Abstract
OBJECTIVE
Adenosine as an additive in blood cardioplegia is cardioprotective in animal studies, but its clinical role in myocardial protection remains controversial. The aim of this study was to investigate whether the addition of adenosine in continuous cold blood cardioplegia would enhance myocardial protection.
METHODS
In a prospective double-blind study comparing adenosine 400 μmol l−1 to placebo in continuous cold blood cardioplegia, 80 patients undergoing isolated aortic valve replacement were randomized into four groups: antegrade cardioplegia with adenosine (n = 19), antegrade cardioplegia with placebo (n = 21), retrograde cardioplegia with adenosine (n = 21) and retrograde cardioplegia with placebo (n = 19). Myocardial arteriovenous differences in oxygen and lactate were measured before, during and after aortic occlusion. Myocardial concentrations of adenine nucleotides and lactate were determined from left ventricular biopsies obtained before aortic occlusion, after bolus cardioplegia, at 60 min of aortic occlusion and at 20 min after aortic occlusion. Plasma creatine kinase (CK-MB) and troponin T were measured at 1, 3, 6, 9, 12 and 24 h after aortic occlusion. Haemodynamic profiles were obtained before surgery and 1, 8 and 24 h after cardiopulmonary bypass. Repeated-measures analysis of variance was used for significance testing.
RESULTS
Adenosine had no effects on myocardial metabolism of oxygen, lactate and adenine nucleotides, postoperative enzyme release or haemodynamic performance. When compared with the antegrade groups, the retrograde groups showed higher myocardial oxygen uptake (17.3 ± 11.4 versus 2.5 ± 3.6 ml l−1 at 60 min of aortic occlusion, P < 0.001) and lactate accumulation (43.1 ± 20.7 versus 36.3 ± 23.0 µmol g−1 at 60 min of aortic occlusion, P = 0.052) in the myocardium during aortic occlusion, and lower postoperative left ventricular stroke work index (27.2 ± 8.4 versus 30.1 ± 7.9 g × m × m−2, P = 0.034).
CONCLUSIONS
Adenosine 400 μmol l−1 in cold blood cardioplegia showed no cardioprotective effects on the parameters studied. Myocardial ischaemia was more pronounced in patients receiving retrograde cardioplegia.
Keywords: Myocardial biopsy, Circulatory haemodynamics, Coronary sinus, Myocardial protection, Myocardial metabolism
INTRODUCTION
The main principle of myocardial protection in cardiac surgery is to preserve myocardial function by preventing ischaemia. No method of cardioplegia has been shown to completely protect the myocardium against ischaemic injury, and cross-clamp times still matter [1].
The endogenous nucleoside adenosine, which is a degradation product of adenosine triphosphate (ATP), is known to possess cardioprotective properties. Adenosine induces vasodilatation and plays an important role in preconditioning and reduction of inflammatory response in ischaemia-reperfusion injury through receptor-mediated mechanisms [2,3]. It may also exert its effect as a substrate in the nucleotide metabolism [4]. Several animal studies have demonstrated enhanced myocardial protection by adenosine as an adjunct to cardioplegia [2,4], but clinical studies have shown conflicting results [5–10]. In the two largest studies, Mentzer et al. [8] found a lower use of dopamine in coronary artery bypass graft (CABG) patients receiving 2 mM adenosine versus placebo in blood cardioplegia, but Cohen et al. [6] found no differences in enzymatic release, incidence of myocardial infarction or inotrope requirement in CABG patients receiving adenosine 15–100 µmol l−1 versus placebo as an adjunct to cardioplegia. In the only study of patients undergoing valve surgery without coronary disease, Liu et al. [7] found a lower enzymatic release and inflammatory response in patients receiving adenosine 1 mmol l−1 in blood cardioplegia versus controls. In one small non-randomized study, Cohen et al. [11] found that adenosine 2 mM as an additive to blood cardioplegia in CABG patients was able to preserve myocardial ATP during aortic occlusion compared with a 15% decrease of ATP concentration in controls. This is the only study of adenosine in blood cardioplegia and its effect on myocardial adenine nucleotide concentration so far, and bearing in mind the difficulty of interpreting myocardial concentrations of nucleotides in the presence of coronary disease with areas of hypoperfusion, this finding warrants further investigation.
This study was conducted among 80 patients undergoing isolated aortic valve replacement. The aim was to investigate whether the addition of adenosine 400 µmol l−1 in continuous cold blood cardioplegia would enhance myocardial protection. The primary endpoint was the preservation of adenine nucleotides during and after aortic occlusion, and secondary endpoints were differences in enzymatic release, oxygen and lactate metabolism and haemodynamic performance.
The concentration of adenosine was chosen on the basis of previous studies which have shown that up to 2 mM adenosine is a safe additive to blood cardioplegia [8,12]. Postulating an aortic occlusion time of 80 min and a continuous infusion of adenosine 400 µmol l−1 in blood cardioplegia at 50 ml min−1, the total amount of adenosine given would be 1.6 mM, thus being within safe limits. Since the aim was to study concentrations of adenine nucleotides in myocardial biopsies, we decided to focus on patients without coronary artery disease undergoing aortic valve replacement. We further decided to use continuous cold blood cardioplegia with or without adenosine, in order to avoid as far as possible any interruption of adenosine supply to the myocardium.
