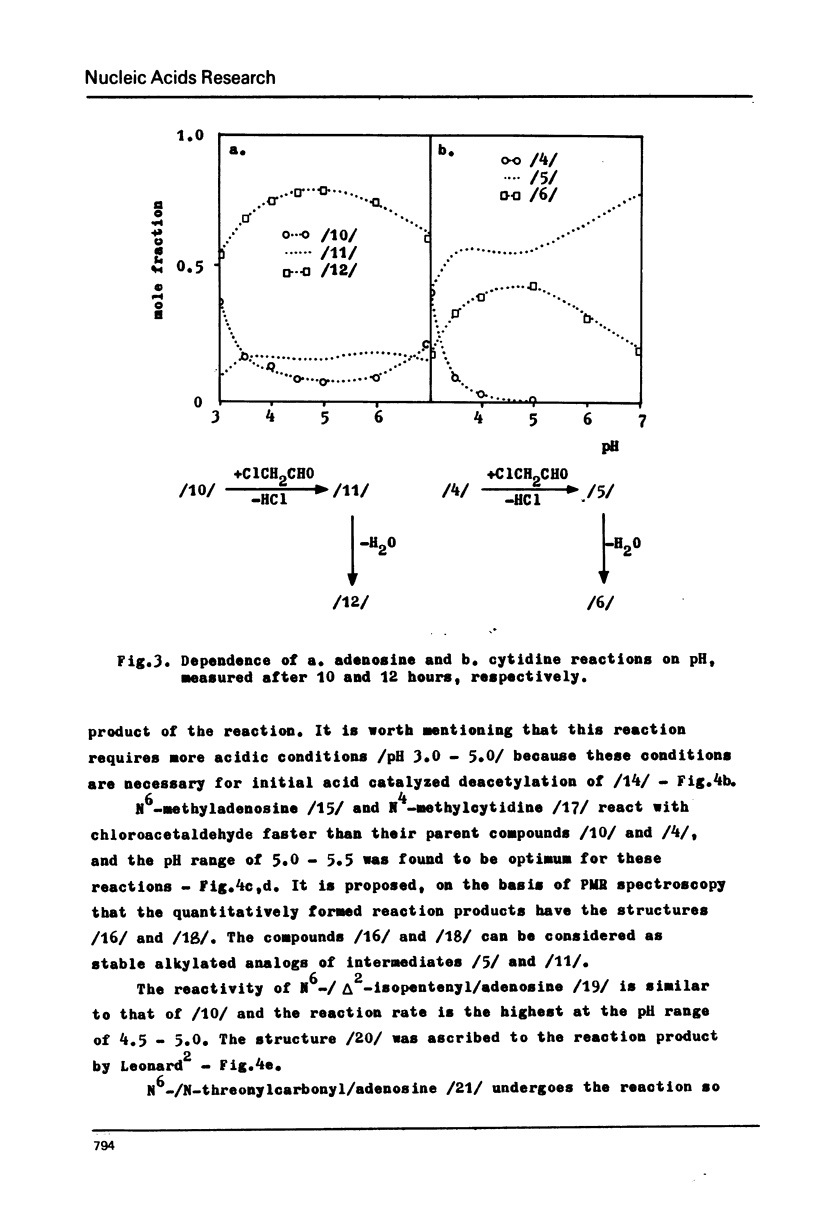
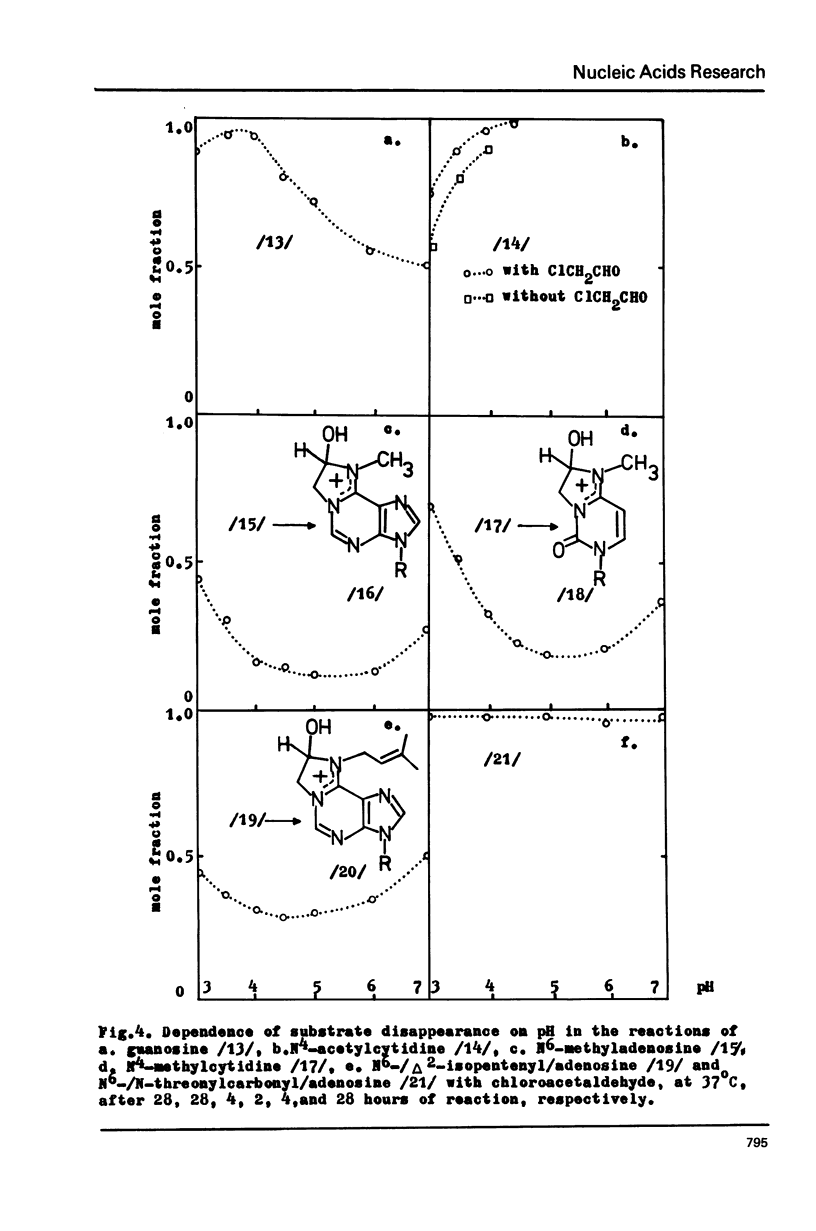
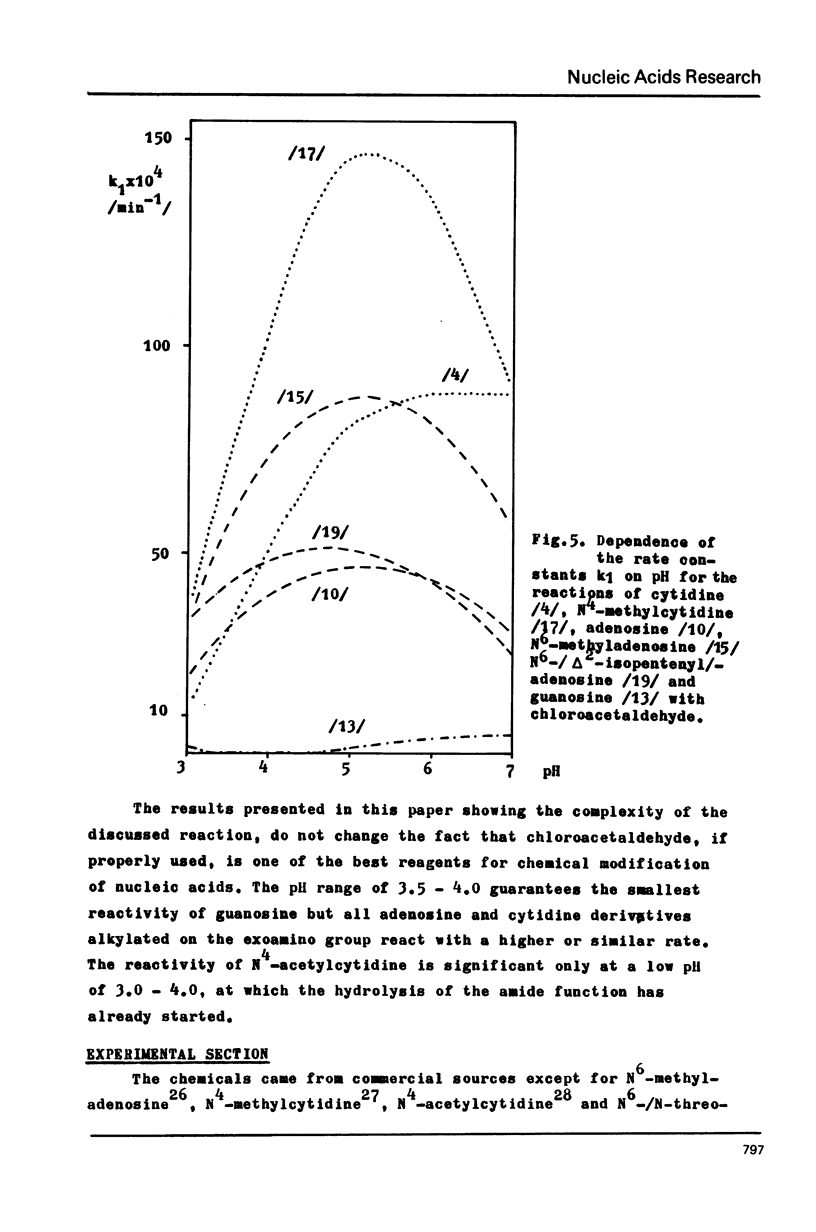
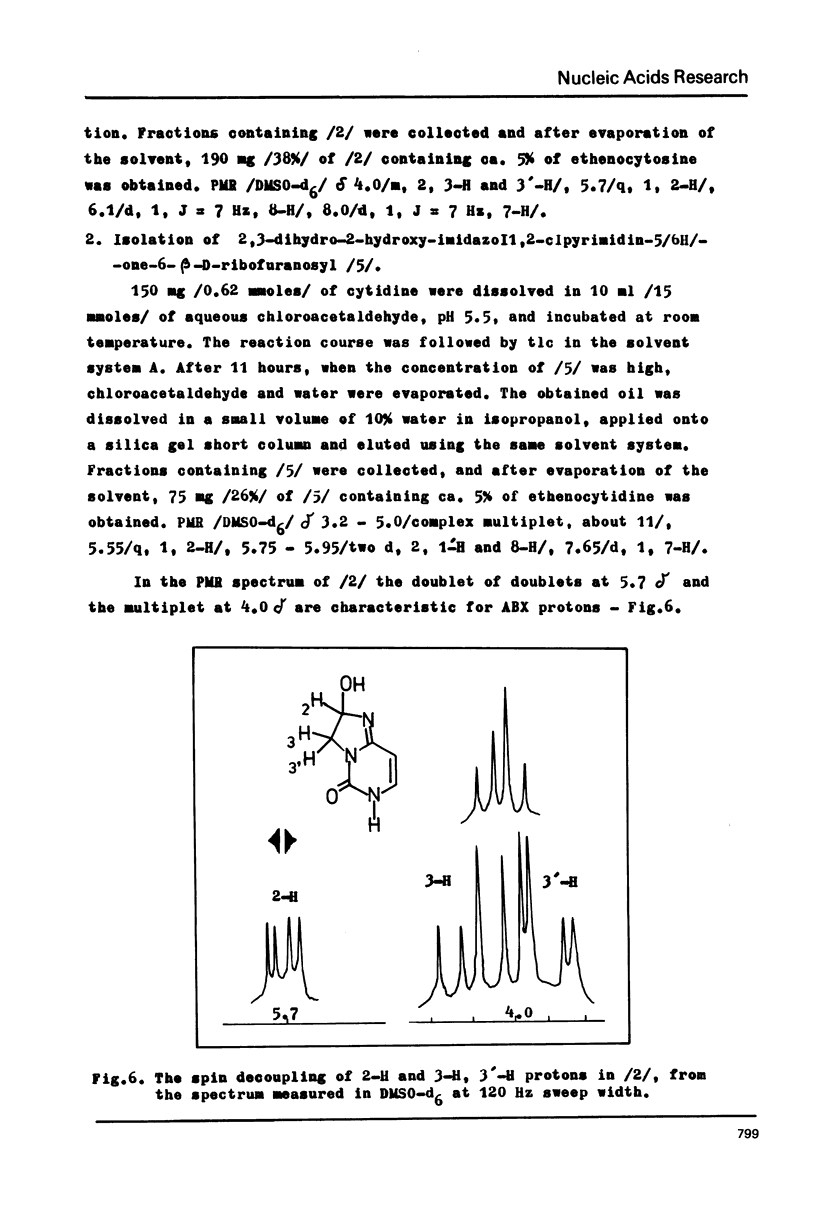
Abstract
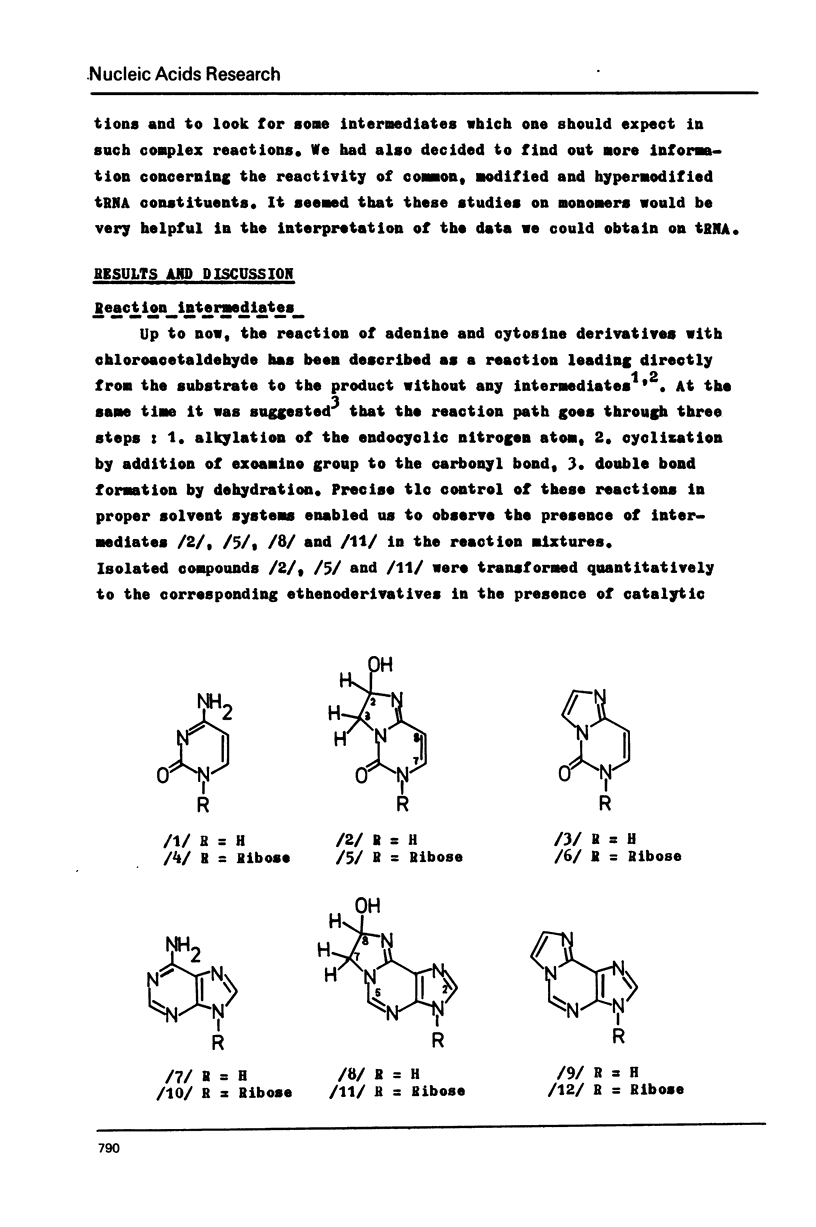
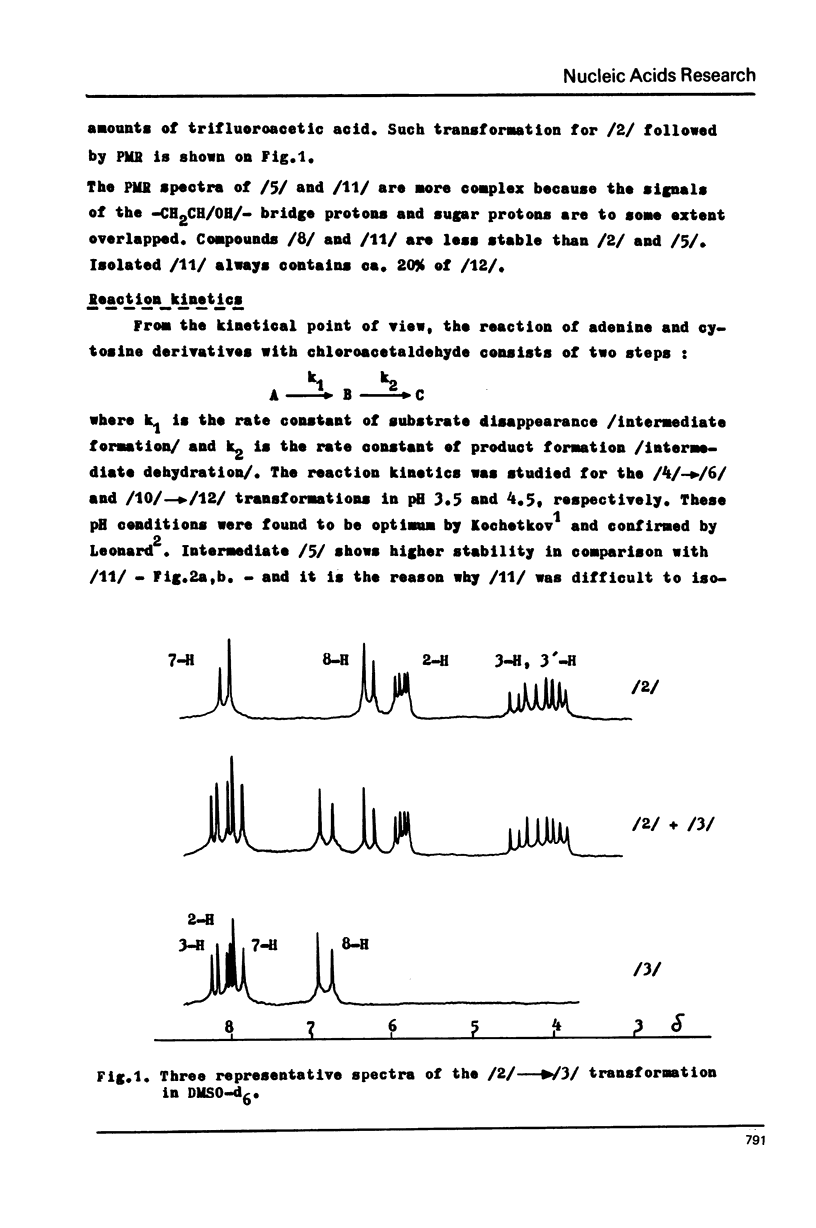
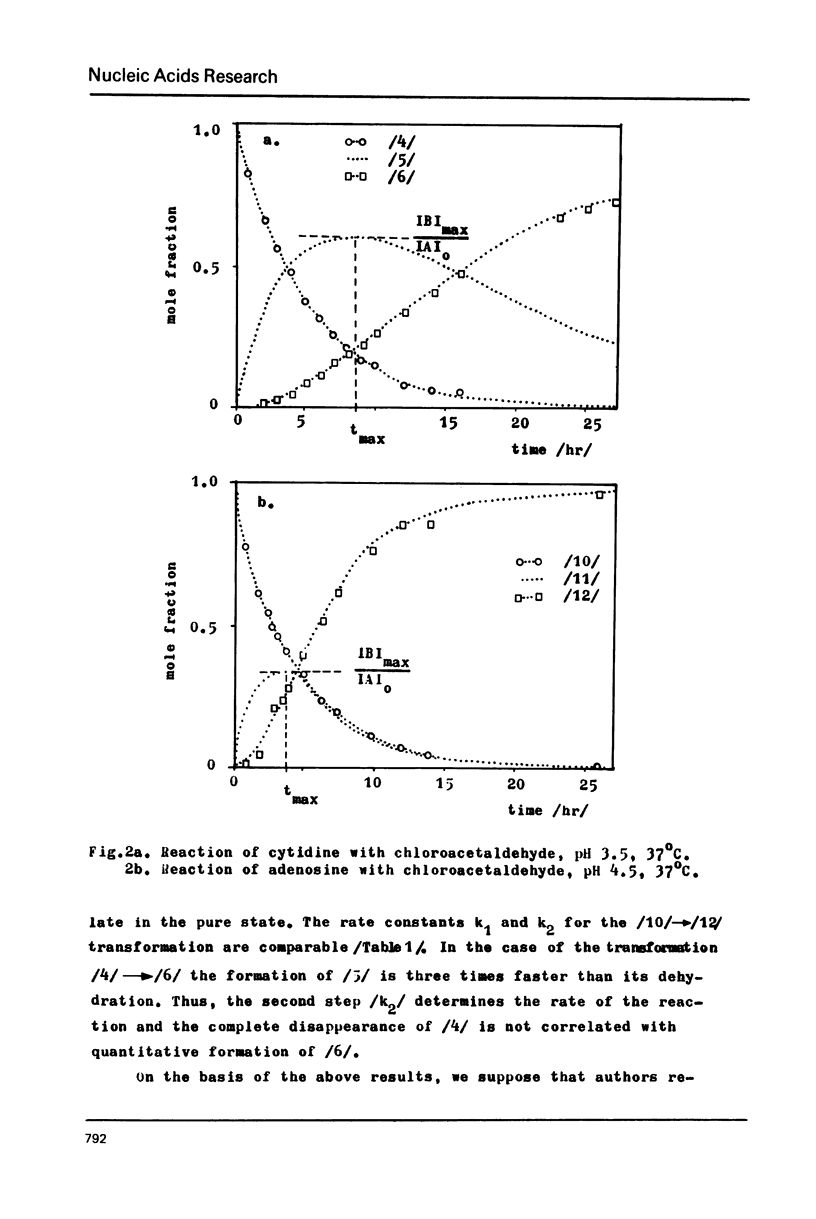
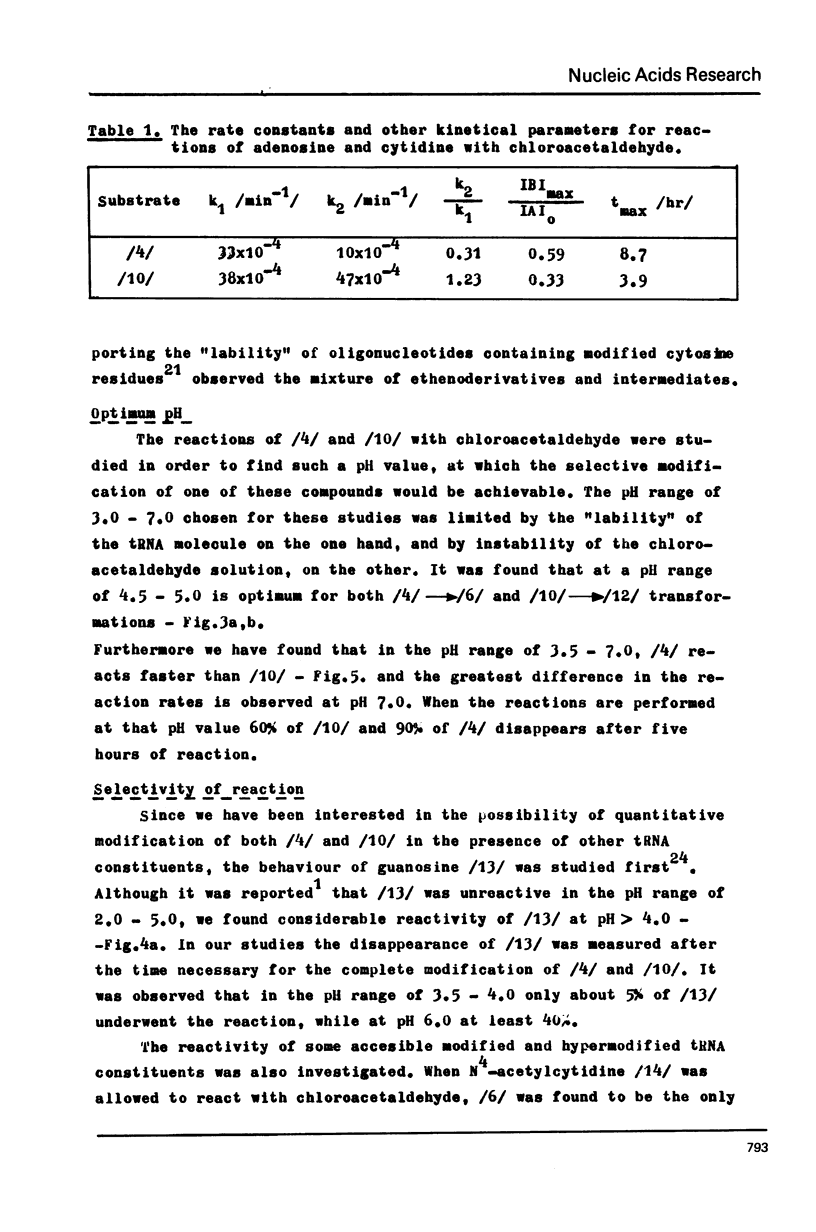
The stable intermediates formed in the reaction of cytosine, cytidine and adenosine with chloracetaldehyde were isolated. The -CH2CH/OH/- bridge between the exo and endo nitrogen atoms of the parent base was found in these compounds by means of PMR