Abstract
We evaluated recent proposals that structures in the medial temporal lobe (MTL)—in particular, perirhinal cortex—support not just memory but certain kinds of perceptual abilities as well. Specifically, it has been suggested that the perirhinal cortex supports the perceptual abilities needed to accomplish visual discrimination performance when the stimuli have complex features and overlapping elements. However, the tasks that have been studied are quite challenging. Stimulus features must be held in working memory while attention shifts among the several parts of the display. When working memory capacity is exceeded, performance must depend on retrieval from long-term memory. Five patients with limited hippocampal lesions and one patient with large MTL lesions were asked to identify the unique object among twin pairs of objects that had a high degree of feature overlap and perceptual similarity. The patient groups performed similarly to controls when there were few objects and features in the displays, but exhibited abrupt declines in performance when the displays contained more objects and more features. Notably, the impairment was observed in memory-impaired patients with hippocampal lesions, not only in association with large MTL lesions that included perirhinal cortex. The pattern of performance suggested that patients encountered difficulty because working memory capacity was exceeded in the more difficult conditions such that performance needed to depend at least in part on long-term memory. Furthermore, when the burden on working memory was removed entirely, the patient with large MTL lesions performed as well as controls. Accordingly, we suggest that deficits on difficult discrimination tasks reported for patients with MTL lesions are due to impaired memory rather than impaired perception.
Keywords: hippocampus, amnesia
The medial temporal lobe (MTL) has long been associated with memory function (1). Early studies suggested that MTL lesions severely impaired the formation of long-term memory, while sparing intellectual and perceptual functions (2). In addition, MTL lesions, as well as more limited hippocampal lesions, spared immediate memory and working memory (2–5). These early findings gave support to the perspective that the ability to acquire new memories is a distinct cerebral function, separable from working memory, perceptual functions, and intellectual ability.
These ideas have been revisited recently with the proposal that MTL structures might be important for perception in addition to memory. For example, studies in monkeys and humans suggested that the perirhinal cortex is needed for discriminating among stimuli with complex features that include overlapping elements (6–15).
One important issue considered previously (16, 17) is that it is difficult to rule out a role for learning and memory in many of the tasks that have been used. For example, learning might contribute to performance in perceptual tasks where stimulus sets are repeated across trials. Indeed, in one study, patients with hippocampal lesions were impaired at discriminating between similar faces or scenes when elements of the stimulus display repeated from trial to trial. However, the same patients were fully intact when each stimulus display was unique (18).
An additional potential issue is that some of the perceptual tasks are quite challenging, and the number and complexity of the stimuli might sometimes exceed the capacity of working memory. In this circumstance, performance should depend substantially on long-term (supraspan) memory. Thus, even when the stimuli to be discriminated are trial-unique and presented simultaneously, working memory might be challenged (and long-term memory might be needed) because of the requirement that attention shift back and forth between multiple stimuli. For example, in one study, an impairment was reported in MTL patients when each trial required discriminating among seven similar objects with overlapping features (12).
An impairment attributable to limited working memory capacity (and impaired long-term memory) rather than perception might be identified in two ways. First, as suggested previously (19), lesions limited to the hippocampus should impair performance, not just large MTL lesions that include perirhinal cortex. Second, an impairment in long-term memory should yield a particular pattern of performance. Specifically, as discussed elsewhere (20, 21), patients with MTL lesions should perform similarly to controls when the burden on working memory is modest (even when stimuli have overlapping features). Then, as the task becomes gradually more difficult, patients should exhibit an abrupt discontinuity in performance at the point where the capacity of working memory is exceeded. Last, controls should begin to make errors at about this same point. This pattern of performance in patients and controls has been reported before in tasks of digit span and object-location association (3, 20), with the interpretation that this pattern reveals an intact immediate (or working) memory in the patients and impaired long-term (supraspan) memory.
In the present study, we applied these same criteria to an object discrimination task using trial-unique stimuli with a high degree of feature overlap. In each display, participants tried to identify the unique object among twin pairs of objects. Six levels of difficulty were created by presenting different numbers of objects and by varying the differences among the objects. If poor performance is attributable to memory impairment, large MTL lesions as well as limited hippocampal lesions should impair performance. In addition, patients should perform similarly to controls until working memory capacity is exceeded. At that point, controls should begin to make errors, and patients with MTL lesions should exhibit an abrupt decline in performance.