In our clinical practice, retrograde cardioplegia is the method of choice during aortic valve surgery, mainly because it does not require cannulation of the coronary ostia and because of its convenience. Retrograde cardioplegia has not, however, proved to be more effective than antegrade cardioplegia, nor have studies indicated that retrograde cardioplegia offers a worse myocardial protection than antegrade delivery [13–15]. To clarify this further, we decided to further randomize the patients to antegrade or retrograde cardioplegia in order to allow for a comparison between these methods of cardioplegic delivery.
PATIENTS AND METHODS
Patient selection
The study was approved by the Regional Ethical Committee, Örebro, Sweden (46600:19). Every participating patient gave written informed consent before surgery. All patients with aortic valve stenosis who were scheduled for primary isolated aortic valve replacement without other concomitant cardiovascular surgical procedures were eligible for the study. Exclusion criteria were emergency surgery, the presence of coronary artery disease and medication with dipyramidol or theophylline.
The sample size was calculated as follows: in one small previous study of CABG patients, myocardial levels of ATP were preserved in patients receiving adenosine-supplemented cardioplegia, in contrast to a 15% reduction in control patients [11]. In this study, we hypothesized that adenosine in cold blood cardioplegia would preserve ATP levels in myocardial biopsies, in contrast to a minimum of 10% reduction in the placebo group. In order to detect this difference with a power of 0.85 and a significance level of 0.05, a minimum of 70 patients were needed. It was, therefore, decided to include 80 patients in the study. All patients were operated on by the same surgeon (C.S.).
After induction of anaesthesia, patients underwent randomization using sealed opaque envelopes in a blocked randomization scheme. The patients were randomized into four groups, with groups 1 and 2 receiving adenosine cardioplegia by antegrade (ADE-ant, n = 19) or retrograde (ADE-ret, n = 21) delivery, and groups 3 and 4 receiving cardioplegia with placebo by antegrade (CTRL-ant, n = 21) or retrograde (CTRL-ret, n = 19) delivery. The patients and all postoperative staff were blinded to treatment allocation.
Surgical procedure and cardiopulmonary bypass management
Anaesthesia was induced with barbiturates and opiates and maintained by inhalation anaesthesia with isoflurane. The extracorporeal circuit consisted of a venous reservoir (Sorin, Mirandola, Italy) primed with 2000 ml of Ringer's acetate, a roller pump, a hollow fibre oxygenator with an integrated heat exchanger (Sorin, Mirandola, Italy) and a polyvinyl tubing system.
All operations were performed through a median sternotomy with standard cannulation of the aorta and a two-stage cannula in the right atrium. Systemic heparinization (300 U kg−1) was used to keep the activated clotting time >480 s, and nonpulsatile flow was kept at 2.4 l min−1 m−2. Moderate hypothermia (28°C) was used during the procedure, and the mean arterial pressure (MAP) was maintained at 50–80 mmHg.
Myocardial protection
Two separate roller pumps mixed cold (10°C) haemodiluted blood with potassium chloride to the desired potassium concentration of 20 mmol l−1. The cardioplegia was delivered as an initial bolus dose of 1000 ml, at a rate of 200 ml min−1, followed by a continuous infusion of 50 ml min−1 which was terminated when the aortotomy was closed. In the antegrade groups, the left coronary ostium was cannulated with a straight 7 mm balloon tipped coronary perfusion catheter (Polystan, 3500 Vaerlose, Denmark). In the retrograde groups, the coronary sinus was cannulated with a 14 F retrograde cardioplegia delivery catheter (Medtronic DLP, Grand Rapids, MI, USA).
In the adenosine groups, adenosine 20 mmol l−1 (Apoteksbolaget, Sweden) dissolved in mannitol 50 mg ml−1 (Apoteksbolaget, Sweden) was infused in the cardioplegia line giving a final adenosine concentration of 400 μmol l−1 and a mannitol concentration of 1 g l−1 in the cardioplegic solution during continuous infusion. The infusion was kept at the same rate during the whole cardioplegia delivery period. In the control groups, the infusion pump contained only mannitol with a final concentration of 1 g l−1 in the cardioplegic solution. The adenosine/mannitol infusion line was connected to the coronary perfusion catheter in the antegrade groups and to a separate lumen in the coronary sinus catheter in the retrograde groups. Topical cooling of the heart was achieved with ice slush.
Sample collection and analysis
Myocardial temperature during aortic occlusion was measured in the apical portion of the interventricular septum by a thin thermistor needle (Myocard Probe, Dideco, Mirandola, Italy).
Blood samples were obtained from the left cubital vein just before aortic occlusion and at 0, 1, 3, 6, 9, 12 and 24 h after aortic occlusion. Plasma concentrations of creatine kinase (CK-MB) and troponin T were determined immunochemically and expressed in μg l−1 (IMx Instruments, Abbott, IL, USA and ES 300 Analyzer, Boehringer, Mannheim, Germany, respectively).
Myocardial arteriovenous differences in oxygen and lactate were measured immediately before aortic occlusion, at 10, 30 and 60 min of aortic occlusion, immediately after the release of aortic occlusion and at 10, 30 and 60 min thereafter. Blood was sampled simultaneously from the radial artery and coronary sinus before and after aortic clamping. During aortic occlusion, blood samples were obtained from the cardioplegia line and the coronary sinus in the antegrade groups and from the cardioplegia line and the left coronary ostium in the retrograde groups. Oxygen and lactate concentrations were analysed using spectrophotometric techniques (ABL 520, Radiometer Copenhagen) and colorimetric methods (TDx instruments, Abbott, IL, USA).
