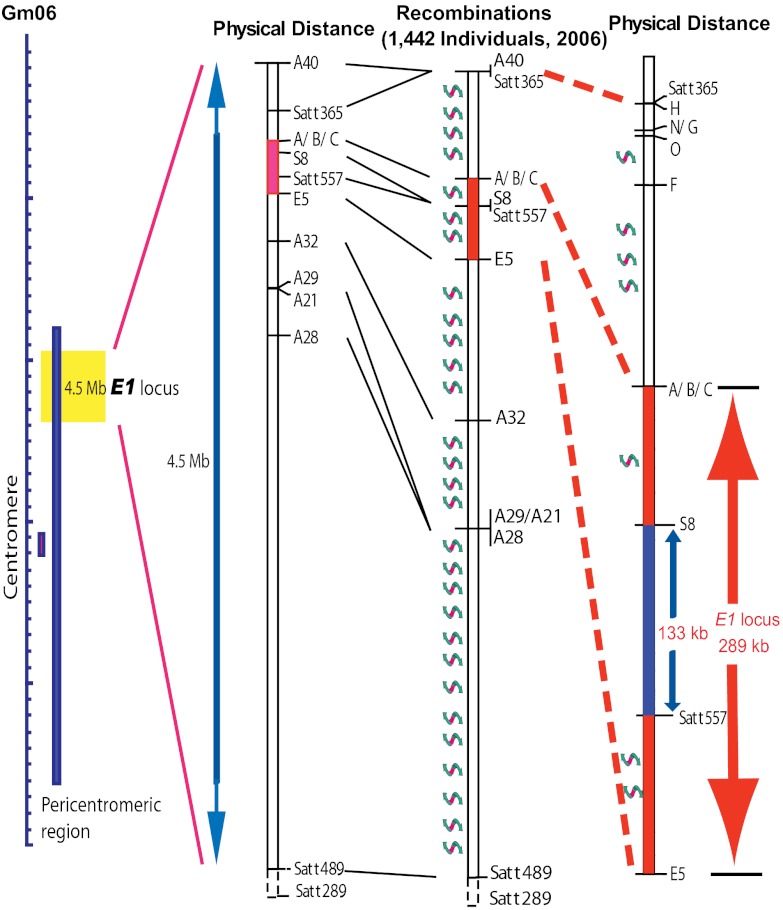
The association between gray pubescence color (t/t) and late maturity (E1/E1) was first perceived in 1927 (18), and confirmed by recombination rates from 1.4% to 4.0% (19, 26). The E1 locus was linked to the Satt365 marker in linkage group (LG) C2, on chromosome 6 (Gm06) (26, 41). Based on the soybean genome sequence (42) (http://www.phytozome.net), the E1 locus resides in a pericentromeric region. Such regions are repeat-rich and gene-poor, with a high ratio of physical to genetic distance. This makes it difficult to precisely locate and characterize pericentromeric genes.
As a result of its photoperiodic responsiveness, the E1 gene was previously assumed to be a member of the GmPHYB family (36). However, this became implausible when none of the GmPHYB members were genetically or physically mapped in proximity to the E1 locus (36, 42). Therefore, we focused on deciphering the molecular basis of the E1 locus through positional cloning in the present study. By using a large population, we delimited the E1 locus to a 17.4-kb region containing a single gene. Our functional characterization of natural variation in cultivars and in ethylmethanesulfonate (EMS)-derived mutants validated the molecular identity of the E1 gene. Moreover, transcriptional profiling of E1 under different environmental conditions and in various genetic backgrounds enabled us to establish a preliminary genetic model for photoperiodic flowering pathway in soybean.
Results
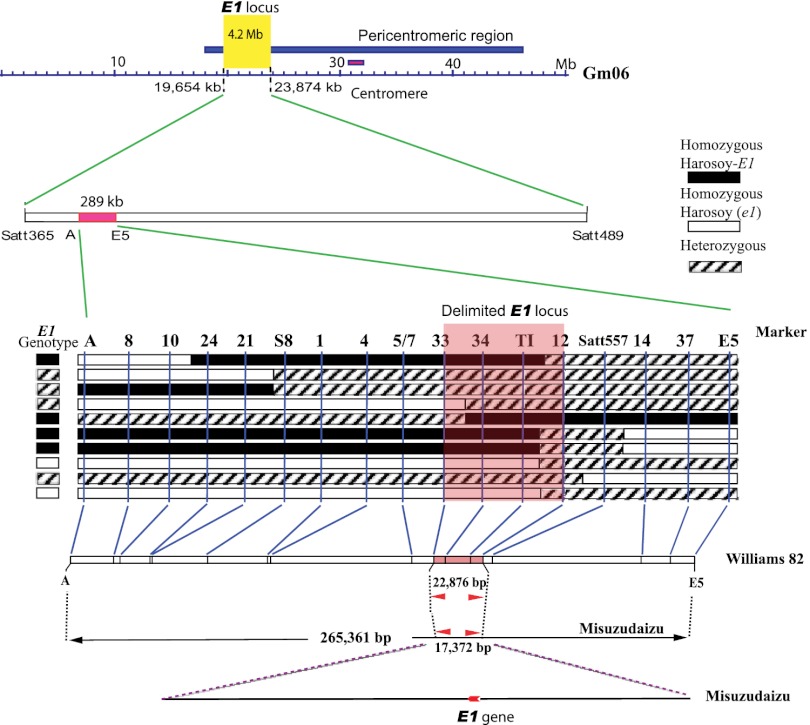
E1 Locus Was Delimited to a 17.4-kb Region That Contained a Single Gene.
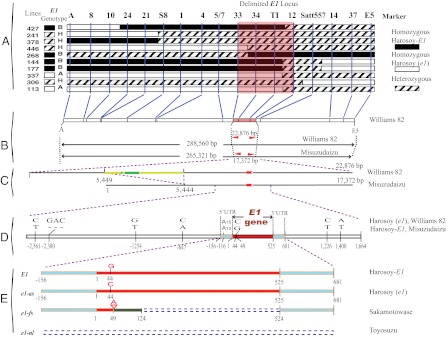
Mapping populations were originally derived from a cross between two E1 NILs, Harosoy-E1 (E1e2E3E4e5) and Harosoy (e1e2E3E4e5), which carry contrasting E1 alleles. Harosoy-E1 required 45.0 ± 0.78 d (mean ± SD) to reach R1 (from emergence to opening of the first flower) stage (43), which was 10 d longer than Harosoy (e1) (34.9 ± 0.83 d) under natural field conditions at Matsudo, Japan (35°78′N, 139°90′E), in 2005. The E1 allele is partially dominant over e1, because F1 plants of the cross between these lines flowered at 41.5 ± 1.16 d after emergence. The E1 locus was initially mapped close to marker Satt557, which is between markers Satt365 and Satt289, by means of quantitative trait locus analysis of flowering time in an F2 population (117 plants) at Matsudo in 2005. Seven recombinants carrying a crossover between markers Satt365 and E5 were identified in 2006 in an F2:3 population consisting of 1,442 individuals derived from 51 F2 plants that were heterozygous at Satt557 (Fig. 1 and Fig. S1). The E1 genotype of each recombinant was determined based on its flowering time in 2006, and was confirmed based on the segregation pattern among its progeny in 2007 (Fig. S1 and Table S1) at Tsukuba, Japan (36°03′N, 140°04′E). No recombination was found between the markers S8 and Satt557, despite a physical distance of 133 kb (Fig. 1). This might be a result of the low recombination rate that often occurs in the pericentromeric region (Fig. 1) (42). At this stage of the mapping study, we were able to delimit the E1 region to an interval of ∼289 kb between the markers A and E5 (Fig. 1). However, given that more than 40 genes were predicted in this 289-kb region using RiceGAAS (44), more intensive fine-scale mapping was conducted. With a simple manual protocol (SI Materials and Methods) for large-scale genotyping of soybean seeds, we successfully screened 13,761 F2:5 seeds harvested from self-crossed F2:4 plants carrying a heterozygous E1 segment, and identified 10 recombinants carrying crossovers within the 289-kb region (Fig. 2A). The E1 genotype of each recombinant was determined by evaluating the phenotypic segregation pattern of the progeny at Tsukuba in 2009 (Fig. S1 and Table S1). The differences in flowering time (R1) among the genotypes were statistically significant (ANOVA, P < 0.001; Table S1).The E1 locus cosegregated with the markers 34 and TI in these recombinants, thus delimiting the candidates for the E1 gene to a region between marker 33 and marker 12.
Fig. 1.
Genetic mapping of the E1 locus. The mapping population was developed by crossing the NILs, Harosoy-E1 and Harosoy (e1). The E1 locus was initially delimited to a 289-kb region (in red) in the pericentromeric region of LG C2 (chromosome Gm06) in the soybean genome (http://www.phytozome.net) from 2006 to 2007. No recombination occurred between markers S8 and Satt557 despite the 133-kb physical distance, indicating a low recombination rate in the E1 region. The twisted arrows represent recombination.
Fig. 2.