Results
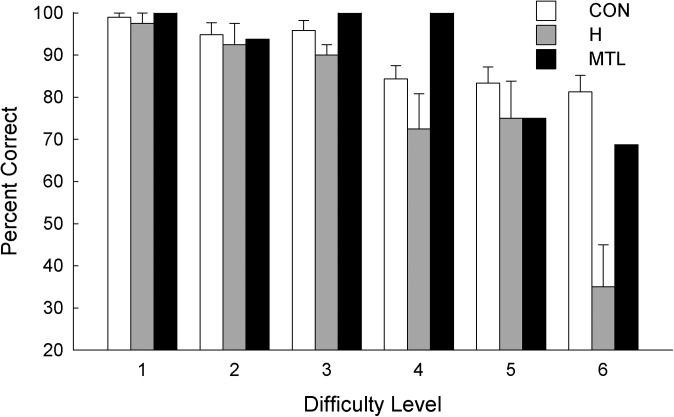
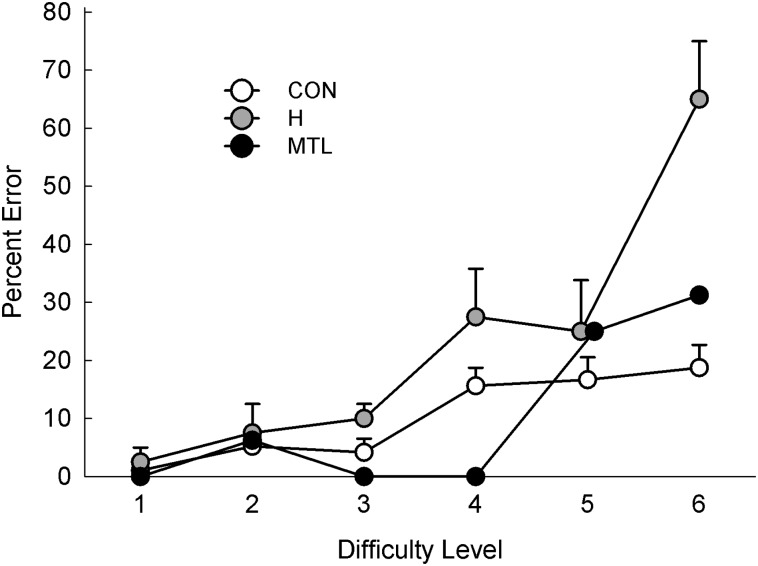
Across all 48 displays, the controls scored 89.8% correct, and the patients with hippocampal lesions scored 77.1% correct (Fig. 1; P < 0.01). Patient G.P. with large MTL lesions performed well overall (89.6% correct). Fig. 2 shows performance across the six difficulty levels (eight trials per level). An ANOVA comparing the hippocampal patients with controls revealed an effect of group [F(1, 15) = 10.7, P < 0.01], an effect of difficulty level [F(5, 75) = 28.4, P < 0.001], and a group × difficulty level interaction [F(5, 75) = 9.2, P < 0.001]. The interaction reflects the fact that the hippocampal patients were impaired relative to controls only in the more difficult conditions. In the easiest conditions (difficulty levels 1–3), 9–11 of the 12 controls scored 100%, and the patients also performed well. At difficulty level 4, most controls made errors for the first time (only 2 of 12 scored 100%), and their accuracy noticeably decreased relative to difficulty level 3 (P < 0.05). Accuracy of the patients also declined for the first time at this same difficulty level (P = 0.05, relative to difficulty level 3). Fig. 3 shows performance across the six difficulty levels as percent error scores and highlights the fact that performance was very good overall until performance of hippocampal patients sharply declined at difficulty level 4.
Fig. 1.
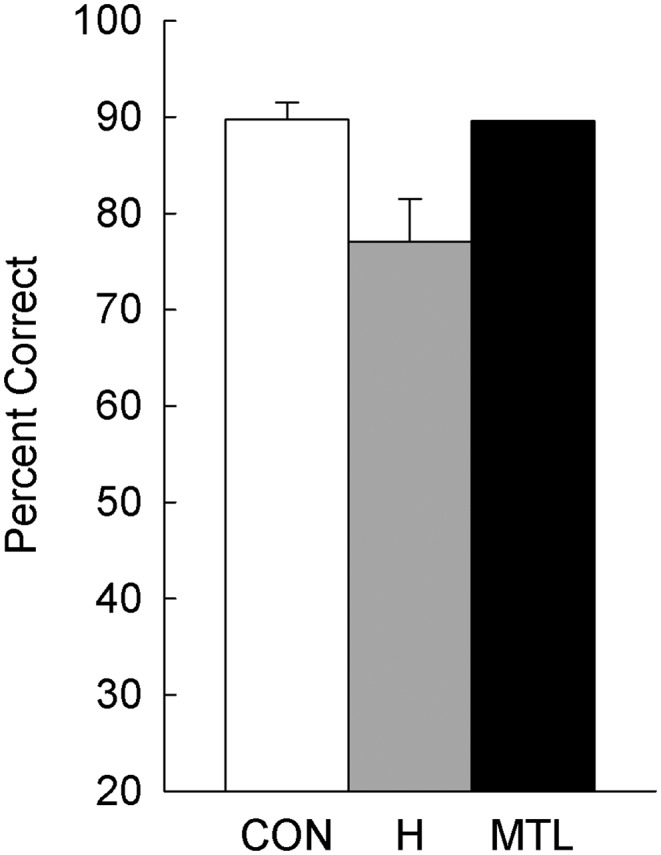
Participants saw 48 unique displays of five or seven objects, and attempted in each case to identify the unique object. Performance of controls (CON, n = 12), patients with hippocampal lesions (H, n = 5), and a patient with large medial temporal lobe lesions (MTL, n = 1). In every display, each appendage appeared on more than one object, such that the unique object was always defined by the conjunction of two appendages. Brackets show SEM.