Biopsies from the left ventricular apex, with an average thickness of 0.8 mm and a length of 5–10 mm, were obtained immediately before aortic clamping (I), after bolus cardioplegia (II), at 60 min of aortic occlusion (III) and at 20 min after the release of aortic occlusion (IV) using an 18 Gauge biopsy needle (Bard, Inc., West Sussex, England). The myocardial biopsies were immediately frozen in liquid nitrogen and stored until analysis at −70°C. The contents of ATP, adenosine diphosphate (ADP) and adenosine monophosphate were determined through high-pressure liquid chromatography and lactate by a fluorometric method [16].
After induction of anaesthesia, a 7.5 Fr Swan-Ganz thermodilution catheter (Baxter, Irvine, CA, USA) was placed in the pulmonary artery, and haemodynamic profiles were obtained before surgery (control) and 1, 8 and 24 h after termination of cardiopulmonary bypass. Cardiac output was assessed by thermodilution (Siemens Sirecust 961, Erlangen, Germany); triplicate measurements were performed and the average was calculated. MAP, mean pulmonary artery pressure (MPAP), central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP) and heart rate (HR) were recorded simultaneously, and derived indices were calculated from standard formulas.
Statistical analysis
To compare the differences in repeated measures of continuous data between the groups, repeated-measures analysis of variance (ANOVA) was used. This method allowed for a comparison between treatment allocation (adenosine versus placebo), cardioplegia delivery effect (antegrade versus retrograde), time effect and interrelations between these. The first myocardial biopsy obtained immediately before aortic clamping and the first haemodynamic profile measured before surgery were regarded as control values and subtracted from the subsequent values before analysis. Arteriovenous differences in oxygen and lactate were analyzed during and after aortic occlusion. Time, treatment allocation (adenosine versus placebo) and the relations between these were tested in all analyses. Post hoc analysis was performed only when the ANOVA showed significant differences. Categorical variables were compared using χ2 tests or Fisher's exact test, while continuous variables were compared using Student's t-test for independent samples. All statistical analyses were performed using Statistica 9.0 (Statsoft, Inc., Tulsa, USA). Probability values of less than 0.05 were regarded statistically significant.
RESULTS
Baseline data
The baseline data are shown in Table 1. The adenosine group had a higher mean age and a greater prevalence of hypertension than the control group.
Table 1:
Baseline data
| Adenosine group (n = 40) | Control group (n = 40) | |
|---|---|---|
| Age (years)a | 72.2 ± 7.8 | 65.7 ± 10.1 |
| Female genderb | 25 (62%) | 20 (50%) |
| BMI (kg m−2)a | 25.3 ± 3.9 | 25.9 ± 3.9 |
| Diabetes mellitusb | 6 (15%) | 7 (18%) |
| Hypertensionb | 17 (42%) | 7 (18%) |
| Preoperative pacemakerb | 2 (5%) | 4 (10%) |
| LVEF (%)a | 64 ± 11 | 63 ± 13 |
BMI, body mass index; LVEF, left ventricular ejection fraction.
aMean ± SD.
bNumber (percent within group).
Clinical outcomes
The clinical outcome is summarized in Table 2. There were two hospital deaths in the study. In the ADE-ant group, a 73-year-old woman developed asystole on the second postoperative day after an initial uneventful postoperative course. Autopsy showed multiple small infarctions in the left ventricle due to calcific emboli from the aortic valve replacement.
Table 2:
Clinical data
| Adenosine group (n = 40) | Control group (n = 40) | P-value | |
|---|---|---|---|
| Time on CPB (min)a | 122 ± 21 | 127 ± 23 | 0.39 |
| Cross-clamp time (min)a | 87 ± 15 | 87 ± 13 | 0.98 |
| Myocardial temperature (°C)a,c | 10.9 ± 1.7 | 11.6 ± 1.8 | 0.11 |
| Postoperative pacemaker implantb | 2 (5%) | 1 (2%) | 0.50 |
| Inotropic support >6 hb | 11 (27%) | 8 (20%) | 0.43 |
| In-hospital mortalityb | 1 (2%) | 1 (2%) | 1.00 |
CPB, cardiopulmonary bypass.
aMean ± SD.
bNumber (percent within group).
cDuring cross-clamp.
In the CTRL-ant group, a 77-year-old woman with a preoperative ejection fraction of 24% required a biventricular assist when weaning from bypass. She died 2 days later due to heart failure. Autopsy showed an inferior myocardial infarction and no obstruction of the coronaries. Permanent pacemaker was required in three female patients due to third-degree atrioventricular blocks, two in the ADE-ant group and one in the CTRL-ret group. Nineteen patients required inotropic support >6 h postoperatively, with no significant differences between the groups.
There were no differences between the groups regarding cardiopulmonary bypass time, aortic cross-clamp time or myocardial temperature during aortic cross-clamp.