Positional cloning and characterization of the E1 locus. (A) Graphical genotypes of 10 recombinants carrying crossovers in the E1 region. The E1 genotype of each recombinant was confirmed based on the phenotypic segregation pattern in its progeny (Fig. S1, Right). The delimited region for the E1 locus is shaded in pink. (B) The corresponding marker positions in physical contigs of Williams 82 and Misuzudaizu. (C) Within the delimited region in Misuzudaizu, a single intron-free gene (red) was identified. A 5,537-bp retrovirus-related sequence (yellow) containing a 1,401-bp putative gag/pol retrovirus gene (green) was inserted ∼4 kb upstream of e1-as in Williams 82. (D) Genetic variation in the E1 allele between Harosoy-E1/Misuzudaizu and Harosoy (e1)/Williams 82. The UTR and coding regions of E1 are marked in blue and red, respectively. (E) Apart from the dominant E1 gene, we identified three recessive e1 genotypes (as, missense point mutation; fs, frameshift mutation; nl, null mutation).
In two physical contigs (Fig. S2) built from two independent BAC libraries, the delimited region respectively corresponds to 17,372 bp for the dominant E1 allele in Misuzudaizu and 22,876 bp for the recessive e1 allele in Williams 82 (Fig. 2 B and C). A single intron-free gene (AB552962, 525 bp, 174 aa) was consistently identified by GenScan (45), GeneMark (46), FGENESH (http://linux1.softberry.com/berry.phtml), and RiceGAAS (44) for the dominant E1 genotype (Misuzudaizu and Harosoy-E1), and was designated E1 (Fig. 2D). In Williams 82 (39) and Harosoy (e1), which carry the recessive e1 allele, a single missense point mutation occurred at nucleotide 44 in the coding region of E1 (Fig. 2D), leading to a substitution of threonine for arginine at amino acid residue 15. We designated this recessive allele as e1-as (AB552963). We also detected 14 SNPs or indels (2–3 bp) between E1 and e1-as in the promoter regions. Moreover, an insertion of a 5,537-bp retrovirus-related sequence, within which a gag/pol protein was predicted by RiceGAAS (44), was inserted in the region 4 kb upstream of e1-as (Fig. 2C), although it is not clear whether this insertion has any functional impact on e1-as. The predicted e1-as protein (174 aa) corresponds to Glyma06g23040.1 (149 aa) in the Williams 82 genome (42) (http://www.phytozome.net), indicating a difference in gene prediction. Our RACE PCR analysis supported our prediction: the full-length cDNA sequence of E1 was 838 bp, with 157 bp and 156 bp for the 5′ and 3′ UTRs, respectively, in Harosoy-E1 and Harosoy (e1) (AB552964 and AB552965; Fig. 2D).
Allelic Variation in E1 Associated with Flowering Time.
Sequences from soybean cultivars with different flowering times were evaluated (Table S2) to identify association between flowering time and allelic variations in E1, and this analysis led to the identification of two additional recessive alleles (Fig. 2E) from the early-flowering cultivars. One allele, designated e1-fs (AB552971), had a 1-bp deletion in codon 17 that resulted in a premature stop codon in Sakamotowase and its derived NILs (20). The second, designated e1-nl, was a null allele in which ∼130 kb (including the entire E1 gene) was deleted in some early-flowering cultivars [i.e., Fiskeby V, Yukihomare (Table S2), Toyosuzu, Toyomusume, Hejian 1, and Heihe 28] known for photoperiod-insensitivity or being cultivated at high latitudes. The absence of the E1 sequence in these cultivars was confirmed by Southern hybridization (SI Materials and Methods and Fig. S3).
To evaluate the flowering time for the different E1 genotypes, we grew the cultivars in a growth chamber under long-day conditions (16 h light/8 h dark). Cultivars with E1 (except the E1/E2/e3/e4 genotype, with R1 of 36 d), e1-as, e1-fs, and e1-nl genotypes flowered ∼70, ∼50, ∼30, and ∼30 d after emergence, respectively (Table S2). The e1-fs and e1-nl genotypes showed a similar early-flowering phenotype, with no apparent delay in flowering time under long-day conditions, despite different genetic compositions at the other E loci (Table S2), suggesting the truncated e1-fs–produced protein is nonfunctional. Cultivars with the e1-as genotype generally had flowering times intermediate between the E1 and e1-fs genotypes both in the growth chamber and in the field, suggesting that e1-as is a leaky allele and may retains partial E1 function. The functional differences among the e1 alleles observed in the present study are consistent with a recent report (47) in which a flowering-time QTL at the E1 locus was detected in a population derived from Harosoy-e3 (e1-as) and Sakamotowase (e1-fs).
EMS-Derived E1 Mutants Show Early-Flowering Phenotype.
To confirm the function of E1 in delaying flowering, we identified mutant lines from EMS-treated libraries by using the Targeting Induced Local Lesions IN Genomes approach (48). We obtained three independent E1 mutants with missense mutations (Fig. S4), i.e., serine to phenylalanine at aa 17 (e1-m1), arginine to lysine at aa 15 (e1-m2), and threonine to isoleucine at aa 65 (e1-m3). The e1-m1 mutation was generated in OLERICHI50, whereas e1-m2 and e1-m3 were generated in Fukuyutaka. Both WT OLERICHI50 and Fukuyutaka carry the E1 allele, yet they have different flowering times (Table 1), which is most likely because of differences in their genetic backgrounds (20, 30). All three mutant lines flowered significantly earlier compared with their respective WT plants (Table 1). In addition, the line with e1-m1/e1-m1 flowered at 33.7 ± 0.47 d (n = 14), vs. 41.6 ± 1.8 d (n = 13) for the WT (OLERICHI50) under natural field conditions at Tsukuba. Under long-day conditions, the flowering times of F2 plants derived from a cross between the homozygous e1-m1 mutant and the WT were significantly correlated with the genetic mutation at the E1 locus (P < 0.01; Table 1). The mutant lines, e1-m2/e1-m2 and e1-m3/e1-m3, both flowered significantly earlier than the WT (Fukuyutaka; Table 1). Similarly, the phenotypes of the progeny of the self-pollinated heterozygous genotypes (E1/e1-m2 or E1/e1-m3) were significantly correlated with the genetic mutations at the E1 locus under natural conditions (P < 0.05; Table 1). The similar phenotype for three independent mutant lines and the significant differences in flowering time among genotypes in the mutant-derived progenies indicated that the mutations at E1, rather than at other loci, were responsible for alternation of the phenotype. Taken together, the results obtained from map-based cloning and the genetic and phenotypic analysis of the natural and artificial mutants strongly supported the hypothesis that E1 is the gene governing flowering time at the E1 locus.
Table 1.
Phenotypic analysis of flowering time in the WT and the progenies of E1 mutants (mut) obtained using EMS mutagenesis
| Flowering time, days after emergence |
||||||
| Mutant line | Mutation in E1 protein‡ | Population | WT/WT | WT/mut | mut/mut | P value§ |
| E1-m1* | Ser17Phe | Homozygous mut × WT, F2 | 69.70 ± 1.37 (12) | 66.80 ± 1.82 (12) | 63.80 ± 1.07 (12) | < 0.001 |
| E1-m2† | Arg15Lys | Heterozygous mut, self-crossed | 47.67 ± 1.25 (9) | 45.38 ± 2.15 (16) | 38.71 ± 1.03 (7) | < 0.001 |
| Arg15Lys | Homozygous mut | — | — | 38.11 ± 1.73 (9) | — | |
| E1-m3† | Thy65Ile | Heterozygous mut, self-crossed | 46.75 ± 1.92 (4) | 43.75 ± 1.64 (8) | 37.33 ± 2.05 (3) | 0.01 < P < 0.05 |
| Thy65Ile | Homozygous mut | — | — | 35.20 ± 1.72 (5) | — | |
| WT | Normal | WT, Fukuyutaka | 48.57 ± 0.90 (7) | — | — | — |
—, not applicable or not available.
*The mutant (mut) line was derived from OLERICHI50. The F2 population had a ratio of 18:47:22 for homozygous WT, heterozygous, and homozygous mutant alleles, respectively, and was thinned to an equal number of plants (n = 12) of each genotype for phenotypic investigation under artificial long-day conditions (15 h light/9 h dark for 30 d after sowing and thereafter 14 h light/10 h dark).
†These mutant lines were derived from Fukuyutaka. The progenies of plants harboring heterozygous or homozygous mutant alleles were grown under natural day length conditions [14:33 (h:min) at emergence to 13:23 at R1 for Fukuyutaka]. WT plants (Fukuyutaka) were grown as a control.
‡The sequence electrograms for representative mutant lines are shown in Fig. S4.
§The statistical significance of the phenotypic differences within each population was evaluated using one-way ANOVA.
In Silico Analysis and in Vivo Subcellular Distribution.