Fig. 2.
Accuracy at identifying the unique object for controls (CON, n = 12), patients with hippocampal lesions (H, n = 5), and a patient with large medial temporal lobe lesions (MTL, n = 1) across six levels of difficulty (eight trials per level). Difficulty (Materials and Methods) was manipulated by varying the number of objects in each display (five or seven), the number of appendages on each object (two or four), the number of body colors in each display (two or one), and the differences among the appendages (relatively salient or more subtle). For difficulty levels 1 and 2, chance = 20.0%; for difficulty levels 3–6, chance = 14.3%. Brackets show SEM.
Fig. 3.
Percent error scores for controls (CON, n = 12), patients with hippocampal lesions (H, n = 5), and a patient with large medial temporal lobe lesions (MTL, n = 1) across six levels of difficulty. Patients first exhibited impairment at difficulty level 4, which is the same level at which controls first made consistent errors.
Patient G.P. with large MTL lesions performed well at difficulty levels 1–4. He was marginally impaired at difficulty level 5 (75.0% vs. 83.3% for controls, P < 0.06), one difficulty level later than the point where controls and hippocampal patients first made errors. G.P. was also impaired at difficulty level 6 (68.8% vs. 81.3%, P < 0.01).
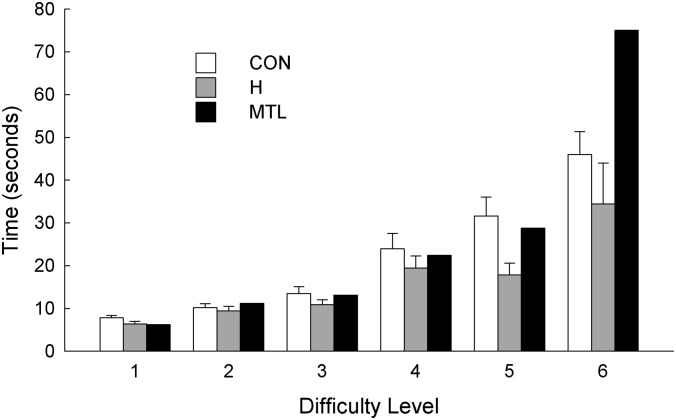
Fig. 4 shows the mean times needed to identify the unique object at each level of difficulty. An ANOVA comparing hippocampal patients and controls revealed only an effect of difficulty level [F(5, 75) = 29.6, P < 0.001]. Overall, the three groups had similar response times and a similar sensitivity to the difficulty level. Note though that at difficulty level 5 hippocampal patients took marginally less time than the controls to make their choices (17.8 vs. 31.6 s, P < 0.08). In addition, G.P. performed very slowly at difficulty level 6. When incorrect choices were made, the participants with hippocampal lesions often identified the unique object correctly when given additional time to choose again. Presumably this was possible because hippocampal lesions leave some residual capacity for supraspan memory. Nonetheless, on one occasion at difficulty level 6, patients H3 and H5 failed to correctly identify the unique object. In addition, patient H2 declined to guess a second time when he erred at difficulty level 6.
Fig. 4.
Time needed to identify the unique object for controls (CON, n = 12), patients with hippocampal lesions (H, n = 5), and a patient with large medial temporal lobe lesions (MTL, n = 1) across six difficulty levels. Brackets show SEM.
Sixteen additional trials (difficulty levels 7 and 8) were given to G.P. and eight controls to more severely challenge G.P.’s performance. Controls found these displays to be difficult, scoring 82.8% and 56.3% correct at difficulty levels 7 and 8, respectively. G.P. scored 75.0% correct at difficulty level 7 and then declined abruptly at difficulty level 8 (25.0% correct; P < 0.05 relative to controls). When invited to try again after his errors, G.P. was able to identify the correct object only once (in a total of eight error trials), even after trying as many as three times. G.P.’s failure to correct himself is consistent with the severity of his memory impairment.