Enzyme release
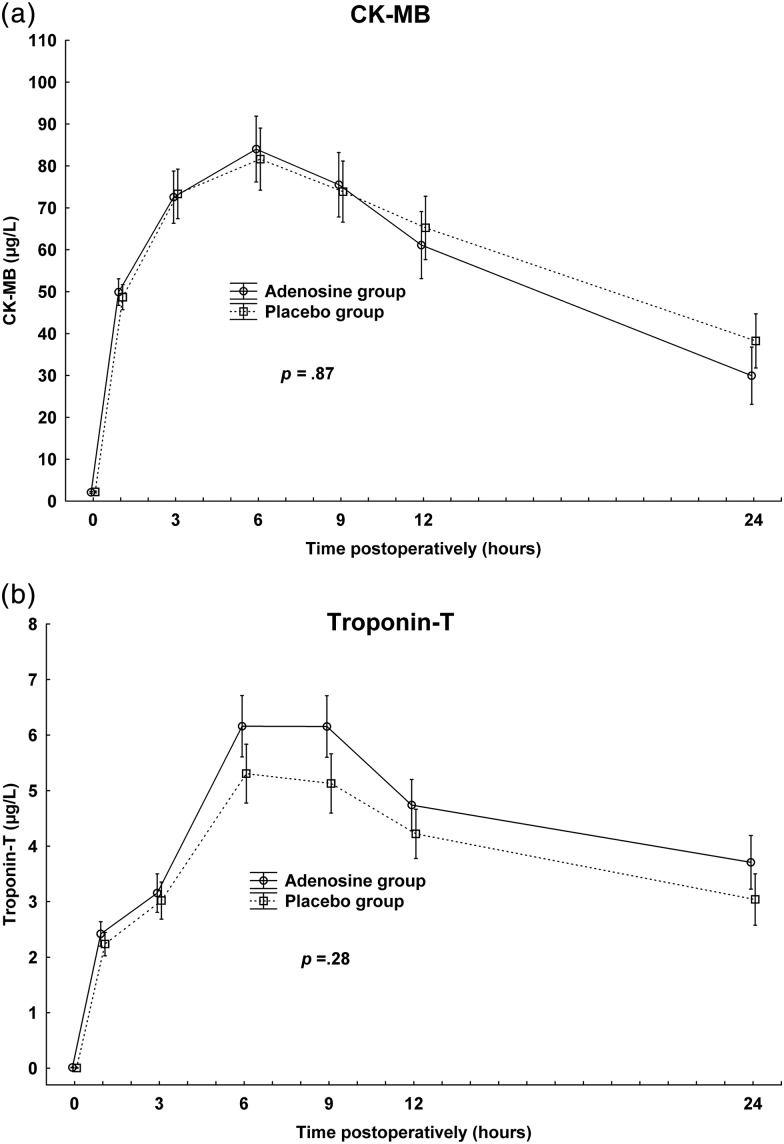
Plasma concentrations of CK-MB and troponin T peaked at 6 h postoperatively, with no significant differences between adenosine and control groups (P = 0.87 and P = 0.28, respectively, Fig. 1).
Figure 1:
Postoperative plasma concentrations of creatine kinase (CK-MB) and troponin T. Vertical bars denote ±standard error of the mean. P-values denote significance test of treatment allocation in repeated-measures ANOVA. (a) CK-MB. (b) Troponin T.
Myocardial arteriovenous differences in oxygen and lactate
There were no significant differences between the adenosine and control groups during or after aortic occlusion (Figs 2a and b). During aortic occlusion, there was a small myocardial uptake of oxygen. The coronary sinus lactate concentrations increased significantly when the aortic occlusion was released (Fig. 2b).
Figure 2:
Coronary arteriovenous (A-V) difference in oxygen and venoarterious (V-A) difference in lactate during and after aortic cross-clamp. Vertical bars denote ±standard error of the mean. P-values denote significance test of treatment allocation in repeated-measures ANOVA. (a) Oxygen. (b) Lactate.
Myocardial adenine nucleotides and lactate
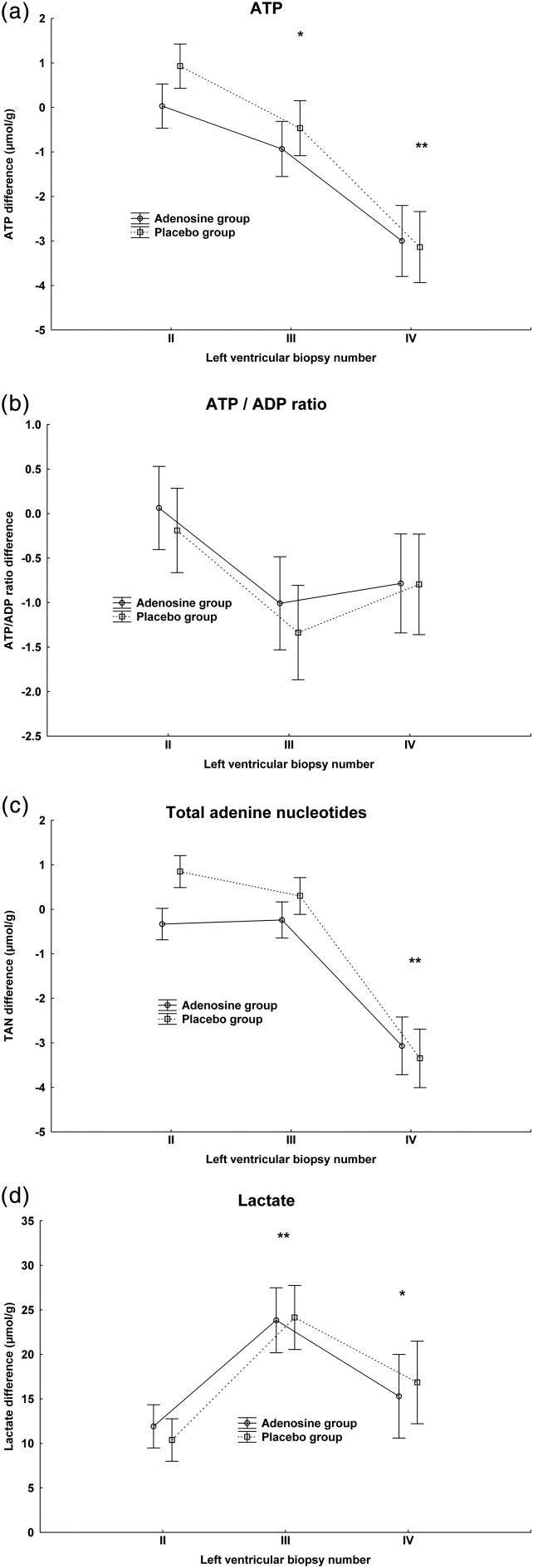
The myocardial biopsy results are shown in Table 3. Overall, there were no significant differences between adenosine and control groups. Myocardial ATP concentration decreased significantly in both groups after 60 min of aortic occlusion and even more 20 min after aortic declamping (Fig. 3a). There were no significant differences over time in ATP/ADP ratio (Fig. 3b) or energy charge (EC). The total adenine nucleotide concentrations fell significantly after aortic occlusion (Fig. 3c). Myocardial lactate concentration increased significantly during aortic occlusion and decreased 20 min after aortic declamping, though still remained significantly higher than the control value before aortic occlusion (Fig. 3d).