spectroscopy. Their acid-induced dehydration resulted in formation of appropriate ethenoderivatives. The rate constants of the intermediate formation and its dehydration were found to be 38x10(-4) and 47x10(-4) /min-1/ for adenosine, and 33x10(-4) and 10x10(-4) /min-1/ for cytidine. The PH range of 4.5--5.0 was found to be optimum for both adenosine and cytidine reactions. The quantitative modification of these two nucleosides in the presence of guanosine may be achieved with high selectivity only at a low pH of 3.0--4.0 N6-methyladenosine and N4-methylcytidine react quantitatively with chloroacetaldehyde and the reaction rate is higher than in the case of the parent nucleosides. The structure of the reaction products was assigned on the basis of PMR spectroscopy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrio J. R., Sattsangi P. D., Gruber B. A., Dammann L. G., Leonard N. J. Species responsible for the fluorescence of 3,N4-ethenocytidine. J Am Chem Soc. 1976 Nov 10;98(23):7408–7414. doi: 10.1021/ja00439a049. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. Fluorescent adenosine and cytidine derivatives. Biochem Biophys Res Commun. 1972 Jan 31;46(2):597–604. doi: 10.1016/s0006-291x(72)80181-5. [DOI] [PubMed] [Google Scholar]
- Jones G. H., Murthy D. V., Tegg D., Golling R., Moffatt J. G. Analogs of adenosine 3',5'-cyclic phosphate. II. Synthesis and enzymatic activity of derivatives of 1,N6-ethenoadenosine 3',5'-cyclic phosphate. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1338–1343. doi: 10.1016/0006-291x(73)90612-8. [DOI] [PubMed] [Google Scholar]
- Kochetkov N. K., Shibaev V. N., Kost A. A. Vzaimodeistvie komponentov DNK s khloratsetal'degidom. Dokl Akad Nauk SSSR. 1973 Dec 21;213(6):1327–1330. [PubMed] [Google Scholar]