At difficulty level 7, G.P. and controls recorded similar response times (68.6 s vs. 57.2 s). At difficulty level 8, G.P. responded more slowly than at difficulty level 7 but faster than controls (108.8 s vs. 150.3 s, P < 0.03).
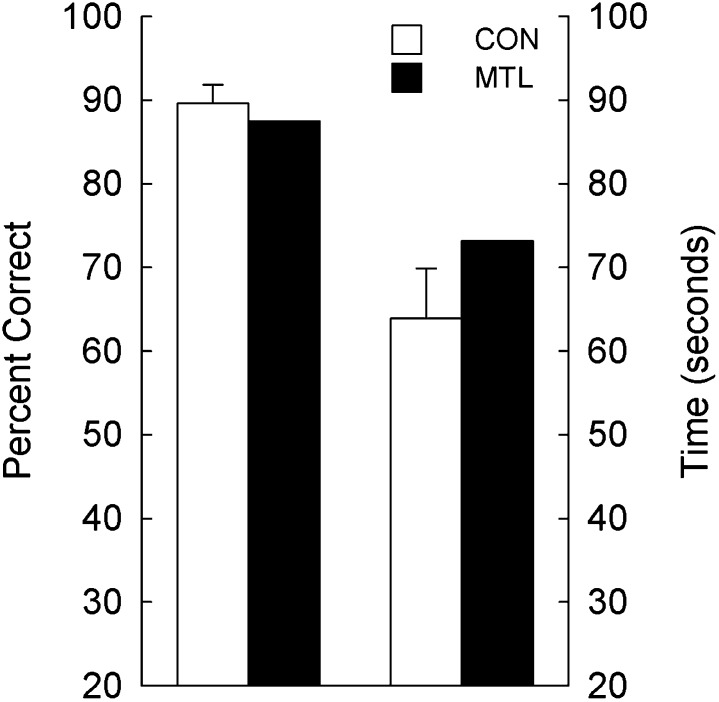
On the final test, which involved difficulty levels 1–8, G.P. and controls used a pencil to draw lines between the twin pairs to remove the need to hold any material in mind while they worked on each display. For difficulty levels 1–4, G.P. scored 96.9% correct (response time = 20.9 s), and controls scored 97.4% correct (response time = 15.2 s). We examined the scores for difficulty levels 5–8 separately because these were the trials at which G.P. encountered difficulty in the earlier tests (Fig. 3 and text). G.P. performed similarly to controls in this condition (Fig. 5). G.P. scored 87.5% correct (response time = 73.2 s), and controls scored 89.6% correct (response time = 63.9 s, range = 50.0–85.3 s) (both P values > 0.10).
Fig. 5.
Accuracy scores and response times for controls (CON, n = 6) and one patient with large medial temporal lobe lesions (MTL, n = 1) across difficulty levels 5–8. In this condition, participants used a pencil to connect the twin pairs, thereby eliminating the need to hold material in mind. Brackets show SEM.
Discussion
Patients with damage to MTL structures were tested for their ability to discriminate among objects with a high degree of feature overlap and perceptual similarity. All groups (controls, patients with hippocampal lesions, and a patient with large MTL lesions) performed similarly on displays of five objects and on displays of seven objects that had only two appendages (difficulty levels 1–3). At difficulty level 4 (seven objects, four appendages), patients with hippocampal lesions exhibited a decline in performance, and controls also made their first errors (10 of 12 controls made errors, compared with 3 of 12 controls at level 3; Figs. 2 and 3). The patient with large MTL lesions (G.P.) performed very well through difficulty level 4 and then exhibited a sharp decline in performance at difficulty level 5.
Two points deserve emphasis. First, an impairment was evident in patients with limited hippocampal lesions, not only in the patient (G.P.) with large MTL lesions that included perirhinal cortex. Inasmuch as hippocampal damage has not previously been linked to the ability to make discriminations among objects, this result appears to favor an account of impaired performance that emphasizes the role of memory. Second, the impairment was evident only in the more difficult conditions (difficulty levels 4–6), even though at every difficulty level the task required discriminating among objects with overlapping features. This finding also suggests that some other component of the task (besides the requirement to make perceptual discriminations) might account for impaired performance. We suggest that in its more difficult conditions the task placed demands on long-term memory, and that the memory impairment of the patients can account for their poor performance.
The pattern of our results is reminiscent of earlier observations of memory-impaired patients with MTL damage (3, 20). For example, the noted patient H.M. was able to repeat back strings of one to six digits without error but then failed at seven digits even after 25 repetitions of the same digit string (3). Notably, controls made their first errors in reporting digits at string length eight. More recently, patient G.P. was asked to recollect across a 1-s interval the location of objects in a display (20). G.P. performed as well as controls when presented with one, two, or three objects, but with four objects he showed an abrupt decline in performance and was unable to perform successfully even after 10 presentations of the same display. Furthermore, the discontinuity in G.P.’s performance occurred at the point where controls made their first errors. These findings suggested that patients performed normally so long as they could rely on their intact immediate (and working) memory, and that they exhibited a decline in performance at the point where working memory capacity was exceeded. At that point, we suggest that performance depended at least in part on long-term memory (21).