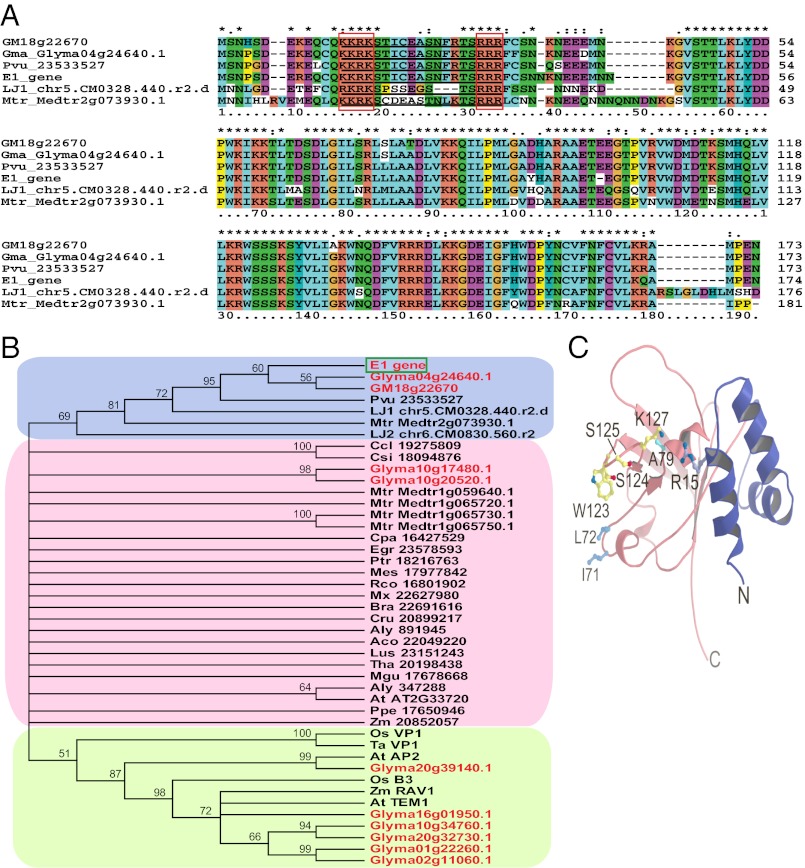
Two E1 paralogues (E1-L genes) Glyma04g24640.1 and Glyma18g22670.1 are present in the genome of soybean variety Williams 82, and each has 94% nucleotide sequence identity to E1 (Fig. 3A). This prediction was supported by the results of Southern hybridization (Fig. S3). However, in contrast to the annotation for Glyma18g22670.1 (156 aa; http://www.phytozome.net), this gene was predicted to encode 173 aa by RiceGAAS, and was referred to as Gm18g22670 in the present study (Fig. 3 A and B). Two other genes, Glyma10g17480.1 and Glyma10g20520.1, shared moderate (56.8% and 52.7%, respectively) amino acid sequence identity to the E1 protein (Fig. 3B). In legumes, sequences highly homologous to E1 were retrieved from Phaseolus vulgaris, Medicago truncatula, and Lotus japonicus (Fig. 3 A and B).
Fig. 3.
Characterization of the E1 protein. (A) Alignment of sequences highly homologous to E1 from legumes (Glyma or GM, G. max; Lja, L. japonicus; Mtr, M. truncatula; Pvu, P. vulgaris). The putative bipartite NLS is underlined, with its two basic domains (KKRK and RRR) marked by red squares. (B) A phylogenetic tree of E1 and its homologous sequences from various plant species. Sequences highly homologous to the E1 protein have a blue background. The sequences containing a standard B3 domain have a green background. Gm18g22670 was predicted by RiceGAAS (41) to have 173 aa, and is 17 aa longer than (but corresponds to) Glyma18g22670.1 (http://www.phytozome.net). Predicted protein sequences of Lja_chr5.CM0328.440.r2.d and Lja_chr6.CM0830.560.r2 were retrieved from http://www.kazusa.or.jp/lotus/blast.html. Other protein sequences were mainly retrieved from phytozome.net (version 8.0). Aly, Arabidopsis lyrata; At, A. thaliana; Bra, Brassica rapa; Ccl, Citrus clementine; Cpa, Carica papaya; Cru, Capsella rubella; Csi, Citrus sinensis; Egr, Eucalyptus grandis; Lja, L. japonicus; Lus, Linum usitatissimum; Mgu, Mimulus guttatus; Mtr, M. truncatula; Os, O. sativa; Pvu, P. vulgaris; Tae, Triticum aestivum; Zm, Zea mays. The gene name or reference number at phytozome.net is indicated after the species abbreviation. Typical B3-domain sequences obtained from the GenBank database (http://www.ncbi.nlm.nih.gov) are from A. thaliana, At AP2 (NP_175483.1) and At TEM1 (NP_173927.1); from O. sativa, Os B3 (EAY75457.1) and Os VP1 (Os01g0911700); from T. aestivum, Tae VP1 (CAB91107.1); and from Z. mays, Zm RAV1 (NP_001141742.1). Their homologues in soybean were also included for comparison. (C) A 3D structural model of the E1 protein generated by using the I-TASSER software (49), which predicts a B3-like fold for the C-terminal residues (aa 55–174, pink) and a helix–turn–helix structure for the N-terminal region (blue). Side chains of I71, L72, A79, W123, S124, S125, and K127 that correspond to the predicted DNA-contacting residues of AtRAV1 are shown in the ball-and-stick representation. In the e1-as protein, Arg is replaced by Thr at the position of the R15 side chain.
No known conserved domain was present in the E1 protein (E < 0.01) based on primary sequences in the National Center for Biotechnology Information Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). An analysis using I-TASSER (49) and Phyre (50) software showed that the E1 protein shared a low level (21–27%) of amino acid sequence identity with B3 domain sequences. The B3 domain has been identified in a number of transcriptional factors specific to plant species (46) (Fig. S5 A and B). Interestingly, the C-terminal region (aa 55–174) carries the minimal B3 domain structure required for DNA contact (51, 52) (Fig. S5A). In fact, there are several genes that contain a B3 domain in the soybean genome (Fig. 3B), although their functions have not been well characterized. In addition, prediction of the 3D structure using I-TASSER also showed a helix–turn–helix structure for the N-terminal region (aa 1–54; Fig. 3C). More importantly, a putative bipartite nuclear localization signal (NLS) with basic domains at each end (KKRK and RRR), separated by 12-aa residues, was identified at aa 13 to 31 (aa 15–33 in the consensus sequence; Fig. 3A). The features identified by in silico analysis indicated that the E1 protein may function in DNA binding or as a transcription factor.
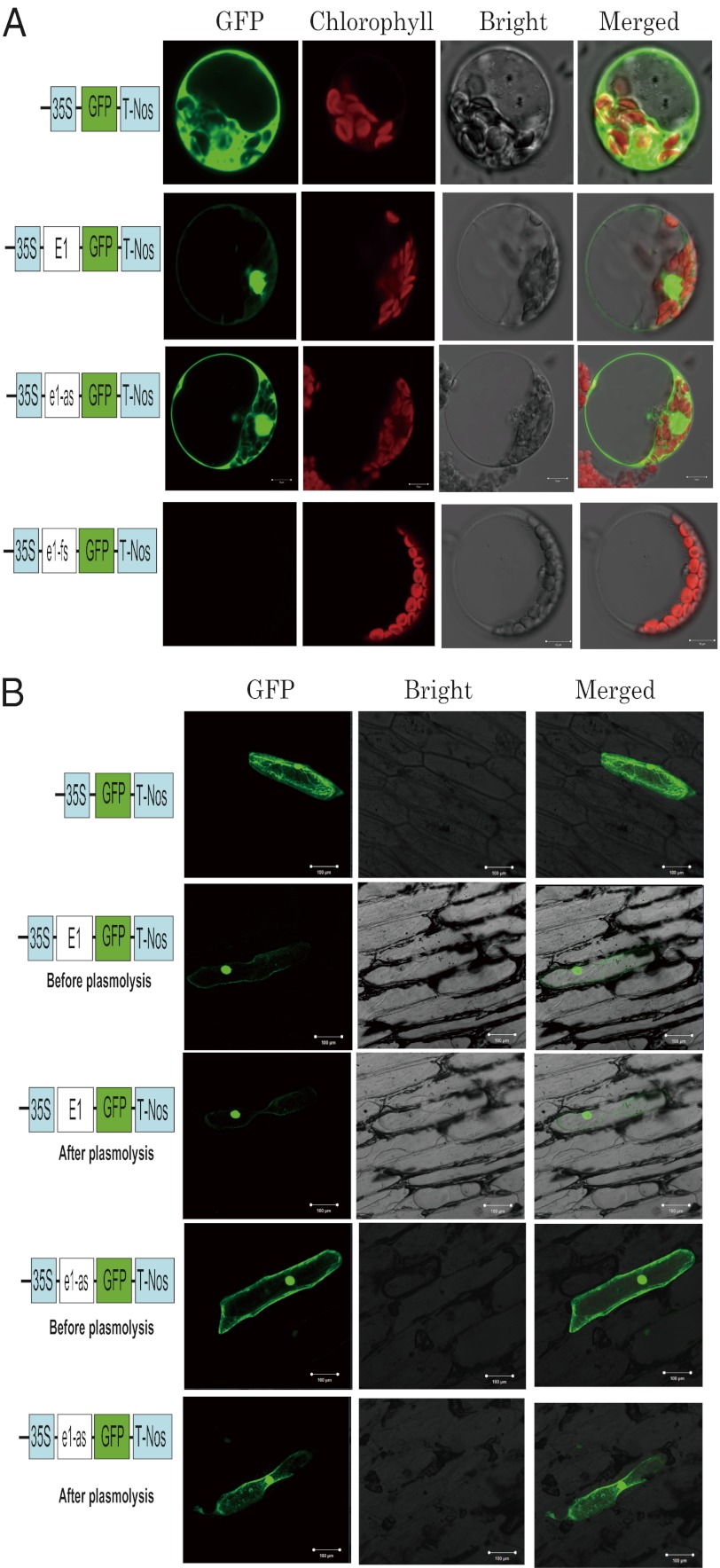
To gain further knowledge regarding the function of the E1 protein, we conducted a transient expression assay to determine its subcellular distribution. We separately fused E1, e1-as, and e1-fs to the eGFP gene and obtained constructs P35S:E1-eGFP, P35S:e1-as-eGFP, and P35S:e1-fs-eGFP, respectively. Confocal imaging showed that the E1-eGFP fusion protein was distributed primarily in the nucleus, with little signal in the cytoplasm either in A. thaliana protoplasts (Fig. 4A) and in onion (Allium cepa) epidermal cells (Fig. 4B). In contrast, the e1-as-eGFP fusion protein was distributed in the nucleus and the cytoplasm of both cell types (Fig. 4 A and B). There was no signal for the e1-fs:eGFP construct (Fig. 4A), indicating that the predicted protein (corresponding to aa 55–174 of the E1 protein) was not produced as a result of the frameshift mutation (e1-fs). This result indicated that an amino acid substitution in the basic domain of the putative bipartite NLS affected nuclear targeting of the E1 protein (53, 54). The changes in subcellular localization may represent a key mechanism underlying difference in flowering time governed by E1 and e1-as.