Table 3:
Concentrations of adenine nucleotides and lactate in myocardial biopsies obtained before aortic occlusion (I), after bolus cardioplegia (II), at 60 min of aortic occlusion (III) and at 20 min after release of aortic occlusion (IV)a
| Adenosine group (n = 40) | Control group (n = 40) | P-valued | ||
|---|---|---|---|---|
| ATP (μmol g−1) | I | 20.75 ± 2.87 | 20.42 ± 4.60 | 0.90 |
| II | 20.85 ± 2.91 | 21.41 ± 4.10 | ||
| III | 19.36 ± 3.21 | 19.66 ± 4.41 | ||
| IV | 17.78 ± 3.52 | 17.01 ± 4.48 | ||
| ADP (μmol g−1) | I | 3.66 ± 1.51 | 3.66 ± 1.71 | 0.78 |
| II | 3.44 ± 0.97 | 3.51 ± 1.40 | ||
| III | 4.38 ± 1.60 | 4.24 ± 1.24 | ||
| IV | 3.54 ± 1.30 | 3.39 ± 1.46 | ||
| AMP (μmol g−1) | I | 0.65 ± 0.55 | 0.86 ± 0.99 | 0.50 |
| II | 0.55 ± 0.39 | 0.75 ± 0.85 | ||
| III | 1.03 ± 0.95 | 1.01 ± 1.02 | ||
| IV | 0.78 ± 0.64 | 0.79 ± 0.75 | ||
| ATP/ADP | I | 6.49 ± 2.51 | 6.66 ± 2.73 | 0.65 |
| II | 6.52 ± 1.90 | 6.72 ± 2.04 | ||
| III | 5.14 ± 2.26 | 5.15 ± 2.08 | ||
| IV | 5.70 ± 2.28 | 5.91 ± 2.65 | ||
| TANb (μmol g−1) | I | 25.06 ± 2.63 | 24.93 ± 4.38 | 0.84 |
| II | 24.85 ± 2.66 | 25.60 ± 4.37 | ||
| III | 24.78 ± 2.35 | 24.92 ± 3.89 | ||
| IV | 22.11 ± 3.13 | 21.19 ± 4.29 | ||
| Energy charge (EC)c | I | 0.90 ± 0.04 | 0.89 ± 0.07 | 0.55 |
| II | 0.91 ± 0.04 | 0.90 ± 0.05 | ||
| III | 0.87 ± 0.07 | 0.87 ± 0.06 | ||
| IV | 0.88 ± 0.06 | 0.88 ± 0.07 | ||
| Lactate (μmol g−1) | I | 14.84 ± 6.72 | 14.75 ± 6.85 | 0.84 |
| II | 26.29 ± 14.77 | 24.27 ± 13.93 | ||
| III | 39.91 ± 20.68 | 39.46 ± 23.59 | ||
| IV | 29.49 ± 24.93 | 30.91 ± 29.67 |
ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; TAN, total adenine dinucleotide.
aData are expressed as mean ± SD.
bTAN = ATP + ADP + AMP.
cEC = (ATP + ½ADP)/(ATP + ADP + AMP).
dSignificance test of effect of treatment allocation in repeated-measures ANOVA.
Figure 3:
Concentration differences of adenine nucleotides and lactate in myocardial biopsies obtained after bolus cardioplegia (II), at 60 min of aortic occlusion (III) and at 20 min after release of aortic occlusion (IV). All values are subtracted from control biopsy before aortic occlusion (I). Vertical bars denote ±standard error of the mean. Post hoc significance test of time differences between biopsies are marked with an asterisk. ATP concentration: *P < 0.05 compared with biopsy II. **P < 0.01 compared with biopsy II. Total adenine nucleotide concentration: P < 0.01 compared with biopsy II. Lactate concentration: *P < 0.05 compared with biopsy I. **P < 0.01 compared with biopsy I.
Haemodynamic profiles
Adenosine had no significant effects on the haemodynamic parameters studied (Table 4). Cardiac index (CI) increased significantly in all groups 8 and 20 h postoperatively compared with control (P < 0.001), with no significant differences between the groups, parallel with a significant decrease in SVR at the same time (P < 0.001). Left ventricular stroke work index (LVSWI) was significantly lower at one hour postoperatively (P < 0.001) in all groups compared with control and subsequent values.
