Abstract
Objectives
The aim of this study was to assess the early and long-term outcomes of a previously introduced technique of reduction aortoplasty for asymmetric ascending aortic dilatation. Different indication criteria for reduction ascending aortoplasty have been previously adopted by others, thus another purpose was to identify the patient profile for whom this approach may be best suited.
Methods
Between January 2001 and December 2010, reduction ascending aortoplasty with “waistcoat technique” was performed in 156 patients (mean age 62±12 years, 61% male) with asymmetric dilatation of the ascending aorta (prevailing at the convexity of the supracoronary tract). Eighty-seven patients had a tricuspid aortic valve (TAV), 69 a bicuspid aortic valve (BAV). Aortoplasty was associated to aortic valve replacement in 60% cases. Preoperative, intraoperative, early postoperative and follow-up data were analysed. Comparisons were performed between groups of valve morphology (TAV versus BAV) and subgroups of baseline valve function. In patients with a follow-up time >1 year the annual growth of the ascending tract was calculated and compared between subgroups. The independent predictors of growth velocity were assessed by multivariable linear regression analysis.
Results
Mean cross-clamp and cardiopulmonary bypass times were 39 ± 18 and 69 ± 29 min, respectively. Hospital death was 1.9%. In no case, postoperative death or any early complication was causally related to the aortoplasty procedure. The mean postoperative ascending diameter was 3.1 ± 0.3 (versus preoperative 5.2 ± 0.8 cm, P < 0.001). Mean follow-up time was 4 ± 2.5 years (maximum 10 years): 7-year survival was 95 ± 2%; 7-year freedom from aortic events 94 ± 4%. Redilatation (ascending diameter exceeding 4.5 cm) occurred in two patients, acute dissection in one: all three preoperatively had significant aortic regurgitation. The mean ascending aortic diameter at last follow-up was 3.4 ± 0.5 cm; median diameter progression was 0.4 mm/year, with no significant difference between TAV and BAV and no patient reaching 0.5 cm/year. With TAV, the only determinant of aortic growth rate was normal preoperative valve function (P = 0.04); with BAV, the degree of regurgitation at preoperative echocardiography (P = 0.001).
Conclusions
Waistcoat aortoplasty proved a safe and durable treatment for patients with asymmetric non-syndromic non-familial ascending aorta dilatation. The technique showed its best durability in aortic stenosis patients and in patients with normofunctional BAV.
Keywords: Aortic aneurysm surgery, Aortic valve disease, Bicuspid aortic valve, Indications, Long-term follow-up
INTRODUCTION
The gold standard in surgery for ascending aorta aneurysms is represented by tube graft replacement; however it has long been debated whether a conservative treatment should also be considered or not among the surgical options [1–3]. Several different variants of reduction ascending aortoplasty (RAA) procedures with or without external wrapping exist [4, 5]: all variants share the rationale of addressing the diameter factor in the Laplace law (or in case of external support, also the wall thickness factors), thus restoring theoretically normal aortic wall tension and preventing further dilatation. As previously reported, in 1998, the senior author of this study (M.C.) developed an original technique of RAA, characterized by the closure of the reduced aorta by a double-suture double-layer technique, in a ‘waistcoat’ fashion [6].
Leaving native tissue in place, RAA operations have been criticized as exposing the patient to a significant risk of redilatation, rupture or dissection, especially in the presence of intrinsic disease of the arterial wall [2, 7]. Nevertheless, series of successful RAA are continuously being reported on, with some authors proposing it only for high-risk patients, with short life expectancy [8], some others for smaller size dilatations, supposedly having more preserved wall structure, especially when other procedures must be concomitantly performed [9, 10]. The congenital bicuspid aortic valve (BAV), which is associated with a peculiar form of aortopathy, has been in turn considered a contraindication [6, 9] or an elective indication [11–13] in previous RAA series.
Between the late 1990s and the early 2000s, studies by both the present authors [6, 14] and others [15], have led to the recognition of a definite anatomical form of aortic dilatation or aneurysm, i.e. the asymmetric dilatation, engendered by the preferential expansion of the convexity of the ascending aorta (right antero-lateral aspect), whereas the symmetric (or ‘fusiform’) dilatations involve uniformly the whole circumference of the aorta. Asymmetric anatomy of the dilatation is typical of the BAV aortopathy [15, 16], however it has been described in nearly 50% of TAV-associated dilatations as well [16]. The above mentioned waistcoat aortoplasty (WA) was specifically introduced for the treatment of this asymmetric form of aortopathy, as it implies resection and reinforcement (partial autologous wrapping) of the convexity [6].
The present study assessed the late outcomes of WA procedures performed during 10 years of experience and searched for variables identifying the patients with the best long-term outcome.
MATERIALS AND METHODS
Patient series
The WA technique was selectively employed when the aneurysm involved the tubular supracoronary tract, with unaffected or less dilated sinuses and normal arch (mid-ascending type [17]), and had asymmetric configuration (dilatation of the convexity) [15], in patients without Marfan syndrome or other known genetically determined connective tissue disorders and with negative family history for aortic diseases. Atherosclerotic aneurysms, usually showing a symmetrical configuration, were therefore systematically treated by other procedures.