Fig. 4.
Subcellular localization of eGFP and of the E1-eGFP, e1-as-eGFP, and e1-fs-eGFP fusion proteins. (A) Arabidopsis protoplasts were transformed with plasmids that express the indicated gene constructs under the control of the constitutive 35S cauliflower mosaic virus (CaMV) promoter and the nopaline synthase terminator (T-Nos). The unfused eGFP coding sequence served as a control. The eGFP fusion proteins (E1-eGFP, e1-as-eGFP, and e1-fs-eGFP) were produced in the protoplasts and observed under a confocal laser-scanning microscope. GFP fluorescence, chlorophyll fluorescence, and bright-field images, and overlays of the green and red images (“merged”), are shown. (B) Subcellular distribution of the E1 and e1-as proteins in onion epidermal cells. E1-eGFP and e1-as fusion proteins (or eGFP alone as a control) were produced transiently under the control of the CaMV 35S promoter and T-Nos in onion epidermal cells and were observed under a confocal microscope. The photographs were taken with a dark field for green fluorescence (Left), with a bright field for the cell morphology (Center), and with a combination approach (merged, Right). Plasmolysis was performed to test for signals from the cell wall. In A and B, each 10 transformants were observed, and the experiments were performed three times, with similar results obtained.
Transcriptional Profiling of E1.
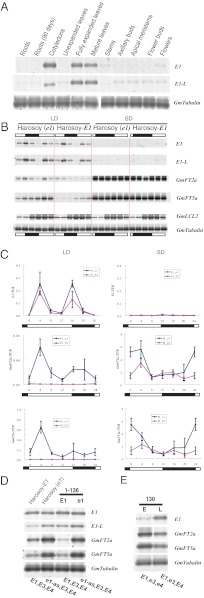
To examine how E1 transcription levels are related to flowering time, we analyzed the expression patterns of E1 and other related genes under different day length conditions. The low in planta abundance of E1 transcripts (demonstrated by RT-PCR) may explain the lack of EST data for E1 in the GenBank database (55, 56). BE608878, the only sequence we found in GenBank might have originated from Glyma04g24640.1, the E1 paralogue, as they are almost identical (405 of 406 bp identity). The expression of E1 was tissue-specific, with high levels in fully expanded leaves (including cotyledons) and low levels in other tissues (Fig. 5A).
Fig. 5.
Expression of E1, E1-L, and GmFTs. (A) Semiquantitative RT-PCR analysis of E1 and E1-L expression levels in different tissues of Harosoy-E1 plant under long-day conditions (18h light/6 h dark). All tissues were sampled on day 90 after emergence except for young roots and cotyledons on day 15. Images shown are representative profiles from three independent experiments. E1-L, E1 paralogues (Glyma04g24640.1/Gm18g22670); GmTubulin, internal control. (B) Semiquantitative RT-PCR analysis of E1, E1-L, GmFT2a, GmFT5a, and GmLCL2 expression levels in the leaves on day 15 after emergence under 12 h light/12 h dark (short day; SD) and 16 h light/8 h dark (long day; LD) conditions. Images shown are representative profiles from three independent experiments. GmFT2a, Glyma16g26660.1; GmFT5a, Glyma16g04830.1; GmLCL2, G. max LHY/CCA1Like2 gene (EU076434); GmTubulin, internal control. Black and white bars represent dark and light periods, respectively. (C) Real-time quantitative RT-PCR analysis of E1, GmFT2a, and GmFT5a expression levels. Relative expression levels to GmTubulin (TUB) are shown. Values represent mean ± SD (n = 3) from three biologically independent leaf samples from different plants. The experiment was repeated once, and similar results were obtained both times. Black and white bars represent dark and light periods, respectively. H_E1, Harosoy-E1; H_e1, Harosoy (e1). (D and E) Semiquantitative RT-PCR analysis of E1 (e1) and GmFTs expression levels in leaves on day 15 after emergence under an intermediate light regime (14.5 h light/9.5 h dark). Images shown are representative profile from three independent experiments. The genotypes at the E1, E3, and E4 loci are indicated at the bottom. The cultivars are Harosoy-E1, Harosoy (e1), NILs for the E1 locus [1-136(E1) and 1–136(e1)], and NILs for the E4 locus (130E and 130L; Table S2).
The expression of the clock-gene homologue GmLCL2 (Glycine max LHY/CCA1 Like2) (39, 57) exhibited a circadian rhythm (Fig. 5B), with no noticeable difference between short- and long-day conditions or between the two NILs (Fig. 5C), which was similar to previous observations (39). In contrast, the transcript abundances of E1 in both Harosoy NILs were low under short-day conditions (12 h light/12 h dark; Fig. 5 B and C). Under long-day conditions (16 h light/8 h dark), diurnal expression of E1 showed a bimodal pattern (Fig. 5 B and C). In the dark, E1 transcription gradually decreased, reaching its minimum before dawn. E1 transcription appeared to be reset at dawn and dusk (i.e., twice per day). The high level of suppression under short-day conditions and the bimodal pattern under long-day conditions suggested that the expression of this gene was regulated by photoperiod. Further study is needed to reveal whether the photoperiodic regulation pathways are entangled with the circadian clock. Interestingly, two E1-L genes (Glyma04g24640.1/Gm18g22670) showed an expression pattern similar to E1 (Fig. 5B), suggesting that these genes might be regulated by a similar mechanism.
Under short-day conditions, the transcriptions of GmFTs (GmFT2a and GmFT5a), two homologues of Arabidopsis FT (38), were strongly activated in soybean NILs carrying E1 or e1-as, with peak transcription occurring shortly before sunrise, followed by a steady decline until the end of the light period (Fig. 5C). Under long-day conditions, the overall transcription of the two GmFTs was lower than that under short-day conditions. Intriguingly, GmFTs in the leaf tissue of the e1-as genotype were expressed at significantly higher levels than that of the E1 genotype, peaking shortly after sunrise, followed by a steady decline until noon, and then increasing again to form a minor peak shortly after sunset (Fig. 5C). Given that there were no significant differences in transcript abundance between E1 and e1-as (Fig. 5 B and D), the defect of function in e1-as might be a result of the point mutation at the protein level.
Other genetic factors might also influence E1 expression. To test this possibility, we analyzed E1 transcription levels by using NILs with different genetic backgrounds. In previous research, E1 and the photoreceptor (i.e., PHYA) genes E3 and E4 responded differently to photoperiod (20, 30). Even under long days, E1 expression was significantly suppressed in NIL 130E (Fig. 5E) and in Kariyutaka (58), both of which carry the double-recessive alleles e3 and e4. This is consistent with previous observations that cultivars carrying either e1 or the double recessive alleles (e3 and e4) showed decreased sensitivity to photoperiod (20, 30, 38). Transcriptional profiling in the present study indicated that a high level of E1 expression requires long-day conditions and functional PHYA genes.
Elevated Expression of E1 Resulted in Late Flowering in Transgenic Soybean.