In the present study, the five patients with hippocampal lesions and patient G.P. exhibited a similar pattern of performance. The hippocampal patients first exhibited a significant decline in performance at difficulty level 4 (when controls first made errors), and G.P. first exhibited a deficit at difficulty level 5. We suggest that the decline in performance at difficulty level 4 resulted from the fact that working memory capacity was exceeded at this point, and performance needed to draw on long-term (supraspan) memory (3, 21). That is, when seven objects with high feature overlap appear in a display (as at difficulty level 4), finding the twin pairs and keeping them in mind as one looks for the unpaired object challenges working memory capacity. Note that there was some variability in the point at which patients made errors, presumably because they could approach the displays in different ways. Accordingly, the solution could sometimes be found readily and sometimes less readily.
An informal observation made during G.P.’s testing also suggests the importance of memory impairment in understanding his performance. G.P. is an intelligent and careful individual who, despite his profound memory impairment and large MTL lesions, performed better than the average hippocampal patient at difficulty levels 1–6. At difficulty level 8, however, his performance fell to 25.0% correct (controls, 56.3% correct). When asked how he went about the task, he stated (with a test display in view), that he would begin with the upper left object and look for its pair, then move to the next object and look for its pair, and so on. When then asked how he was able to keep the pairs in memory, he replied, “Well, that’s the problem I was having.”
In a final condition, we eliminated any burden on memory by asking participants to use a pencil to draw lines between the twin pairs. In this way, participants did not need to remember the pairs they had identified as they moved through the display to find the unique object. If MTL lesions that include perirhinal cortex impair perceptual ability on tasks like these, patient G.P. should be less accurate than controls and/or have longer response times. Instead, G.P. performed as accurately and as rapidly as controls in this condition (Fig. 5). This finding provides particularly strong evidence that large MTL lesions spare perceptual abilities.
It is notable that impaired perceptual performance in patients with large MTL lesions has recently been described in tasks where working memory capacity would not appear to be limiting. In one study, a patient with MTL lesions was impaired at judging whether line drawings represented “possible” objects that could potentially exist in three dimensions (22). In another study, two patients (one of whom also participated in the first study) were impaired at making figure-ground judgments about familiar and unfamiliar objects (23). In both cases, damage to the perirhinal cortex was thought to be responsible for the impairment.
As discussed previously (24, 25), a lingering and challenging issue concerns the locus and extent of brain damage in the patients under study and the possibility that damage outside the MTL might contribute to perceptual impairment. Of particular interest is the status of lateral temporal cortex, a region involved in high-order visual perceptual functions (26) and semantic knowledge (27). In this regard, it is noteworthy that patient MTL3, who participated in the two above-mentioned studies (22, 23), has significant volume loss in the right hemisphere in temporopolar cortex, anterior fusiform gyrus, and the anterior half of lateral temporal cortex. This additional damage makes it difficult to isolate the impairment to perirhinal cortex. Volumetric data were not available for the second patient (MTL2) (23). However, visual ratings for both patients (using a 0–4 scale) were provided in earlier work (12), and MTL2’s rating for lateral temporal cortex was even poorer than the corresponding rating for MTL3. Note, however, that the ratings provide an uncertain estimate because they were based on a single section and averaged across both hemispheres and across both the anterior and posterior half of lateral temporal cortex. The more useful volumetric data now provided for MTL3 were calculated separately for the anterior and posterior half of lateral temporal cortex in each hemisphere.
In summary, we evaluated proposals that MTL lesions that include perirhinal cortex impair the ability to discriminate among similar objects with overlapping features. Three observations suggest that the impairment exhibited after MTL lesions reflects impaired memory rather than impaired perception. First, impairment was observed in patients with hippocampal lesions, not just in association with large MTL lesions that included perirhinal cortex. Second, an impairment appeared only in the more difficult conditions, even though all task conditions required discriminating among objects with overlapping elements. Third, performance of the patients first declined significantly at the point where controls made their first errors. We suggest, consistent with earlier interpretations of this pattern of performance (3, 20), that controls began to make errors when working memory capacity was exceeded, and patients were impaired because performance then depended importantly on long-term memory. If so, impaired memory associated with MTL lesions can account for performance on difficult discrimination tasks without proposing an additional impairment in perception.
Materials and Methods
Participants.