Thus, between January 2001 and December 2010, 156 patients (mean age 62 ± 12 years, 61% male, 44% BAV) underwent WA, either isolated (29%) or associated to other procedures: aortic valve replacement (AVR; 60%), AVR and coronary artery bypass grafting (CABG) (6%), and AVR and mitral valve repair or replacement (5%).
All patients underwent preoperative and pre-discharge trans-thoracic or trans-oesophageal echocardiography, including assessment of aortic diameters at annular, sinusal, sinotubular, tubular and arch level. In 37% of cases, a preoperative computed tomographic (CT) scan or magnetic resonance imaging (MRI) was also available. Dilatation was defined as a ratio ≥1.5 (or 1.4 for BAV patients) between observed and normal diameter. Asymmetry was defined based on three-dimensional reconstructions of CT-scan or MRI, when available, otherwise on surgical inspection: frequently in those cases, eye inspection revealed a whitish appearance of the adventitia of the aorta convexity, where the wall proved focally thinned after incision. When visible wall alterations were circumferentially distributed and whenever diffuse wall weakness was suspected, Dacron graft replacement was preferred. Maximal aneurysm diameters ranged between 4.6 and 6 cm in most cases, whereas greater aneurysms were generally excluded, mostly being associated to atherosclerotic aetiology, symmetric configuration or Marfan syndrome. However, to 14 higher risk patients with elderly age and multiple comorbidities, WA was proposed as an alternative to graft replacement, regardless of great aneurysm dimensions (>6 cm).
Surgical technique
The WA technique, represented in Fig. 1, has been already described in detail elsewhere [6]. In 48 patients (31%) with moderate dilatation of the sinuses associated to supracoronary aneurysm, sinus longitudinal plication was associated: this was accomplished including in the pledgeted U stitches for valve prosthesis implantation a portion of the sub-coronary sinus wall [6]. In cases with associated multiple CABG, the saphenous vein grafts were proximally anastomosed at the concavity, i.e. the area facing the pulmonary artery.
Figure 1:
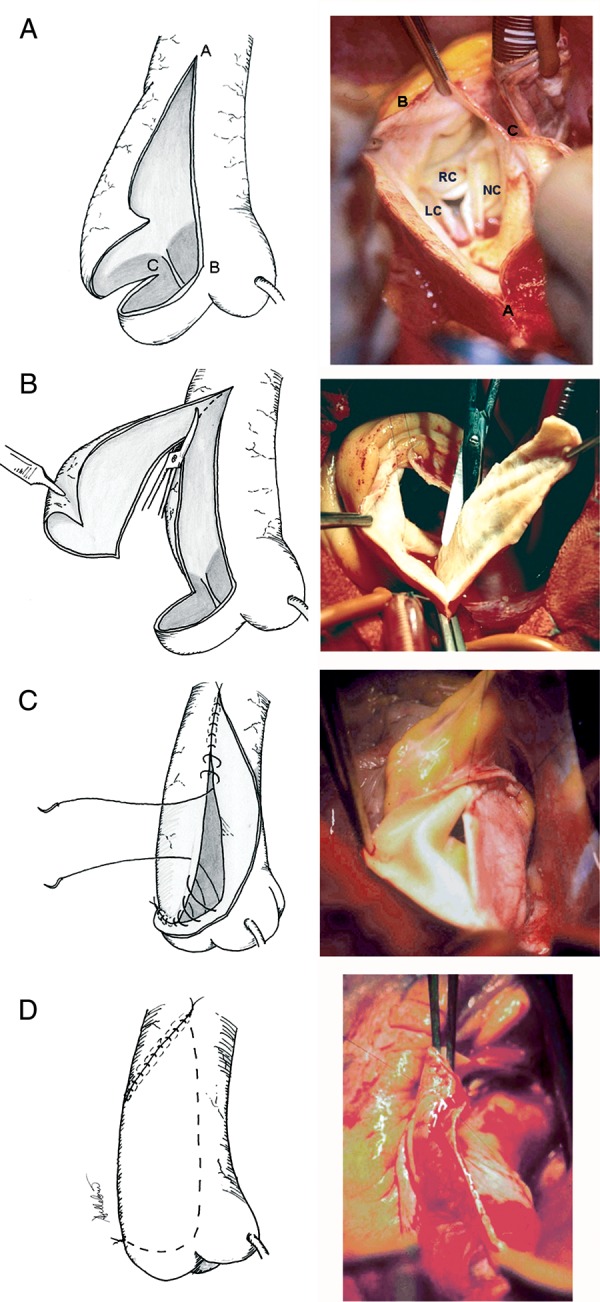
WA procedure. (A) Opening of the tubular tract by a longitudinal incision on its anterior aspect (proximally, the incision was skewed to the right, and in few cases with some minor degree of sinus dilatation, generally with aortic regurgitation, it partly involved the non-coronary sinus); (B) excision (usually 2–3 cm large) of the subtended right antero-lateral portion of the ascending aorta; (C) reconstruction (further contributing to diameter reduction): continuous running or mattress sutures passed through the right margin of the resection and then through the intimal aspect of the aortic wall on the left side, so to close the aorta creating a flap of the left antero-lateral aortic wall, about 2–3 cm in its maximal width; (D) covering of the reconstructed aortic convexity, by securing the free margin of the flap to the right postero-lateral aspect of the ascending aorta with a running or interrupted suture. (A–C) Limits of the aortotomy line; LC, RC and NC denote left coronary, right coronary and non-coronary sinus, respectively.