- Lee C. H., Wetmur J. G. Physical studies of chloroacetaldehyde labelled fluorescent DNA. Biochem Biophys Res Commun. 1973 Feb 5;50(3):879–885. doi: 10.1016/0006-291x(73)91327-2. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Tolman G. L. Fluorescent nucleosides and nucleotides. Ann N Y Acad Sci. 1975 Aug 8;255:43–58. doi: 10.1111/j.1749-6632.1975.tb29212.x. [DOI] [PubMed] [Google Scholar]
- Papas T. S., Chirikjian J. G., Pry T. W., Massicot J. G., Irwin R. D., Chirigos M. A. Effect of chemically modified 70S RNA from avian myeloblastosis virus (AMV) upon the activity of AMV DNA polymerase. J Virol. 1974 Nov;14(5):1108–1114. doi: 10.1128/jvi.14.5.1108-1114.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattsangi P. D., Leonard N. J., Frihart C. R. 1,N2-ethenoguanine and N2,3-ethenoguanine. Synthesis and comparison of the electronic spectral properties of these linear and angular triheterocycles related to the Y bases. J Org Chem. 1977 Sep 30;42(20):3292–3296. doi: 10.1021/jo00440a020. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Location of accessible bases in Escherichia coli formylmethionine transfer RNA as determined by chemical modification. Biochemistry. 1976 Dec 28;15(26):5769–5775. doi: 10.1021/bi00671a013. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Villar-Palasi C., Gilman A. G. Fluorescent modification of adenosine 3',5'-monophosphate: spectroscopic properties and activity in enzyme systems. Science. 1972 Jul 21;177(4045):279–280. doi: 10.1126/science.177.4045.279. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Weber G. Fluorescent modification of adenosine-containing coenzymes. Biological activities and spectroscopic properties. Biochemistry. 1972 Sep 12;11(19):3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- Spencer R. D., Weber G., Tolman G. L., Barrio J. R., Leonard N. J. Species responsible for the fluorescence of 1:N6-ethenoadenosine. Eur J Biochem. 1974 Jun 15;45(2):425–429. doi: 10.1111/j.1432-1033.1974.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Tolman G. L., Barrio J. R., Leonard N. J. Chloroacetaldehyde-modified dinucleoside phosphates. Dynamic fluorescence quenching and quenching due to intramolecular complexation. Biochemistry. 1974 Nov 19;13(24):4869–4878. doi: 10.1021/bi00721a001. [DOI] [PubMed] [Google Scholar]