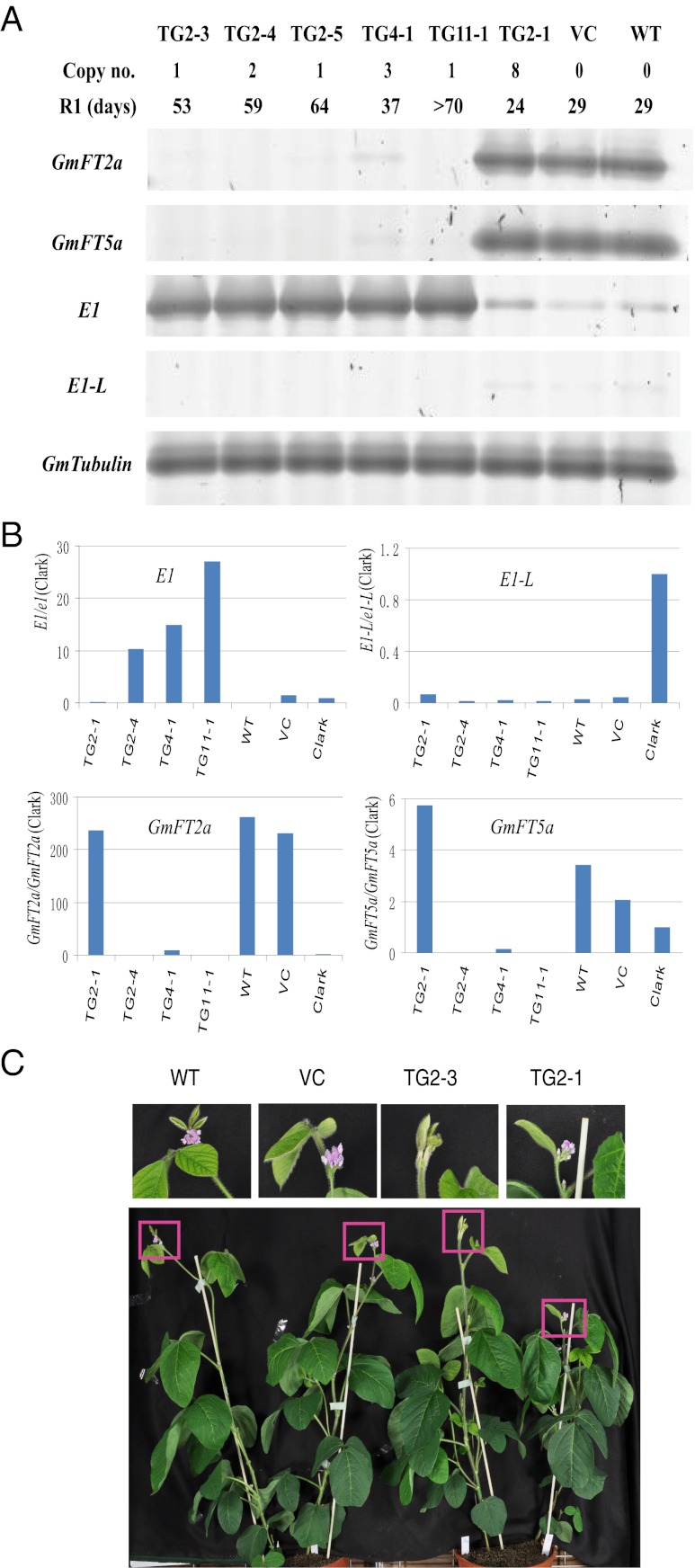
Transgenic plants with high expression of exogenous E1 were evaluated to further characterize the function of the E1 protein. The early-flowering Japanese cultivar Kariyutaka (E1/e3/e4) showed low levels of E1 expression under natural field conditions at Tsukuba and under long-day conditions in a growth chamber with light provided by white fluorescent lights supplemented by incandescent light. Because Kariyutaka is highly amenable to Agrobacterium-mediated transformation (58) and expresses E1 at extremely low levels (Fig. 6), this cultivar was selected for transformation of E1 under its natural promoter to test whether the E1 expression level was associated with the flowering phenotype. A 4,332-bp fragment (AB552966) containing a 2,668-bp upstream sequence from the start codon, the E1 coding region, and a 1,139-bp downstream sequence from the stop codon was amplified from Harosoy-E1 and cloned into the pMDC123-GFP vector (58). We characterized nine transgenic T1 plants derived from three independent T0 plants under long-day conditions (16 h light/8 h dark). In six transgenic plants (derived from two different T0 plants) that harbored one or two copies of the transgene, higher levels of E1 transcripts in the leaves were observed compared with the WT or vector control (Table S3). In the same cDNA samples, lower levels of GmFT transcripts were detected (Fig. 6 A and B). As expected, all six plants displayed much later flowering than the WT and the vector control (Fig. 6C). Conversely, very low levels of E1 transcript abundance coupled with high levels of GmFT transcription were observed in two plants that carried seven or more copies of the transgene, and both showed early flowering (Fig. 6 A and B and Table S3). One plant with three copies of the transgene displaying relatively high E1 transcription and low GmFT transcription exhibited an intermediate flowering time (Table S3). Because high levels of E1 transcripts were always associated with lower GmFT transcription and later flowering, we hypothesize that E1 regulates flowering time through its repression of GmFT transcription, at least partially, under long-day conditions.
Fig. 6.
High expression of E1 in transgenic lines leads to late flowering. (A) Semiquantitative RT-PCR analysis of E1, E1-L and GmFT expression levels in transgenic plants as a function of the copy number of the E1 transgene. Images shown are representative profiles from three technical replicates as a result of the limitation of plant materials. T1 plants TG2-1 to TG2-5 were derived from the T0 transgenic plant TG2, whereas T1 plants TG4-1 and TG11-1 were derived from TG4 (T0) and TG11 (T0), respectively (Table S3). VC, transformation vector only (i.e., vector control); WT, Kariyutaka; R1, days from emergence to opening of the first flower. (B) Real-time quantitative RT-PCR analysis of E1, GmFT2a, GmFT5a, and E1-L expression levels in the transgenic plants using the same biological samples as A. cDNA samples from the cultivar Clark under the same growth conditions were used as a control, whose expression level was set to 1 for all genes analyzed to linearly adjust the corresponding gene expression levels in transgenic plants. Values represent means (n = 3), except for TG2-1 (n = 1). (C) No flower had been produced by day 30 in a transgenic plant (TG2-3) with high E1 expression, whereas flowers emerged in the VC, the WT, and a transgenic plant (TG2-1) with low E1 expression.
Discussion
Function of the E1 Protein.
Our in silico analysis suggests that E1 is among a clade of sequences distantly related to the genes that encode the plant-specific B3 domain. The B3 superfamily encompasses many well characterized families and some poorly understood ones with diverse functions in plant growth and development (52). Well characterized functions are available for the LAV family [LEAFY COTYLEDON2 (LEC2)-ABSCISIC ACID INSENSITIVE3 (ABI3)-VAL], the ARF (AUXIN RESPONSE FACTOR) family, the RAV (RELATED TO ABI3 and VP1) family, and the REM (REPRODUCTIVE MERISTEM) family (52). Some genes that encode the B3 domain are involved in the control of flowering. Ectopic expression of OsLFL1 (O. sativa LEC2 and FUSCAS3 Like 1) in the LAV family resulted in late flowering via suppression of Ehd1 expression by binding to its promoter (59). TEMPRANILLO genes (TEM1 and TEM2) in the RAV family directly suppress FT genes in Arabidopsis (60), in which the quantitative balance between the activator CO and the suppressor TEM determines the expression levels of FT. Mochida et al. (61) performed in silico analysis of transcription factor repertories in soybean and deposited their data in LegumeTFDB (http://legumetfdb.psc.riken.jp) (62). In this database, the categories that include a B3 domain (RAV and ARF) contain more than 230 genes, including homologues of AtTEM genes (e.g., Glyma01g22260.1, Glyma02g11060.1, Glyma20g32730.1, and Glyma10g34760.1; Fig. 3B). However, we found no information on E1 or its two paralogues in this database. E1 (corresponding to Glyma06g23040.1; http://www.phytozome.net) was classified into a “hypothetical gene family” at the Rosid node, and into a “domain of unknown function (DUF313) family” at the Angiosperm node (http://www.phytozome.net; version 8.0). We could not predict the function of E1 based on its low similarity (21–27% at the amino acid level) to TEM1 or any other well characterized B3 genes (Fig. 3B and Fig. S5B). However, homologues of E1 from model legumes have not yet been characterized in vivo.