Follow-up methods
All preoperative and early postoperative data were prospectively collected. Follow-up data were obtained by either review of outpatient clinic charts or telephone interview with the patient or referring practitioner with the subsequent collection of the latest echocardiography reports. When echocardiographic data were considered inadequate for study purposes (for lacking measurement of the ascending tubular tract diameter, or because more than 6 months had passed since last examination), echocardiography was performed at the authors' institution. Following WA, redilatation was defined as an aortic diameter exceeding 4.5 cm [18]. The growth rate of the aorta in the follow-up was calculated.
Statistical analysis
Comparisons were performed between TAV and BAV patients by unpaired t-test (or Mann–Whitney U test, for non-normally distributed data) and chi-square test. Within each group of valve morphology, comparisons between subgroups of baseline valve function (normal, stenosis, regurgitation) were performed by one-way ANOVA test (with Bonferroni correction), or by Kruskal–Wallis test. Baseline aortic valve function was defined, to the purposes of the present analysis, as normal if degrees of stenosis and regurgitation were not greater than mild, otherwise stenotic if the degree of stenosis was greater than the degree of regurgitation, regurgitant if the degree of regurgitation was greater than the degree of stenosis.
Multivariable binary logistic and linear regression models were developed to find out predictors of any aortic event (re-dilatation, rupture or dissection) and of aortic growth rate in the follow-up, respectively: included covariates were age, gender, anthropometrics, comorbidities, preoperative sinus and tubular tract diameter, TAV/BAV, valve function, degree of stenosis, degree of regurgitation, postreduction diameter, type of valve prosthesis implanted (mechanical, biological), sinus plication.
The Kaplan–Meier method was used to estimate actuarial freedom from events and the log-rank test to compare actuarial curves between groups. Significance was set at 0.05 for all P-values. Analysis was performed with SPSS v. 13.0.
RESULTS
Preoperative features
TAV and BAV groups differed significantly in terms of age, gender distribution and prevalence of hypertension (Table 1). Preoperative aortic diameters at the measured levels did not significantly differ (Table 1). Mean annulus diameter in the overall series was 2.5 ± 0.5 cm; mean arch diameter was 3.0 ± 0.5 cm. See Table 1 for comparisons between subgroups of valve function.
Table 1:
Preoperative clinical and echocardiographic data
| Valve function | Age | Gender (M) | Sinuses (cm) | Sino-tubular (cm) | Ascending (cm) | Hypertension | Diabetes | COPD | CRF | |
|---|---|---|---|---|---|---|---|---|---|---|
| TAV (n = 87) | Normal (n = 36) | 66 ± 8 | 13 (36%) | 3.5 ± 0.4 | 3.4 ± 0.5 | 5.3 ± 0.6 | 29 (81%) | 2 (5.6%) | 7 (19%) | 2 (5.6%) |
| Stenosis (n = 12) | 61 ± 11 | 7 (58%) | 3.6 ± 0.6 | 3.4 ± 0.6 | 5.1 ± 0.5 | 8 (67%) | – | 2 (17%) | 1 (8.3%) | |
| Regurgitation (n = 39) | 66 ± 8 | 24 (61%) | 4.3 ± 0.7 | 4.2 ± 0.8 | 5.4 ± 0.9 | 31 (79%) | 2 (5.1%) | 11 (28%) | 5 (13%) | |
| P (TAV)a | 0.15 | 0.07 | <0.001* | <0.001* | 0.64 | 0.51 | 0.72 | 0.57 | 0.61 | |
| BAV (n = 69) | Normal (n = 10) | 51 ± 19 | 6 (60%) | 3.8 ± 0.3 | 3.7 ± 0.3 | 5.1 ± 0.4 | 5 (50%) | – | 2 (20%) | – |
| Stenosis (n = 37) | 60 ± 13 | 28 (76%) | 3.8 ± 0.9 | 3.4 ± 0.5 | 5.1 ± 0.6 | 17 (46%) | 3 (8.1%) | 6 (16%) | 1 (2.7%) | |
| Regurgitation (n = 22) | 54 ± 11 | 17 (77%) | 4.2 ± 0.6 | 3.9 ± 0.7 | 5.4 ± 1.3 | 14 (64%) | 4 (18%) | 3 (14%) | 2 (9.1%) | |
| P (BAV)a | 0.13 | 0.60 | 0.19 | 0.007* | 0.34 | 0.22 | 0.16 | 0.31 | 0.55 | |
| Overall | 62 ± 12 | (61%) | 3.9 ± 0.6 | 3.7 ± 0.7 | 5.3 ± 0.8 | 104 (67%) | 11 (7%) | 31 (20%) | 11 (7%) | |
| All TAV | 65 ± 9 | 44 (51%) | 3.9 ± 0.7 | 3.7 ± 0.8 | 5.3 ± 0.7 | 68 (78%) | 4 (4.6%) | 20 (23%) | 8 (9.2%) | |
| All BAV | 57 ± 14 | 51 (74%) | 3.9 ± 0.6 | 3.6 ± 0.6 | 5.2 ± 0.9 | 36 (52%) | 7 (10%) | 11 (16%) | 3 (4.3%) | |
| P (TAV versus BAV) | <0.001* | <0.001* | 0.002* | 0.65 | 0.33 | 0.43 | 0.001* | 0.21 | 0.11 | 0.22 |
All data are reported as count (percentage) or mean ± SD. BAV, bicuspid aortic valve; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; TAV, tricuspid aortic valve.
aANOVA test between valve function subgroups.