Six memory-impaired patients participated (Table 1). Of these patients, five have damage thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex). K.E. became amnesic after an episode of ischemia associated with kidney failure and toxic shock syndrome. L.J. (the only female) became amnesic during a 6-mo period in 1988 with no known precipitating event; her memory impairment has been stable since that time. R.S. and G.W. became amnesic after drug overdoses and associated respiratory failure. J.R.W. became amnesic after cardiac arrest. Estimates of MTL damage were based on quantitative analysis of magnetic resonance images compared with data from 19 controls (11 for L.J.) (28, 29). K.E., L.J., R.S., G.W., and J.R.W. have an average bilateral reduction in hippocampal volume of 49%, 46%, 33%, 48%, and 44%, respectively (all values >3 SDs from the control mean). The volume of the parahippocampal gyrus (temporopolar, perirhinal, entorhinal, and parahippocampal cortices) is reduced by 17%, −8%, 1%, 12%, and 6%, respectively (all values within 2 SDs of the control mean).
Table 1.
Characteristics of memory-impaired patients
| WMS-R |
||||||||
| Patient | Age, y | Education, y | WAIS-III IQ | Attention | Verbal | Visual | General | Delay |
| K.E. | 70 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | 74 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| R.S. | 55 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| G.W. | 52 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| J.R.W. | 48 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
| G.P. | 61 | 16 | 98 | 102 | 79 | 62 | 66 | 50 |
The Wechsler Adult Intelligence Scale III (WAIS-III) and the Wechsler Memory Scale–Revised (WMS-R) yield mean scores of 100 in the normal population with a SD of 15. The WMS-R does not provide numerical scores for individuals who score below 50. IQ scores for R.S. and J.R.W. are from the Wechsler Adult Intelligence Scale–Revised.
One patient (G.P.) has severe memory impairment resulting from viral encephalitis. G.P. has demonstrated virtually no new learning since the onset of his amnesia, and during repeated testing over many weeks he does not recognize that he has been tested before (30). G.P. has a bilateral reduction in hippocampal volume of 96%. The volume of the parahippocampal gyrus is reduced by 93%, with sparing limited to the parahippocampal cortex. Nine coronal MR images from each patient, together with detailed descriptions of the lesions, can be found in Squire et al. (31).
Twelve healthy controls (nine male) served as controls for the memory-impaired patients. Controls averaged 63.1 ± 3.3 y of age and had 14.4 ± 0.5 y of education. All procedures were approved by the Institutional Review Board at the University of California at San Diego, and participants gave written informed consent.
Materials and Procedure.
The main part of the test consisted of 48 unique displays of five or seven nonsense objects, termed Fribbles, as used in earlier studies of object perception (12, 31). The Fribbles were computer generated using Bryce 5 software (Corel Corp.) and were composed of a main body and two or four appendages. Each display contained either two or three twin pairs and one unique object that did not have a twin (Fig. 6). The unique object could appear in any location in the display.
Fig. 6.
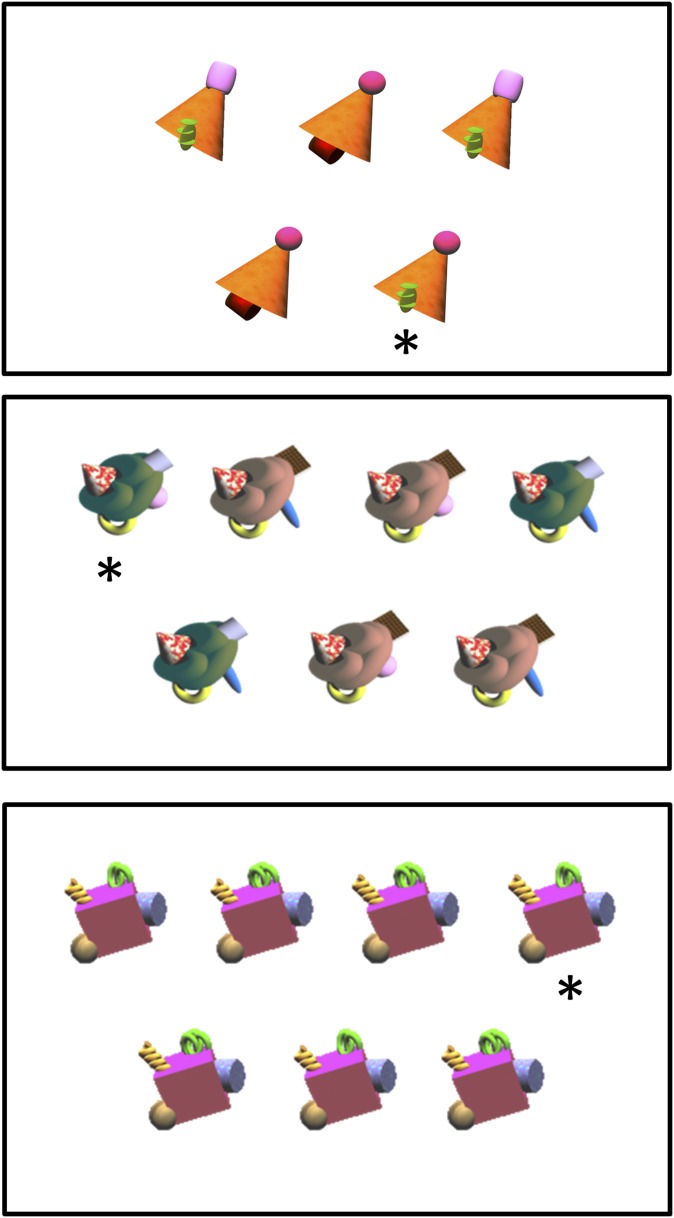