Table 4:
Haemodynamic thermodilution measurements recorded before surgery (baseline) and 1, 8 and 20 h after termination of extracorporeal circulationa
| Adenosine group (n = 40) | Control group (n = 40) | P-valueb | ||
|---|---|---|---|---|
| CI (l min−1 m−2) | Baseline | 2.0 ± 0.4 | 2.3 ± 0.6 | 0.97 |
| 1 h | 2.1 ± 0.5 | 2.3 ± 0.6 | ||
| 8 h | 2.7 ± 0.6 | 3.0 ± 0.6 | ||
| 20 h | 2.8 ± 0.5 | 3.0 ± 0.5 | ||
| LVSWI (g m m−2) | Baseline | 28.5 ± 8.6 | 30.7 ± 8.4 | 0.67 |
| 1 h | 22.8 ± 6.7 | 23.1 ± 7.3 | ||
| 8 h | 26.5 ± 5.8 | 31.1 ± 9.7 | ||
| 20 h | 28.3 ± 7.2 | 32.3 ± 9.2 | ||
| MAP (mmHg) | Baseline | 75 ± 11 | 74 ± 12 | 0.28 |
| 1 h | 73 ± 11 | 74 ± 12 | ||
| 8 h | 72 ± 10 | 76 ± 11 | ||
| 20 h | 74 ± 12 | 76 ± 14 | ||
| MPAP (mmHg) | Baseline | 21 ± 8 | 19 ± 6 | 0.59 |
| 1 h | 20 ± 5 | 19 ± 6 | ||
| 8 h | 21 ± 5 | 18 ± 4 | ||
| 20 h | 20 ± 5 | 19 ± 4 | ||
| SVR (dyne s cm−5) | Baseline | 1644 ± 567 | 1384 ± 437 | 0.22 |
| 1 h | 1524 ± 477 | 1411 ± 643 | ||
| 8 h | 1126 ± 319 | 1068 ± 298 | ||
| 20 h | 1102 ± 289 | 1011 ± 225 | ||
| PVR (dyne s cm−5) | Baseline | 187 ± 91 | 143 ± 74 | 0.68 |
| 1 h | 181 ± 90 | 134 ± 64 | ||
| 8 h | 173 ± 84 | 142 ± 55 | ||
| 20 h | 149 ± 65 | 119 ± 37 | ||
| PCWP (mmHg) | Baseline | 13 ± 6 | 12 ± 5 | 0.68 |
| 1 h | 12 ± 5 | 12 ± 6 | ||
| 8 h | 11 ± 4 | 9 ± 4 | ||
| 20 h | 11 ± 4 | 11 ± 4 | ||
| CVP (mmHg) | Baseline | 7 ± 4 | 6 ± 3 | 0.20 |
| 1 h | 8 ± 4 | 8 ± 4 | ||
| 8 h | 7 ± 3 | 7 ± 3 | ||
| 20 h | 8 ± 4 | 8 ± 4 | ||
| HR (beats min−1) | Baseline | 61 ± 16 | 64 ± 15 | 0.36 |
| 1 h | 77 ± 12 | 84 ± 16 | ||
| 8 h | 84 ± 15 | 90 ± 14 | ||
| 20 h | 84 ± 13 | 85 ± 12 |
CI, cardiac index; CVP, central venous pressure; HR, heart rate; LVSWI, left ventricular stroke work index; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary pressure; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance.
aData are expressed as mean ± SD.
bSignificance test of effect of treatment allocation in repeated-measures ANOVA with baseline value subtracted from subsequent values.
Antegrade versus retrograde cardioplegia
There were no significant differences between the antegrade and retrograde groups in myocardial enzyme release (CK-MB and troponin T), myocardial concentration of adenine nucleotides or arteriovenous differences in lactate. Myocardial oxygen uptake was significantly higher in the retrograde groups during aortic occlusion (P < 0.001, Fig. 4a), and the myocardial concentration of lactate also tended to be higher in the retrograde groups (P = 0.052, Fig. 4b). Postoperatively, LVSWI was higher (P = 0.034, Fig. 4c), and CI tended to be higher (P = 0.067), in patients receiving antegrade cardioplegia.
Figure 4:
Differences between antegrade and retrograde cardioplegia. Vertical bars denote ±standard error of the mean. P-value denotes significance test of cardioplegia direction allocation in repeated-measures ANOVA. (a) Myocardial arteriovenous (A-V) differences in oxygen during aortic occlusion. (b) Concentration of lactate in myocardial biopsies obtained after bolus cardioplegia (II), at 60 min of aortic occlusion (III) and at 20 min after release of aortic occlusion (IV). All values are subtracted from control biopsy obtained before aortic occlusion (I). (c) LVSWI difference in the postoperative period. All time points subtracted from preoperative value.
DISCUSSION
The major finding of this study can be summarized as follows: adenosine 400 µmol l−1 in continuous cold blood cardioplegia did not enhance myocardial protection in patients undergoing aortic valve replacement as measured by myocardial enzyme release, adenine nucleotide and oxygen/lactate metabolism or haemodynamic performance. These findings are in accordance with two randomized trials comprising a total of 418 patients, showing no significant differences in myocardial enzyme release and haemodynamic performance between the adenosine and placebo groups [6,10]. In a randomized study of 253 CABG patients, Mentzer et al. [8] found a lower incidence of inotropic support in patients receiving adenosine as an additive to blood cardioplegia compared with controls, but enzymatic release was not reported. Only one study has focused on valve surgery patients; Liu et al. found lower release of troponin-I, interleukin-6 and interleukin-8 at 10 min and 24 h after declamping in patients receiving adenosine 1 mmol l−1 in blood cardioplegia compared with controls. However, this study showed no difference in CK-MB release, and the differences between adenosine and placebo groups were overall quite subtle [7]. Our study could not verify the results from the small, non-randomized study of adenosine in blood cardioplegia in CABG patients, showing preserved myocardial ATP concentration compared with controls [11]. Instead, in this study, the levels of ATP and total adenine nucleotides fell significantly during and after aortic occlusion, despite continuous cold blood cardioplegia and adenosine supplementation. This finding indicates that adenosine in blood cardioplegia may not be acting as a substrate for ATP resynthesis, as proposed from animal studies [4].