*indicates statistical significance.
Intraoperative variables
Significant differences were observed between BAV and TAV groups in terms of prevalence of stenosis, cross-clamp times (related to decalcification manoeuvres) and type of prostheses (Table 2).
Table 2:
Intra-operative variables
| Valve function | CPB (min) | X-clamp (min) | Prosthetic type (bio) | Sinus plication | MV operation | CABG | Redo | |
|---|---|---|---|---|---|---|---|---|
| TAV (n = 87) | Normal (n = 36) | 47 ± 10 | 20 ± 6 | – | – | – | – | 1 (2.8%) |
| Stenosis (n = 12) | 70 ± 21 | 43 ± 22 | 6 (50%) | 3 (25%) | 3 (25%) | 1 (8.3%) | – | |
| Regurgitation (n = 39) | 89 ± 40 | 50 ± 19 | 12 (31%) | 22 (56%) | 4 (10%) | 4 (10%) | 2 (5.1%) | |
| P (TAV)a | <0.001* | <0.001* | <0.001* | <0.001* | 0.015* | 0.12 | 0.84 | |
| BAV (n = 69) | Normal (n = 10) | 39 ± 11 | 17 ± 5 | – | – | – | 1 (10%) | – |
| Stenosis (n = 37) | 74 ± 18 | 48 ± 10 | 9 (24%) | 10 (27%) | 1 (2.7%) | 4 (11%) | 1 (2.7%) | |
| Regurgitation (n = 22) | 71 ± 10 | 46 ± 7 | 2 (9.1%) | 13 (59%) | – | – | 1 (4.5%) | |
| P (BAV)a | <0.001* | <0.001* | <0.001* | 0.002* | 0.57 | 0.27 | 0.68 | |
| Overall | 69 ± 29 | 39 ± 18 | 29 (19%) | 58 (37%) | 8 (5.1%) | 10 (6.4%) | 5 (3.2%) | |
| All TAV | 69 ± 35 | 37 ± 21 | 18 (21%) | 35 (40%) | 7 (8%) | 5 (5.7%) | 3 (3.4%) | |
| All BAV | 69 ± 19 | 43 ± 14 | 11 (16%) | 23 (33%) | 1 (1.4%) | 5 (7.2%) | 2 (2.9%) | |
| P (TAV versus BAV) | 0.97 | 0.04* | <0.001* | 0.60 | 0.04* | 0.99 | 0.85 |
All data are reported as count (percentage) or mean ± SD. AVR, aortic valve replacement; BAV, bicuspid aortic valve; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; MV, mitral valve; TAV, tricuspid aortic valve; X-clamp, aortic cross-clamp.
aANOVA test.
*indicates statistical significance.
Early postoperative outcomes
Hospital mortality was 1.9% (three patients). Causes of death included low cardiac output syndrome at weaning from the cardiopulmonary bypass in one patient, multiorgan failure in one, pneumonia in one. Complications occurring during hospital stay are reported in Table 3, also stratified for valve morphology and function. In none of the cases, postoperative complications (including bleeding) were found to be causally related with the WA procedure. Median amount of drainages was 512 ml (IQR 400–680) during first postoperative day, 210 ml (IQR 130–270) during the second day. Median intensive care unit (ICU) stay was 2 days (IQR 2–3), median postoperative hospital stay was 9 days (IQR 5–11). Postoperative reduction of aortic diameters was significant (overall 3.1 ± 0.3 versus 5.2 ± 0.8 cm; P < 0.001). No significant difference was observed in terms of mean post-procedural reduction in ascending diameter between TAV and BAV patients (−2.1 ± 0.6 versus −2.2 ± 0.9 cm; P = 0.29).
Table 3:
Early postoperative outcomes
| Valve function | Hospital death | Cardiac eventsb | Respiratory events | Neurological events | Renal failurec | Reoperation for bleeding | |
|---|---|---|---|---|---|---|---|
| TAV (n = 87) | Normal (n = 36) | 1 (2.8%) | – | 1 (2.9%) | – | – | 1 (2.9%) |
| Stenosis (n = 12) | – | 1 (8.3%) | – | – | – | 1 (8.3%) | |
| Regurgitation (n = 39) | 2 (5.1%) | 6 (15%) | 5 (13%) | 1 (2.6%) | 4 (10%) | 1 (2.6%) | |
| P (TAV)a | 0.35 | 0.04* | 0.19 | 0.86 | 0.02* | 0.64 | |
| BAV (n = 69) | Normal (n = 10) | – | – | – | – | – | – |
| Stenosis (n = 37) | – | 2 (5.4%) | 1 (2.7%) | – | 1 (2.7%) | – | |
| Regurgitation (n = 22) | – | 1 (4.5%) | 1 (4.5%) | – | 1 (4.5%) | – | |
| P (BAV)a | – | 0.84 | 0.77 | – | 0.56 | – | |
| Overall | 3 (1.9%) | ||||||
| All TAV | 3 (3.4%) | 7 (8%) | 6 (6.9%) | 1 (1.1%) | 4 (4.6%) | 3 (3.4%) | |
| All BAV | – | 3 (4.3%) | 2 (2.9%) | – | 2 (2.9%) | – | |
| P (TAV versus BAV) | 0.25 | 0.27 | 0.22 | 0.55 | 0.45 | 0.25 |
All data are reported as count (percentage). BAV, bicuspid aortic valve; TAV, tricuspid aortic valve.
aANOVA test.
bCardiac events included: atrial fibrillation (4, 2.6%), pericardial effusion (3, 1.9%), pace-maker implantation (2, 1.3%), low output (1, 0.6%).
cNeed for haemofiltration/dialysis.