Sample displays. The task was to identify the unique object (asterisk). (Top) A representative display from difficulty level 1. This display consisted of two twin pairs and one unique object. Each appendage of the unique object always appeared on another twin pair, so that more than one appendage always needed to be considered to distinguish the unique object from the twin pairs. (Middle and Bottom) Representative displays from difficulty level 4 and 6, respectively. Each display consisted of three twin pairs and one unique object. At these difficulty levels, two appendages of the unique object appeared in the same form on every other object. A third appendage of the unique object appeared on one of the twin pairs in the display, and the fourth appendage of the unique object appeared on a different twin pair. Thus, as at all difficulty levels, every appendage appeared on more than one object, and the unique object could not be identified by the presence of a single feature. Instead, the conjunction of two appendages defined the unique object.
The ability to discriminate among the different objects in a display was manipulated by varying the number of objects in each display (five or seven), the number of appendages on each object (two or four), the number of body colors in each display (two different colors or only one color), and the differences among the appendages (relatively salient or more subtle). The easiest displays consisted of five objects with two appendages (difficulty level 1, eight displays) or five objects with four appendages (difficulty level 2, eight displays). The displays of medium difficulty consisted of seven objects with two appendages (difficulty level 3, eight displays) or seven objects with four appendages (difficulty level 4, eight displays). At difficulty levels 1–4, all of the differences among appendages were relatively salient. In addition, half the displays contained objects made with the same body color, and half contained objects made of two body colors. The most difficult displays consisted of seven objects made with the same body color and with four appendages whose differences were relatively salient (difficulty level 5, eight displays) or seven objects made with the same body color and with four appendages whose differences were more subtle (difficulty level 6, eight displays). For difficulty level 6, the differences among the appendages were made more subtle by manipulating their size or shape. One of the eight displays at difficulty level 6 was identical to the display illustrated in an earlier study (figure 2c in ref. 12).
Participants were told that they would see pictures of objects on the computer screen and that one object in each display did not have a twin pair. The task was to point to the unique object. The 48 displays were presented in blocks of eight trials, beginning with difficulty level 1 and progressing to difficulty level 6. Each appendage of the unique object always appeared on at least one of the twin pairs in the display. Accordingly, to identify the unique object, it was necessary at all difficulty levels to identify the conjunction of two different appendages. A written reminder of the instructions was present throughout testing. Performance was self-paced, and participants identified their choice by pointing to the computer screen. Accuracy and response times were recorded, and the results to be reported were based on these data. However, feedback was provided after each choice, and after incorrect choices participants were given the opportunity to choose again to determine if correct performance was possible when more time was available. The patients with hippocampal lesions and the controls were tested once. Patient G.P. with large MTL lesions was tested on two occasions separated by 1 mo.
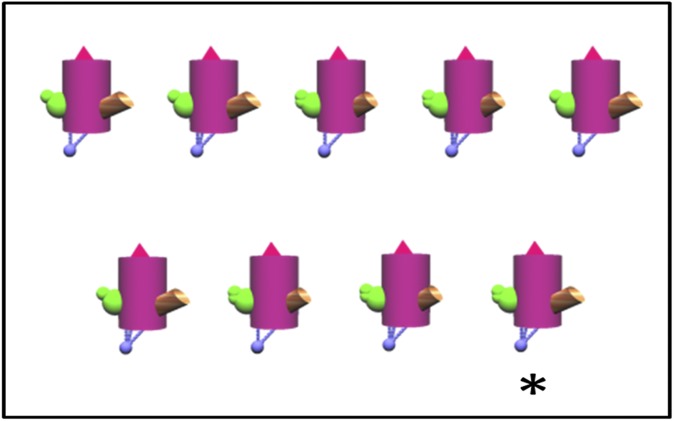
Sixteen additional displays were subsequently constructed for patient G.P. to further increase task difficulty. Eight of these displays consisted of nine objects (four twin pairs and one unique object), four appendages, a single body color, and relatively salient differences among the appendages (difficulty level 7). Eight similar displays were also constructed with nine objects, but for these displays the differences among the appendages were more subtle (difficulty level 8; Fig. 7).