Although our knowledge of the E1 protein is limited, we obtained evidence of nuclear localization. Previous research demonstrated that both basic domains in the bipartite NLS are essential for nuclear targeting (54), particularly at the position of the arginine residue in the first basic domain (63). In contrast, intervening amino acids can tolerate some point mutations and insertions without any functional change (54). In the present study, the point mutation from arginine to threonine at position 15 in E1 (Fig. 3A) occurs at exactly the first basic domain of KKRK (which changes to KKTK) in the bipartite NLS, leading to different subcellular distributions between E1 and e1-as (Fig. 4). Likewise, the change from arginine to lysine at the same position in the e1-m2 mutant might also affect nuclear localization, although this hypothesis was not validated in vivo. The replacement of a highly conserved serine residue next to the NLS by aspartate, which mimics phosphorylation, might also result in decreased nuclear import (64). Similarly, the serine to phenylalanine mutation in e1-m1 occurs at aa 17, the position (Fig. S4A) next to the first basic domain, which might lead to changes in the phosphorylation state, and thus in flowering time. In addition, the replacement of threonine by isoleucine at aa 65 in the e1-m3 mutant (Fig. S4A) occurred in a β-strand of the B3 domain that is conserved among several B3 proteins (Fig. S5), although the functional mechanism is not yet clear. The presence of a putative DNA-binding B3 domain and a helix–turn–helix structure with a bipartite NLS suggests that the E1 protein might function as a transcription factor; however, further analysis is needed to reveal the functional mechanism by which the E1 protein regulates flowering time.
Genomewide duplications occurred ∼59 and 13 Mya in soybean (42). The high levels of sequence similarity at aa level among the E1 and E1-L genes indicate that these genes might have resulted from a lineage-specific duplication in soybean. Intriguingly, the expression pattern of E1-L in Harosoy-E1 is similar to that of E1 (Fig. 5 A, B, and D). However, the physiological function of the E1-L genes in soybean needs to be clarified. To our knowledge, no QTLs for flowering time have been located at either of the E1-L anchored regions (http://www.comparative-legumes.org) (65). Because the putative bipartite NLS and helix–turn–helix structure in the N-terminal region of the two E1-L proteins are almost identical to those of E1, the eight amino acid changes or indels in the C-terminal region (aa 31–173) might result in a subfunctionalization between E1 and E1-L.
Natural Variation in E1.
The latitudinal distribution of a plant or a cultivar is strongly associated with its photoperiod sensitivity. In rice, polymorphism in Hd1 protein, the type of Hd3a promoter and the level of Ehd1 expression were related to diversity of flowering time and geographic adaptation in cultivated rice species (66). Ebana et al. (67) found that functional polymorphisms in several identified key genes (Hd1, RFT1, and Ghd7) contributed greatly to the diversity of rice heading dates. These results demonstrated that independent mutations in key genes could explain a large proportion of the phenotypic variation in rice. Likewise, the allelic variation in E1 was clearly related to the flowering time phenotype in the present study. If E1 is considered the fully functional WT allele, the e1-as allele represents a partially functioning allele and the e1-fs and e1-nl alleles are probably nonfunctional. The early-flowering Swedish cultivar Fiskeby V carries e1-nl, whereas Sakamotowase (e1-fs) and Toyosuzu (e1-nl) were developed in Hokkaido, Japan’s northern island. Similarly, the Hejian 1 and Heihe 28 cultivars (e1-nl) were bred in Heilongjiang, a northern province of China. At these high latitudes, photoperiod insensitivity is generally a prerequisite for successful cultivation of a soybean cultivar. Various mutations of E1 might therefore have been preserved by human selection for cultivars adapted to a local photoperiod regime. Further genomic association study of the allelic variations at E1 and other E loci among a large number of soybean cultivars and natural accessions of the wild species Glycine soja from different geographic locations will help us to understand the domestication process.
Flowering-time genes are generally considered to have pleiotropic effects on important traits such as yield, plant height, and stress tolerance. QTLs, such as those associated with chilling response (68), seed yield, and chemical composition (http://www.soybase.org), are tightly linked to the E1 locus. Further characterization will reveal whether these influences are from E1 itself or from independent neighboring genes.
Putative Function of E1 in Photoperiodic Flowering of Soybean.
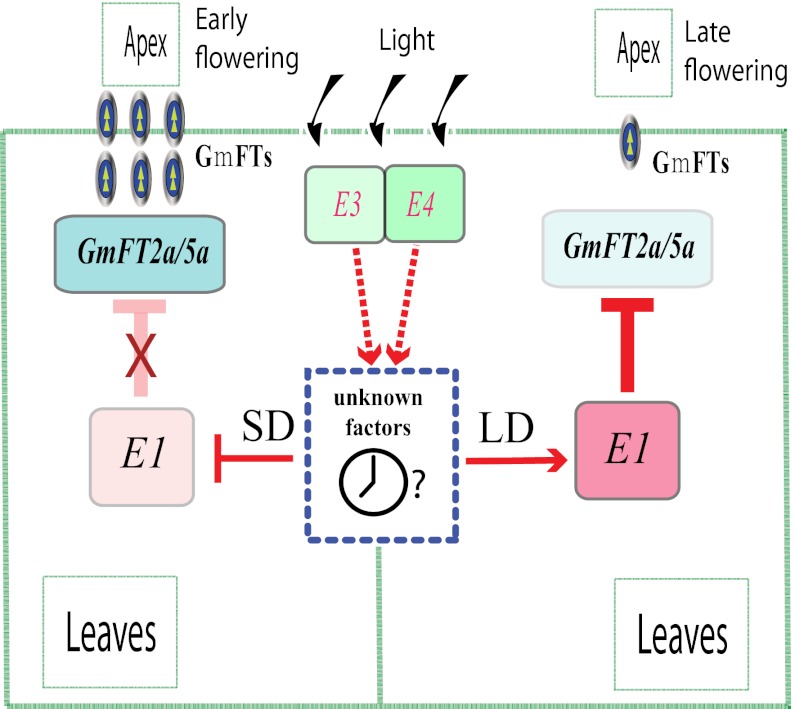
Light signals are mainly perceived and mediated by several photoreceptors, including phytochromes and cryptochromes, mainly in the leaves (69). A strong influence of phytochrome A on flowering signaling was observed in pea (Pisum sativum), a long-day plant (70), and in rice, a short-day plant (71). In soybean, both GmPHYA2 (E4) and GmPHYA3 (E3) suppress GmFTs (GmFT2a and GmFT5a) independently or jointly under long-day conditions (38). Moreover, comparison of the transcriptional profiles in soybean seedlings between the E1 NILs showed that the E1 locus suppresses flowering by mediating the expression levels of GmFTs (40). In the present study, the lower expression of E1 in Kariyutaka and other NILs with loss-of-function alleles of GmPHYA (e3 and e4) was coupled with an elevated expression of GmFT2a or GmFT5a. Research on soybean plants under various photoperiod and light quality conditions showed that E1, E3, and E4 are all involved in photoperiod responses (20, 30).
Allelic variations at the E loci, especially at E1, E3, and E4, could therefore provide considerable genetic plasticity that would allow soybean to be cultivated at a range of latitudes (30). In a mapping population with an e3 background, photoperiod insensitivity was observed in the e1E4, E1e4, and e1e4 genotypes, indicating that GmPHYA2 (E4) and E1 might concurrently mediate photoperiodic flowering, at least partially, via a shared pathway (20). Similarly, in the E3E4 or e3E4 genetic background, a major QTL was detected at the E1 locus (41). When the red to far-red ratio was changed from 2.0 to 1.0, strong responses to long-day conditions for both GmPHYA loci (E3, E4) and the strongest response for the E1 locus were observed (30). The high sensitivity of E1 to light quality might result from convergence of different signaling pathways regulated by GmPHYA2 (E4) and GmPHYA3 (E3), and potentially by other photoreceptor genes. The expression of E1 was induced under long-day conditions, with no significant difference between the E1 and e1-as alleles. In addition, high-level expression of E1 was inversely related to GmFT expression. Therefore, we propose that E1 is a part of the phytochrome A signaling pathway and controls two functionally coordinated GmFT genes (GmFT2a and GmFT5a; Fig. 7). In this scenario, long-day conditions are necessary for the induction of E1 expression, whereas loss-of-function alleles at E3 or E4 can result in some degree of suppression of E1 transcription and correspondingly elevated GmFT expression, leading to relatively early flowering.
Fig. 7.
A proposed flowering-time gene network in soybean. Transcription of E1 antagonistically determines the expression level of GmFTs (GmFT2a and GmFT5a), thereby controlling photoperiodic flowering. Arrows represent stimulation of gene expression; T-shaped symbols represent inhibition of gene expression; X represents the negation of inhibition/promotion; SD, short day length; LD, long day length.
In the present study, we proposed a gene network for the regulation of flowering in soybean, but more work will be necessary to integrate other factors into this network [e.g., to account for the function of the cryptochrome (i.e., GmCRY), a flavin-containing blue light photoreceptor in flowering]. GmCRY1a protein showed a photoperiod-dependent rhythmic expression pattern that was correlated with photoperiodic flowering and the latitudinal distribution cline of soybean cultivars (72).
To further understand the function of E1, we will need to identify the other genes and factors involved in the E1-mediated photoperiodic flowering pathway, and to further investigate how these components control recognition of the critical day length for flowering in soybean. Successfully deciphering of the E1 gene will lead to a greater understanding of the exquisite coordination within soybean’s photoperiodic flowering-gene network, possibly leading to the establishment of a model for related plant species.