*indicates statistical significance.
Clinical follow-up
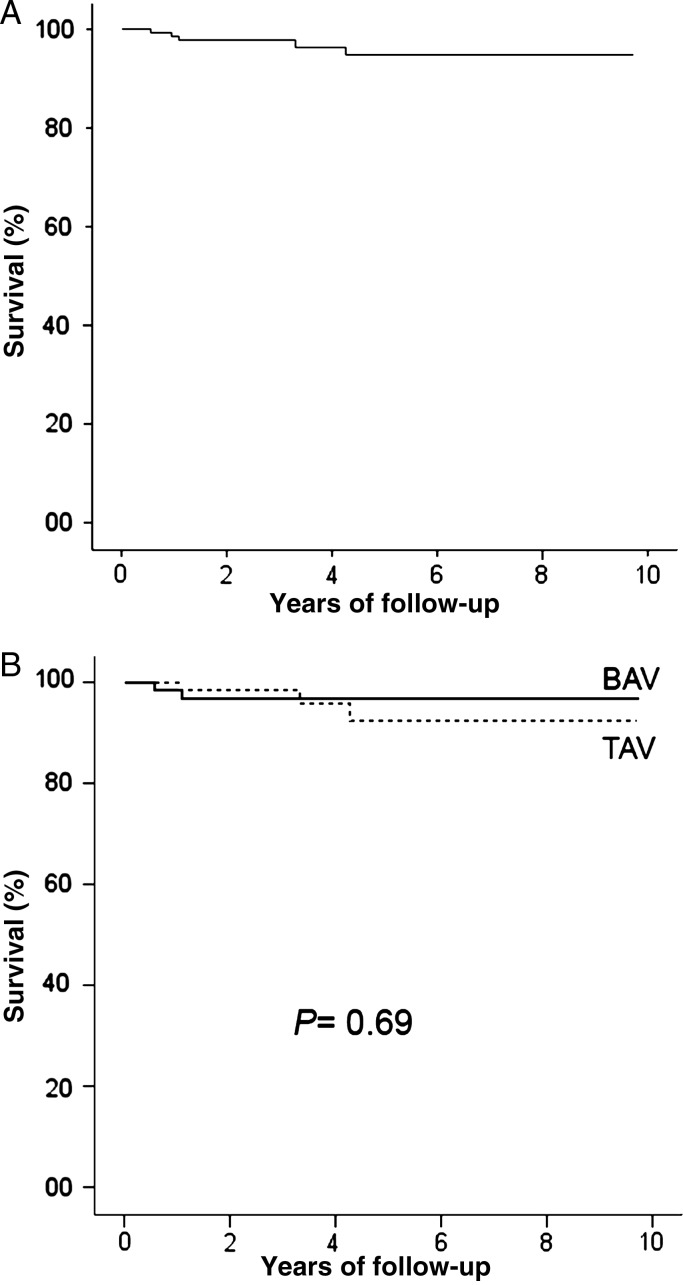
Clinical follow-up was 98.7% complete (151 patients). Mean follow-up time was 4 ± 2.5 years (30% patients had more than 5 years of follow-up). The Kaplan–Meier survival curve is presented in Fig. 2; 7-year survival was 95 ± 2%. There were five late deaths, considered cardiac-related in two cases: one sudden death, one acute myocardial infarction. Other causes were non-embolic non-haemorrhagic stroke (one patient), gastric cancer (one patient) and abdominal occlusion (one patient). Actuarial survival did not differ between TAV and BAV patients (Fig. 2).
Figure 2:
Actuarial survival following WA: (A) in the overall study group; (B) TAV versus BAV.