It could be argued that the timing of adenosine supplementation during aortic occlusion in order to prevent ischaemia is not optimal, and studies of pre- and post-ischaemic administration of adenosine in heart surgery patients have been conducted [5,9,17–19]. However, the two largest studies of pre-ischaemic delivery of adenosine found no differences in either enzymatic release or clinical performance [5,9]. The only study of post-ischaemic administration of adenosine was conducted in 60 valve surgery patients, where an intraarterial injection of 0.3–0.5 mM adenosine at cross-clamp removal was linked to a lower troponin-I release and decreased the need for postoperative inotropic support [17]. This finding is interesting, but has to be confirmed in other studies.
To summarize, this study of aortic valve patients was unable to demonstrate any beneficial effects of adenosine in blood cardioplegia, and together with previous studies showing no or only subtle effects of adenosine-supplemented blood cardioplegia, it seems reasonable to argue that the clinical value of adenosine as an adjunct to blood cardioplegia during cardiac surgery is questionable.
The design of this study also enabled a comparison between antegrade and retrograde cardioplegia. We found a higher myocardial oxygen uptake and a higher myocardial lactate concentration in the retrograde groups during aortic occlusion, together with a lower left ventricular stroke work index in the postoperative period. Cardiac venous anatomy can lead to inhomogeneous retrograde delivery of cardioplegia [20], and increased lactate production in coronary surgery patients receiving retrograde cardioplegia has been demonstrated [21]. On the other hand, studies of cardioplegia delivery and adenine nucleotide metabolism have found no significant differences between antegrade and retrograde routes [13–15]. The observed differences between antegrade and retrograde cardioplegia in myocardial metabolism during aortic occlusion and haemodynamic performance in the postoperative period are interesting and warrant further investigation.
Limitations
This study focused on patients with left ventricular hypertrophy and normal coronary arteries undergoing aortic valve replacement. The results thus cannot be transferred to patients with coronary artery disease.
In this study, continuous cold blood cardioplegia was studied. Although proved to be effective in myocardial protection [22], this is a technique that may affect visualization in the operative field. In the protocol, only the left coronary ostium was cannulated in the antegrade groups. Insufficient protection of the myocardium cannot be ruled out as a cause of death in one patient in the study. Our present protocol is therefore always to perfuse both coronary arteries in antegrade cardioplegic delivery.
ACKNOWLEDGEMENTS
We thank Jane Strand, Laila Örtensjö, Kristina Bodin and Barbara Norman for valuable technical assistance and support.
Funding
This work was supported by the Research Committee of Örebro University Hospital.
Conflict of interest: none declared.
REFERENCES
- 1.Doenst T, Bugger H, Schwarzer M, Faerber G, Borger MA, Mohr FW. Three good reasons for heart surgeons to understand cardiac metabolism. Eur J Cardiothorac Surg. 2008;33:862–71. doi: 10.1016/j.ejcts.2008.02.015. doi:10.1016/j.ejcts.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Hudspeth DA, Nakanishi K, Vinten-Johansen J, Zhao ZQ, McGee DS, Williams MW, Hammon JW., Jr. Adenosine in blood cardioplegia prevents postischemic dysfunction in ischemically injured hearts. Ann Thorac Surg. 1994;58:1637–44. doi: 10.1016/0003-4975(94)91650-0. doi:10.1016/0003-4975(94)91650-0. [DOI] [PubMed] [Google Scholar]
- 3.Vinten-Johansen J, Zhao ZQ, Corvera JS, Morris CD, Budde JM, Thourani VH, et al. Adenosine in myocardial protection in on-pump and off-pump cardiac surgery. Ann Thorac Surg. 2003;75:S691–9. doi: 10.1016/s0003-4975(02)04694-5. doi:10.1016/S0003-4975(02)04694-5. [DOI] [PubMed] [Google Scholar]
- 4.Bolling SF, Childs KF, Ning XH. Adenosine's effect on myocardial functional recovery: substrate or signal? J Surg Res. 1994;57:591–5. doi: 10.1006/jsre.1994.1188. doi:10.1006/jsre.1994.1188. [DOI] [PubMed] [Google Scholar]
- 5.Belhomme D, Peynet J, Florens E, Tibourtine O, Kitakaze M, Menasche P. Is adenosine preconditioning truly cardioprotective in coronary artery bypass surgery? Ann Thorac Surg. 2000;70:590–4. doi: 10.1016/s0003-4975(00)01502-2. doi:10.1016/S0003-4975(00)01502-2. [DOI] [PubMed] [Google Scholar]
- 6.Cohen G, Feder-Elituv R, Iazetta J, Bunting P, Mallidi H, Bozinovski J, et al. Phase 2 studies of adenosine cardioplegia. Circulation. 1998;98:II225–33. [PubMed] [Google Scholar]