Fig. 7.
Sample display from difficulty level 8 that was given only to patient G.P. and eight controls. Each trial consisted of nine objects, four twin pairs and one unique object (asterisk), with the same body color and four appendages. One appendage of the unique object appeared in the same form on every other object. The second, third, and fourth appendages of the unique object each appeared on two other twin pairs in the display. Thus, it was necessary to identify the conjunction of three different features to identify the unique object.
These displays were given on a single occasion to patient G.P. and eight controls (mean = 62.8 ± 3.5 y of age; 14.1 ± 0.5 y of education), who had earlier taken the 48-display test described above. The procedure was the same as in the 48-display task. The 16 displays for difficulty levels 7 and 8 were preceded by 12 displays, two each from difficulty levels 1–6. In this way, participants progressed through gradually more difficult displays before encountering the 16 new displays that comprised difficulty levels 7 and 8 and that were expected to be quite challenging.
Last, all 64 displays (difficulty levels 1–8) were presented on a subsequent occasion to patient G.P. and six controls (mean = 60.7 ± 4.7 y of age; 14.2 ± 0.5 y of education), who had earlier taken the 48-display test. In this condition, the displays were presented on individual sheets of paper, and participants were asked to draw lines between each twin pair using a pencil. In this way, we intended to eliminate the need to hold any material in mind (i.e., eliminate any role for memory) as participants worked on each display to identify the unique object.
Acknowledgments
We thank Jennifer Frascino and Christine Smith for assistance, and Jill Leutgeb for helpful suggestions. This work was supported by the Medical Research Service of the Department of Veterans Affairs and National Institute of Mental Health Grant MH24600.
Footnotes
The authors declare no conflict of interest.
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 3.Drachman DA, Arbit J. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol. 1966;15:52–61. doi: 10.1001/archneur.1966.00470130056005. [DOI] [PubMed] [Google Scholar]
- 4.Baddeley AD, Warrington EK. Amnesia and the distinction between long- and short-term memory. J Verbal Learn Verbal Behav. 1970;9:176–189. [Google Scholar]
- 5.Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. J Neurosci. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs visual object identification. J Neurosci. 1998;18:2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- 8.Buckley MJ, Booth MC, Rolls ET, Gaffan D. Selective perceptual impairments after perirhinal cortex ablation. J Neurosci. 2001;21:9824–9836. doi: 10.1523/JNEUROSCI.21-24-09824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- 10.Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: Testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- 11.Barense MD, et al. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Lee AC, Barense MD, Graham KS. The contribution of the human medial temporal lobe to perception: Bridging the gap between animal and human studies. Q J Exp Psychol B. 2005;58:300–325. doi: 10.1080/02724990444000168. [DOI] [PubMed] [Google Scholar]
- 14.Lee AC, et al. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- 15.Lee AC, et al. Perceptual deficits in amnesia: Challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia. 2005;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Hampton RR. Monkey perirhinal cortex is critical for visual memory, but not for visual perception: Reexamination of the behavioural evidence from monkeys. Q J Exp Psychol B. 2005;58:283–299. doi: 10.1080/02724990444000195. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki WA. Perception and the medial temporal lobe: Evaluating the current evidence. Neuron. 2009;61:657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, et al. Memory, visual discrimination performance, and the human hippocampus. J Neurosci. 2011;31:2624–2629. doi: 10.1523/JNEUROSCI.5954-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham KS, Barense MD, Lee ACH. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Jeneson A, Mauldin KN, Squire LR. Intact working memory for relational information after medial temporal lobe damage. J Neurosci. 2010;30:13624–13629. doi: 10.1523/JNEUROSCI.2895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learn Mem. 2012;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee AC, Rudebeck SR. Human medial temporal lobe damage can disrupt the perception of single objects. J Neurosci. 2010;30:6588–6594. doi: 10.1523/JNEUROSCI.0116-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barense MD, Ngo JKW, Hung LHT, Peterson MA. Interactions of memory and perception in amnesia: The figure-ground perspective. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr347. 10.1093/cercor/bhr347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci. 2006;26:2235–2240. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki WA. Untangling memory from perception in the medial temporal lobe. Trends Cogn Sci. 2010;14:195–200. doi: 10.1016/j.tics.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: Medial vs. lateral temporal lobe. Proc Natl Acad Sci USA. 2004;101:6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005;46:799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squire LR, et al. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams P, Simons DJ. Detecting changes in novel, complex three-dimensional objects. Vis Cogn. 2000;7:297–322. [Google Scholar]