Materials and Methods
Plant Materials and Phenotypic Flowering Time Parameters.
Cultivars were obtained from the Japanese National Institute of Agrobiological Sciences Genebank and from the US Department of Agriculture Agricultural Research Service National Plant Germplasm System (Table S2). The phenotypic parameter flowering time (R1) was defined as the time from emergence to opening of the first flower (43).
Fine Mapping of E1 Locus.
We crossed two NILs, Harosoy-E1 (L68-694, E1e2E3E4e5, PI547707) and Harosoy (e1) (L58-266, e1e2E3E4e5, PI547676), in 2004. We confirmed the production of true F1 hybrid seed by using heterozygous alleles in the E1 region based on the Satt365 and Satt557 markers. The F2 plants (117 plants) in 2005 and an F2:3 population of 1,442 individuals in 2006 were planted at Matsudo, Japan (35°78′N, 139°90′E). From 2007 to 2009, all field trials were performed at National Institute of Agrobiological Sciences (36°02′N, 140°11′E) at Tsukuba, Japan. We genotyped all progenies by using the derived cleaved amplified polymorphic sequence marker TI (Fig. S4), which can distinguish E1 from e1-as, to determine whether allelic variation in E1 was correlated with phenotypic differences in 2009 (Fig. S1).
BAC Contig Construction and Marker Development.
Two parallel contigs (Fig. S2) for dominant and recessive E1 genotypes were developed from the Misuzudaizu (E1) BAC library (73) and the Williams 82 (e1-as) GM_WBb BAC library (https://www.genome.clemson.edu) using locus-specific markers (Table S4). BAC screening and BAC clone information are given in SI Materials and Methods.
Simple-sequence-repeat marker information was retrieved from SoyBase (http://www.soybase.org) and elsewhere (74, 75). Amplified fragment length polymorphism markers were generated following a standard protocol (75). All markers (Table S4) used for fine-scale mapping of the E1 locus were developed by targeting polymorphisms between the contigs (Fig. S2) representative of the two E1 genotypes.
Sequence Alignment and Phylogenetic Analysis.
Sequences of E1 and its homologues were aligned by using Clustal X2, and phylogenetic analysis was performed by using MEGA4. Sequence acquisitions and parameters used in Clustal X2 and MEGA4 are provided in SI Materials and Methods.
Sequence Variation at E1 Locus in Different Cultivars.
We amplified genomic regions (∼4 kb) including the promoter and coding regions of E1 from Harosoy-E1, Harosoy (e1), Sakamotowase, Williams 82, Misuzudaizu, and Mashidougong 503 by using the 4K primer (Table S4). These sequences were deposited in DNA Data Base in Japan under accession numbers AB552966 to AB552971.
Screening and Characterization of EMS-Derived Mutations.
Details of mutagenesis of OLERICHI50 and Fukuyutaka, Targeting Induced Local Lesions In Genomes screening of the mutant libraries, and phenotypic characterization of the mutants are given in SI Materials and Methods.
Transformation of E1 into Kariyutaka.
We amplified a 4,332-bp fragment (AB552966) from Harosoy-E1 using the 4K primer pair (Table S4), cloned it into the pMDC123-GFP vector (58), and transformed this construct into Kariyutaka. The transformation followed a previously described procedure (58). The flowering times of the transgenic plants were evaluated in a growth chamber with cool white fluorescent light plus incandescent lamps (235–270 μmol⋅s−1⋅m−2) under a 16 h light/8 h dark photoperiod at a constant temperature of 25 °C.
RNA Preparation and Quantitative PCR.
Plants were grown in growth chambers. RNA was extracted by using the TRIzol (Life Technologies) method. cDNA synthesis was performed by using ReverTra Ace (Toyobo).
Semiquantitative RT-PCRs.
PCR products were resolved in nondenaturing polyacrylamide gel and fluorescently stained.
Real-time quantitative RT-PCR.
Transcript levels were quantified by using real-time quantitative RT-PCR (iCycle iQ; BioRad). Details are given in SI Materials and Methods.
Subcellular Localization.
To obtain a C terminus fusion plasmid, we amplified a cDNA fragment containing the coding region without a stop codon by means of PCR using the primers 5′-CCATCGATAGATGAGCAACCCTTCAGATGAAAGG-3′ (forward) and 5′-GACTAGTATAATTCTCTGGCATAGCTTGTTTAAGG-3′ (reverse) from plasmids (pGEM-T Easy; Promega) containing the E1, e1-as, or e1-fs sequence. The PCR product was then inserted downstream of the CaMV 35S promoter and in-frame with the 5′ terminus of the eGFP gene in the backbone of the pBSK vector (76). The recombinant fusion plasmids were introduced into onion epidermal cells by means of particle bombardment, and were expressed in Arabidopsis protoplasts by means of polyethylene glycol-mediated transfection, as described previously (77, 78).
Supplementary Material
Acknowledgments
We thank Profs. S. Y. Chen and W. K. Zhang (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for instruction in and generous help with functional analysis; N. Jiang (Michigan State University), E. R. Cober (Agriculture and Agri-Food Canada), J. D. Faris (US Department of Agriculture Agricultural Research Service), L. Yan (Oklahoma State University), T. Izawa (National Institute of Agrobiological Sciences), and B. Larkins (University of Arizona) for critical reading of an early draft of this paper; K. Saeki (Nara Women’s University) for interpretation of the results; and J. Abe (Hokkaido University) and B. Liu and F. Kong (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences) for providing primer information. This study was supported by Ministry of Agriculture, Forestry and Fisheries of Japan Genomics for Agricultural Innovation Grants DD-2040 and SOY2003; Japan Grant-in-Aid for Scientific Research (A) 18208001; and Chinese Academy of Sciences Hundred Talents Program and Grant KZCX2-EW-303.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Base in Japan, http://www.ddbj.nig.ac.jp (DDBJ accession nos. AB552962, AB552963, AB552971, AP011812, AP011814–AP011820, and AP011823).
See Author Summary on page 12852 (volume 109, number 32).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117982109/-/DCSupplemental.
References
- 1.Jung C, Müller AE. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009;14:563–573. doi: 10.1016/j.tplants.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- 3.Garner WW, Allard HA. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J Agric Res. 1920;18:553–606. [Google Scholar]
- 4.Samach A, et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- 5.Robson F, et al. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001;28:619–631. doi: 10.1046/j.1365-313x.2001.01163.x. [DOI] [PubMed] [Google Scholar]
- 6.Izawa T. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot. 2007;58:3091–3097. doi: 10.1093/jxb/erm159. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji H, Taoka K, Shimamoto K. Regulation of flowering in rice: Two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol. 2011;14:45–52. doi: 10.1016/j.pbi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 9.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu J, Warthmann N, Küttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- 12.Notaguchi M, et al. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 2008;49:1645–1658. doi: 10.1093/pcp/pcn154. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Naim O, et al. The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 2006;46:462–476. doi: 10.1111/j.1365-313X.2006.02706.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayama R, Agashe B, Luley E, King R, Coupland G. A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis. Plant Cell. 2007;19:2988–3000. doi: 10.1105/tpc.107.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JY, Yu JP, McIntosh L, Kende H, Zeevaart JAD. Isolation of a CONSTANS ortholog from Pharbitis nil and its role in flowering. Plant Physiol. 2001;125:1821–1830. doi: 10.1104/pp.125.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lifschitz E, et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA. 2006;103:6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayama R, Coupland G. The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol. 2004;135:677–684. doi: 10.1104/pp.104.042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen FV. Inheritance studies in soybeans. II. Glabrousness, color of pubescence, time of maturity, and linkage relations. Genetics. 1927;12:519–529. doi: 10.1093/genetics/12.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard RL. Two major genes for time of flowering and maturity in soybeans. Crop Sci. 1971;11:242–244. [Google Scholar]
- 20.Abe J, et al. Photoperiod-insensitive Japanese soybean landraces differ at two maturity loci. Crop Sci. 2003;43:1300–1304. [Google Scholar]
- 21.Buzzell RI. Inheritance of a soybean flowering response to fluorescent-daylength conditions. Can J Genet Cytol. 1971;13:703–707. [Google Scholar]
- 22.Buzzell RI, Voldeng HD. Inheritance of insensitivity to long day length. Soybean Genet Newsl. 1980;7:26–29. [Google Scholar]
- 23.McBlain BA, Bernard RL, Cremeens CR, Korczak JF. A procedure to identify genes affecting maturity using soybean isoline testers. Crop Sci. 1987;27:1127–1132. [Google Scholar]
- 24.Saindon G, Beversdorf WD, Voldeng HD. Adjustment of the soybean phenology using the E4 locus. Crop Sci. 1989;29:1361–1365. [Google Scholar]
- 25.Bonato ER, Vello NA. E6 a dominant gene conditioning early flowering and maturity in soybeans. Genet Mol Biol. 1999;22:229–232. [Google Scholar]
- 26.Cober ER, Voldeng HD. A new soybean maturity and photoperiod-sensitivity locus linked to E1 and T. Crop Sci. 2001;41:698–701. [Google Scholar]