- 7.Liu R, Xing J, Miao N, Li W, Liu W, Lai YQ, et al. The myocardial protective effect of adenosine as an adjunct to intermittent blood cardioplegia during open heart surgery. Eur J Cardiothorac Surg. 2009;36:1018–23. doi: 10.1016/j.ejcts.2009.06.033. doi:10.1016/j.ejcts.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Mentzer RM, Jr., Birjiniuk V, Khuri S, Lowe JE, Rahko PS, Weisel RD, et al. Adenosine myocardial protection: preliminary results of a phase II clinical trial. Ann Surg. 1999;229:643–9. doi: 10.1097/00000658-199905000-00006. discussion 649–650 doi:10.1097/00000658-199905000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinne T, Laurikka J, Penttila I, Kaukinen S. Adenosine with cold blood cardioplegia during coronary revascularization. J Cardiothorac Vasc Anesth. 2000;14:18–20. doi: 10.1016/s1053-0770(00)90049-1. doi:10.1016/S1053-0770(00)90049-1. [DOI] [PubMed] [Google Scholar]
- 10.Shalaby A, Rinne T, Jarvinen O, Saraste A, Laurikka J, Porkkala H, et al. Initial results of a clinical study: adenosine enhanced cardioprotection and its effect on cardiomyocytes apoptosis during coronary artery bypass grafting. Eur J Cardiothorac Surg. 2008;33:639–44. doi: 10.1016/j.ejcts.2007.12.049. doi:10.1016/j.ejcts.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Cohen G, Shirai T, Weisel RD, Rao V, Merante F, Tumiati LC, et al. Optimal myocardial preconditioning in humans. Ann NY Acad Sci. 1999;874:306–19. doi: 10.1111/j.1749-6632.1999.tb09246.x. doi:10.1111/j.1749-6632.1999.tb09246.x. [DOI] [PubMed] [Google Scholar]
- 12.Mentzer RM, Jr., Rahko PS, Molina-Viamonte V, Canver CC, Chopra PS, Love RB, et al. Safety, tolerance, and efficacy of adenosine as an additive to blood cardioplegia in humans during coronary artery bypass surgery. Am J Cardiol. 1997;79:38–43. doi: 10.1016/s0002-9149(97)00262-2. doi:10.1016/S0002-9149(97)00262-2. [DOI] [PubMed] [Google Scholar]
- 13.Carrier M, Pelletier LC, Searle NR. Does retrograde administration of blood cardioplegia improve myocardial protection during first operation for coronary artery bypass grafting? Ann Thorac Surg. 1997;64:1256–61. doi: 10.1016/S0003-4975(97)00900-4. doi:10.1016/S0003-4975(97)00900-4. [DOI] [PubMed] [Google Scholar]
- 14.Kaukoranta PK, Lepojarvi MV, Kiviluoma KT, Ylitalo KV, Peuhkurinen KJ. Myocardial protection during antegrade versus retrograde cardioplegia. Ann Thorac Surg. 1998;66:755–61. doi: 10.1016/s0003-4975(98)00459-7. doi:10.1016/S0003-4975(98)00459-7. [DOI] [PubMed] [Google Scholar]
- 15.Lotto AA, Ascione R, Caputo M, Bryan AJ, Angelini GD, Suleiman MS. Myocardial protection with intermittent cold blood during aortic valve operation: antegrade versus retrograde delivery. Ann Thorac Surg. 2003;76:1227–33. doi: 10.1016/s0003-4975(03)00840-3. doi:10.1016/S0003-4975(03)00840-3. [DOI] [PubMed] [Google Scholar]
- 16.Norman B, Sabina RL, Jansson E. Regulation of skeletal muscle ATP catabolism by AMPD1 genotype during sprint exercise in asymptomatic subjects. J Appl Physiol. 2001;91:258–64. doi: 10.1152/jappl.2001.91.1.258. [DOI] [PubMed] [Google Scholar]
- 17.Jin ZX, Zhou JJ, Xin M, Peng DR, Wang XM, Bi SH, et al. Postconditioning the human heart with adenosine in heart valve replacement surgery. Ann Thorac Surg. 2007;83:2066–72. doi: 10.1016/j.athoracsur.2006.12.031. doi:10.1016/j.athoracsur.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Lee HT, LaFaro RJ, Reed GE. Pretreatment of human myocardium with adenosine during open heart surgery. J Card Surg. 1995;10:665–76. doi: 10.1111/j.1540-8191.1995.tb00657.x. doi:10.1111/j.1540-8191.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei M, Kuukasjarvi P, Laurikka J, Honkonen EL, Kaukinen S, Laine S, et al. Cardioprotective effect of adenosine pretreatment in coronary artery bypass grafting. Chest. 2001;120:860–5. doi: 10.1378/chest.120.3.860. doi:10.1378/chest.120.3.860. [DOI] [PubMed] [Google Scholar]
- 20.Ruengsakulrach P, Buxton BF. Anatomic and hemodynamic considerations influencing the efficiency of retrograde cardioplegia. Ann Thorac Surg. 2001;71:1389–95. doi: 10.1016/s0003-4975(00)01991-3. doi:10.1016/S0003-4975(00)01991-3. [DOI] [PubMed] [Google Scholar]
- 21.Yau TM, Ikonomidis JS, Weisel RD, Mickle DA, Hayashida N, Ivanov J, et al. Which techniques of cardioplegia prevent ischemia? Ann Thorac Surg. 1993;56:1020–8. doi: 10.1016/0003-4975(95)90007-1. doi:10.1016/0003-4975(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 22.Bomfim V. Myocardial protection during aortic valve replacement: a clinical and laboratory study of intra-operative myocardial metabolism. Scand J Thorac Cardiovasc Surg Suppl. 1981;29:1–26. [PubMed] [Google Scholar]