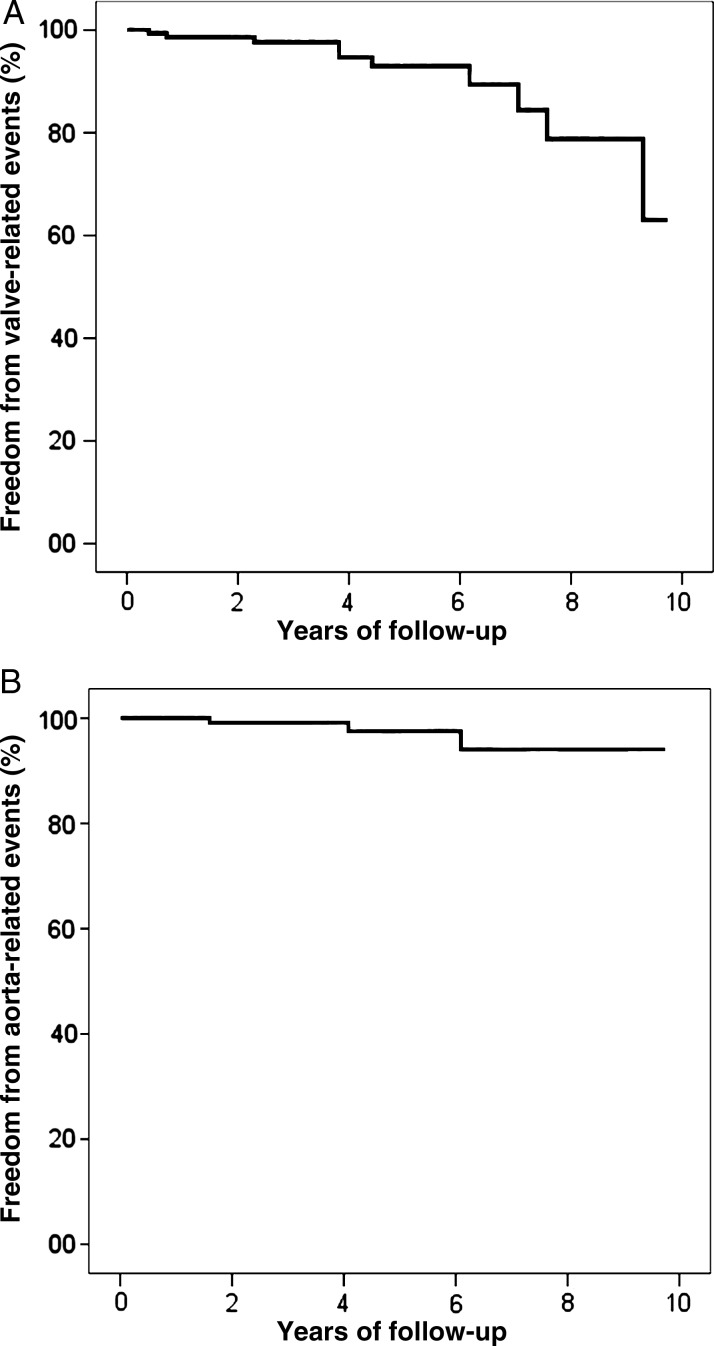
As regards valve-related events in the follow-up period: endocarditis occurred in four patients (linearized rate 0.7%/patient-year); thromboembolism in three TAV and two BAV patients (linearized rate 0.9%/patient-year); haemorrhage in one BAV patient (0.18%/patient-year). In two patients, undergoing reoperation for endocarditis, the aortic diameter had remained normal by the time of the redo procedure (Fig. 3).
Figure 3:

Specimens were taken from the tailored double-layer convexity of the ascending aorta in a patient undergoing aortic valve replacement 6 years following isolated WA. Histology showed no sign of medial degeneration and perfect fusion of the two overlapped wall layers (Elastic van Gieson staining, 100× magnification).
As far as aortic events are concerned: acute aortic type A dissection, with entry tear at the distal ascending level, occurred 6 years following WA and AVR in one regurgitant TAV patient (0.18%/patient-year), who was successfully re-operated; ascending aorta aneurysm recurrence was observed in two patients (one patient in the regurgitant BAV subgroup, successfully reoperated with Bentall technique 1.6 years postoperatively, and one patient in the regurgitant TAV subgroup, who refused reoperation in her 80s, 4 years postoperatively: linearized rate 0.36%/patient-year). Actuarial curves of freedom from valve- and aorta-related events are shown in Fig. 4. In particular, freedom from aortic complications at 7 and 10 years was 94 ± 4%.
Figure 4:
Freedom from valve- (A) and aorta-related (B) adverse events in the overall study population.
No predictor of aortic events was found by multivariable analysis.
Echocardiographic follow-up
Fifteen patients were excluded from any analysis of their change in aortic dimensions because of their echocardiographic follow-up time not reaching at least 1 year. In the resulting population (n = 136), there was no significant difference in follow-up length between TAV and BAV patients (3.3 ± 2 versus 3.9 ± 2 years; P = 0.13). As to the completeness of echocardiographic follow-up, it was 97.8% (three missing) for ascending tract diameter data, 80% (27 missing) for sinus-sinotubular diameters. The mean diameter at last follow-up (3.3 ± 0.5 cm in the overall population) was compared with postoperative as shown in Table 4. BAV patients with regurgitation at baseline had greater aortic dimensions at last follow-up compared to those with normal valve function or stenosis (P = 0.09 and P = 0.01 by post hoc test with Bonferroni correction).