- 27.Cober ER, Molnar SJ, Charette M, Voldeng HD. A new locus for early maturity in soybean. Crop Sci. 2010;50:524–527. [Google Scholar]
- 28.Ray JD, Hinson K, Mankono EB, Malo FM. Genetic control of a long-juvenile trait in soybean. Crop Sci. 1995;35:1001–1006. [Google Scholar]
- 29.Cober ER, Tanner JW, Voldeng HD. Genetic control of photoperiod response in early-maturing near-isogenic soybean lines. Crop Sci. 1996a;36:601–605. [Google Scholar]
- 30.Cober ER, Tanner JW, Voldeng HD. Soybean photoperiod-sensitivity loci respond differentially to light quality. Crop Sci. 1996b;36:606–610. [Google Scholar]
- 31.Watanabe S, et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics. 2009;182:1251–1262. doi: 10.1534/genetics.108.098772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, et al. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics. 2008;180:995–1007. doi: 10.1534/genetics.108.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61:11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe S, et al. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics. 2011;188:395–407. doi: 10.1534/genetics.110.125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huq E, Tepperman JM, Quail PH. GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc Natl Acad Sci USA. 2000;97:9789–9794. doi: 10.1073/pnas.170283997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasma IM, Shoemaker RC. Mapping flowering time gene homologs in soybean and their association with maturity (E) loci. Crop Sci. 2003;43:319–328. [Google Scholar]
- 37.Hecht V, et al. Conservation of Arabidopsis flowering genes in model legumes. Plant Physiol. 2005;137:1420–1434. doi: 10.1104/pp.104.057018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong FJ, et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol. 2010;154:1220–1231. doi: 10.1104/pp.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakare D, Kumudini S, Dinkins RD. Expression of flowering-time genes in soybean E1 near-isogenic lines under short and long day conditions. Planta. 2010;231:951–963. doi: 10.1007/s00425-010-1100-6. [DOI] [PubMed] [Google Scholar]
- 40.Thakare D, Kumudini S, Dinkins RD. The alleles at the E1 locus impact the expression pattern of two soybean FT-like genes shown to induce flowering in Arabidopsis. Planta. 2011;234:933–943. doi: 10.1007/s00425-011-1450-8. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka N, et al. Fine mapping of the FT1 locus for soybean flowering time using a residual heterozygous line derived from a recombinant inbred line. Theor Appl Genet. 2005;110:634–639. doi: 10.1007/s00122-004-1886-3. [DOI] [PubMed] [Google Scholar]
- 42.Schmutz J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 43.Fehr WR, Caviness CE, Burmood DT, Pennington JS. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971;11:929–931. [Google Scholar]
- 44.Sakata K, et al. RiceGAAS: An automated annotation system and database for rice genome sequence. Nucleic Acids Res. 2002;30:98–102. doi: 10.1093/nar/30.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 46.Lukashin AV, Borodovsky M. GeneMark.hmm: New solutions for gene finding. Nucleic Acids Res. 1998;26:1107–1115. doi: 10.1093/nar/26.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu BH, Abe J. QTL mapping for photoperiod insensitivity of a Japanese soybean landrace Sakamotowase. J Hered. 2010;101:251–256. doi: 10.1093/jhered/esp113. [DOI] [PubMed] [Google Scholar]
- 48.McCallum CM, Comai L, Greene EA, Henikoff S. Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiol. 2000;123:439–442. doi: 10.1104/pp.123.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy A, Kucukural A, Zhang Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley LA, Sternberg MJE. Protein structure prediction on the Web: A case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 51.Yamasaki K, et al. Solution structure of the B3 DNA binding domain of the Arabidopsis cold-responsive transcription factor RAV1. Plant Cell. 2004;16:3448–3459. doi: 10.1105/tpc.104.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swaminathan K, Peterson K, Jack T. The plant B3 superfamily. Trends Plant Sci. 2008;13:647–655. doi: 10.1016/j.tplants.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 54.Raikhel N. Nuclear targeting in plants. Plant Physiol. 1992;100:1627–1632. doi: 10.1104/pp.100.4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong CE, Singh MB, Bhalla PL. Molecular processes underlying the floral transition in the soybean shoot apical meristem. Plant J. 2009;57:832–845. doi: 10.1111/j.1365-313X.2008.03730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libault M, et al. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010;63:86–99. doi: 10.1111/j.1365-313X.2010.04222.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, et al. Analysis of clock gene homologs using unifoliolates as target organs in soybean (Glycine max) J Plant Physiol. 2009;166:278–289. doi: 10.1016/j.jplph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Sato H, Yamada T, Kita Y, Ishimoto M, Kitamura K. Production of transgenic plants and their early seed set in Japanese soybean variety, Kariyutaka. Plant Biotechnol. 2007;24:533–536. [Google Scholar]
- 59.Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL. Ectopic expression of OsLFL1 in rice represses Ehdl by binding on its promoter. Biochem Biophys Commun. 2007;360:251–256. doi: 10.1016/j.bbrc.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 60.Castillejo C, Pelaz S. The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr Biol. 2008;18:1338–1343. doi: 10.1016/j.cub.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 61.Mochida K, et al. In silico analysis of transcription factor repertoire and prediction of stress responsive transcription factors in soybean. DNA Res. 2009;16:353–369. doi: 10.1093/dnares/dsp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mochida K, et al. LegumeTFDB: An integrative database of Glycine max, Lotus japonicus and Medicago truncatula transcription factors. Bioinformatics. 2010;26:290–291. doi: 10.1093/bioinformatics/btp645. [DOI] [PubMed] [Google Scholar]
- 63.Abel S, Theologis A. A polymorphic bipartite motif signals nuclear targeting of early auxin-inducible proteins related to PS-IAA4 from pea (Pisum sativum) Plant J. 1995;8:87–96. doi: 10.1046/j.1365-313x.1995.08010087.x. [DOI] [PubMed] [Google Scholar]
- 64.Birkenbihl RP, Jach G, Saedler H, Huijser P. Functional dissection of the plant-specific SBP-domain: overlap of the DNA-binding and nuclear localization domains. J Mol Biol. 2005;352:585–596. doi: 10.1016/j.jmb.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Gonzales MD, et al. The Legume Information System (LIS): An integrated information resource for comparative legume biology. Nucleic Acids Res. 2005;33(database issue):D660–D665. doi: 10.1093/nar/gki128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi Y, Teshima KM, Yokoi S, Innan H, Shimamoto K. Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci USA. 2009;106:4555–4560. doi: 10.1073/pnas.0812092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebana K, et al. Uncovering of major genetic factors generating naturally occurring variation in heading date among Asian rice cultivars. Theor Appl Genet. 2011;122:1199–1210. doi: 10.1007/s00122-010-1524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Funatsuki H, Kawaguchi K, Matsuba S, Sato Y, Ishimoto M. Mapping of QTL associated with chilling tolerance during reproductive growth in soybean. Theor Appl Genet. 2005;111:851–861. doi: 10.1007/s00122-005-0007-2. [DOI] [PubMed] [Google Scholar]
- 69.Lin C. Photoreceptors and regulation of flowering time. Plant Physiol. 2000;123:39–50. doi: 10.1104/pp.123.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weller JL, Beauchamp N, Kerckhoffs LH, Platten JD, Reid JB. Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J. 2001;26:283–294. doi: 10.1046/j.1365-313x.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 71.Takano M, et al. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell. 2005;17:3311–3325. doi: 10.1105/tpc.105.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Q, et al. Association of the circadian rhythmic expression of GmCRY1a with a latitudinal cline in photoperiodic flowering of soybean. Proc Natl Acad Sci USA. 2008;105:21028–21033. doi: 10.1073/pnas.0810585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia Z, Sato H, Watanabe S, Kawasaki S, Harada K. Construction and characterization of a BAC library of soybean. Euphytica. 2005;141:129–137. [Google Scholar]
- 74.Song QJ, et al. A new integrated genetic linkage map of the soybean. Theor Appl Genet. 2004;109:122–128. doi: 10.1007/s00122-004-1602-3. [DOI] [PubMed] [Google Scholar]
- 75.Xia Z, et al. An integrated high-density linkage map of soybean with RFLP, SSR, STS, and AFLP markers using A single F2 population. DNA Res. 2007;14:257–269. doi: 10.1093/dnares/dsm027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li HJ, et al. POD1 regulates pollen tube guidance in response to micropylar female signaling and acts in early embryo patterning in Arabidopsis. Plant Cell. 2011;23:3288–3302. doi: 10.1105/tpc.111.088914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheen J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 2001;127:1466–1475. [PMC free article] [PubMed] [Google Scholar]
- 78.Xie ZM, et al. Soybean Trihelix transcription factors GmGT-2A and GmGT-2B improve plant tolerance to abiotic stresses in transgenic Arabidopsis. PLoS ONE. 2009;4:e6898. doi: 10.1371/journal.pone.0006898. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]