Table 4:
Echocardiographic follow-up.
| Valve function | Postoperative sinus diameter (cm) | Postoperative STJ diameter (cm) | Postoperative ascending diameter (cm) | Follow-up sinus diameter (cm) | Follow-up STJ diameter (cm) | Follow-up ascending diameter (cm) | |
|---|---|---|---|---|---|---|---|
| TAV (n = 70) | Normal (n = 28) | 3.4 ± 0.5 | 3.3 ± 0.6 | 3.2 ± 0.2 | 3.5 ± 0.4 | 3.2 ± 0.5 | 3.5 ± 0.4 |
| Stenosis (n = 10) | 3.2 ± 0.4 | 3.0 ± 0.5 | 3.0 ± 0.3 | 3.6 ± 0.4 | 3.1 ± 0.6 | 3.1 ± 0.3 | |
| Regurgitation (n = 32) | 3.5 ± 0.7 | 3.3 ± 0.8 | 3.3 ± 0.4 | 3.6 ± 0.7 | 3.4 ± 0.6 | 3.4 ± 0.6 | |
| P (TAV)a | 0.49 | 0.38 | 0.12 | 0.82 | 0.47 | 0.06 | |
| BAV (n = 63) | Normal (n = 9) | 3.6 ± 0.4 | 3.4 ± 0.4 | 3.1 ± 0.6 | 3.7 ± 0.5 | 3.4 ± 0.3 | 3.3 ± 0.7 |
| Stenosis (n = 34) | 3.4 ± 0.4 | 3.0 ± 0.7 | 3.0 ± 0.3 | 3.4 ± 0.5 | 3.0 ± 0.6 | 3.1 ± 0.4 | |
| Regurgitation (n = 20) | 3.5 ± 0.4 | 3.1 ± 0.6 | 3.0 ± 0.4 | 3.7 ± 0.8 | 3.2 ± 0.3 | 3.6 ± 0.6 | |
| P (BAV)a | 0.20 | 0.45 | 0.81 | 0.25 | 0.38 | 0.017* | |
| Overall | 3.4 ± 0.5 | 3.2 ± 0.7 | 3.1 ± 0.4 | 3.6 ± 0.6 | 3.2 ± 0.6 | 3.3 ± 0.5 | |
| All TAV | 3.4 ± 0.5 | 3.3 ± 0.7 | 3.2 ± 0.4 | 3.6 ± 0.5 | 3.3 ± 0.6 | 3.4 ± 0.5 | |
| All BAV | 3.4 ± 0.4 | 3.1 ± 0.6 | 3.0 ± 0.5 | 3.5 ± 0.6 | 3.1 ± 0.5 | 3.2 ± 0.5 | |
| P (TAV versus BAV) | 0.62 | 0.68 | 0.12 | 0.99 | 0.22 | 0.17 |
All data are reported as mean ± SD. BAV, bicuspid aortic valve; STJ, sino-tubular junction; TAV, tricuspid aortic valve.
aANOVA test.
*indicates statistical significance.
Median velocity of diameter progression at the ascending level was 0.4 mm/year (IQR = 0–1.1 mm/year). In no patient was a growth rate ≥0.5 cm/year observed. Growth velocity was not significantly different in BAV versus TAV patients (0.37 mm/year, IQR = 0–0.97 versus 0.54 mm/year, IQR = 0–1.49; P = 0.21). However, interesting differences emerged when stratifying for baseline valve function. In the TAV group, when baseline valve function was normal, postoperative aortic growth was 1.2 mm/year (IQR = 0.5–1.7); 0 mm/year (IQR = 0–0.3) when the valve had been replaced for stenosis, 0.35 mm/year (IQR = 0–0.67) for regurgitation (P = 0.003). Differently, in the BAV group, patients with preoperative regurgitation showed a significantly faster aortic growth (1.3 mm/year, IQR = 0.3–2.7) compared to those with functionally normal valves (0.4 mm/year, IQR = 0–0.8) or with stenosis (0.2 mm/year, IQR = 0–0.5; P = 0.001). In the overall cohort, no significant predictor of diameter progression velocity emerged; however, in the TAV group, the only determinant was normal preoperative valve function (β = 0.76, P = 0.04) and in the BAV group, the preoperative degree of regurgitation (β = 0.44, P = 0.001).
DISCUSSION
RAA for ascending aortic aneurysms has long been a debated topic. Pivotal questions are still unanswered, including: (1) Is a conservative alternative needed within the surgical armamentarium for ascending aneurysms? (2) Which of the diverse proposed technical variants best combines safety and effectiveness? (3) Which are the correct indications (i.e. which type of patient shows the best long-term results)? [1, 2, 7]
The series described here provides further contribution to the abovementioned debate, representing the largest experience of unsupported RAA ever reported so far, with consistent indications throughout (asymmetric dilatation involving the mid-ascending tract, in non-syndromic non-familiar forms of aortopathy), and nearly full follow-up completeness.
The results presented here conform with previous reports on different RAA techniques showing low rates of early postoperative complications [9–12] and short ICU and hospital stay periods [19]. Supporters of the RAA techniques emphasize that the presence of a tubular prosthesis at the ascending level induces the loss of the normal Windkessel function of the aorta, i.e. the capability of the native aortic wall of storing energy from the blood flow during systole and releasing it during diastole [9], potentially causing overstress and dilatation of the sinuses and even impairing ventricular performance [19, 20]. However, this is an argument against RAAs with external reinforcement by Dacron wrapping as well [9]. Moreover, wrapping with Dacron fabric suppresses the rationale of not placing any material prone to infection inside the mediastinum. The WA technique does not imply any use of Dacron, inasmuch as it is selectively indicated for asymmetric forms of aneurysms, whose most dilated, stressed and diseased portion is the convexity [14–16, 21, 22]: therefore, it provides a tailored autologous reinforcement, not circumferential, but limited to the repaired convexity. Avoidance of Dacron wrapping also prevents the previously reported phenomena of under-the-wrap erosion of the ascending wall, possibly occurring with or without distal dislocation of the wrap [1, 23]. Concomitantly, the double-suture technique allows for tension relief from the main closure suture, which otherwise would represent a site of possible acute disruption. Of note, the second suture of WA is not passed full-thickness through the aortic wall, but through the adventitial layer only, so as to avoid creating further source of bleeding or site of weakness. However, it must be admitted that this was not a comparative study, therefore it could only show excellent results with WA, but not its superiority versus graft replacement or the other previously proposed RAA variants.
As regards the long-term follow-up, no significant factor emerged from regression models predicting redilatation/dissection, probably because of the very low number of events. However, the predictors of faster growth of the ascending aorta were identified: the degree of regurgitation for BAV patients and the normal aortic valve function for TAV subjects. Patients with these features may have an increased risk of redilatation, provided a follow-up time lasting enough. Notably, the observed median growth rate (0.4 mm/year) was very low, and consistent with another recent report [18], and in no patient did it reach the 5 mm/year threshold for reoperation according to current recommendations. The low aortic growth rates observed may also suggest that, at least in the aortic stenosis subgroups, follow-up imaging controls could be safely performed at longer intervals than currently recommended.
Preoperative regurgitation has been already pointed out as associated to worse outcome of unsupported RAA [1], suggesting the hypothesis that regurgitation may be associated with particular aortic wall fragility. In the present study, all three aortic events (one dissection, two recurrences) occurred in patients with aortic regurgitation at baseline; however, regurgitant TAV patients did not show any significant difference in aortic growth rate compared to those with stenotic TAV. Conversely, while neither BAV nor aortic regurgitation were risk factors per se for redilatation following WA, BAV insufficiency resulted to be a marker of more insidious, probably genetically caused, aortopathy (median progression rate of 1.3 mm/year), also in accordance with previously drawn theories [1, 17, 22].
Although the number of normofunctional BAV patients was quite small, the finding of similar growth rates and no recurrence in the normally functioning BAV as in the stenotic BAV subset is consistent with the hypothesis, supported by both anatomo-clinical [16, 17] and biomechanical evidence [21], that the so called normofunctional BAV is intrinsically stenotic: haemodynamics may have a major pathogenetic role in the asymmetric dilatations with non-regurgitant BAV, as the presence of a BAV is enough to cause increased wall stress at the convexity [21, 22, 24].
Previous analyses identified larger post-reduction [13] aortic diameter as associated with aneurysm recurrence: this factor did not result to be a determinant of faster growth rate in the present study. In the work by Bauer et al. [13], including 115 BAV patients, nine subjects presenting redilatation following unsupported RAA had a mean postoperative diameter of 4.1 cm; conversely, in none of the BAV patients in the present series, the post-reduction diameter exceeded 3.9 cm. According to the literature, it is crucial to reduce the aorta to a postoperative diameter ≤3.5 cm [1, 3, 13], which was nearly constantly achieved by WA in part through the resection and in part by adequately tailoring the autologous flap.
Preoperative diameter >5.5 cm was found by Polvani et al. [25] to predict redilatation following unsupported RAA: notably, with the autologous reinforcement, neither there was significant difference in aortic events nor in the aortic growth between patients with preoperative diameter ≤5.5 cm or >5.5 cm (0.40 versus 0.37 mm/year; P = 0.41). Therefore, aneurysms greater than 5.5 cm seem to be not a correct contraindication, at least in the setting of non-syndromic non-familial asymmetrical aneurysms treated by WA.
LIMITATIONS
The present study was limited by its descriptive nature and an effort would be necessary, as advocated by Sievers 7 years ago [2], to gather prospective experiences from multiple centres in a randomized comparison with the conventional treatment modalities. The present study results would suggest carefully selecting cohorts for such trials, taking into account the configuration of the aorta and importantly the morpho-functional status of the aortic valve, to have comparable data.
Moreover, it must be acknowledged that patients were not all centrally followed-up: for some of them the last echocardiography report performed elsewhere less than 6 months before was accepted. As a consequence, the analysis of aortic growth rate focused on the ascending tubular tract only, because bi-dimensional measurements of the other levels were not always available. However, the tubular tract is the level where dilatation is maximal in asymmetric aneurysms and where the greatest size reduction occurs with WA.
CONCLUSIONS
The present study showed that WA is a viable and durable option when a non-syndromic non-familial aneurysm has asymmetrical configuration, involving the convexity. The BAV does not represent a contraindication to this technique of RAA: the most appropriate candidates to WA proved to be those with BAV and TAV stenosis, followed by normofunctional BAV and regurgitant TAV. Future studies should aim to find more refined markers of the risk for rapid aortic growth and aneurysm recurrence.
Conflict of interest: none declared.
REFERENCES
- 1.Robicsek F, Cook JW, Reames MK, Sr, Skipper ER. Size reduction ascending aortoplasty: is it dead or alive? J Thorac CardioVasc Surg. 2004;128:562–70. doi: 10.1016/j.jtcvs.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Sievers HH. Reflections on reduction ascending aortoplasty's liveliness. J Thorac CardioVasc Surg. 2004;128:499–501. doi: 10.1016/j.jtcvs.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Gill M, Dunning J. Is reduction aortoplasty (with or without external wrap) an acceptable alternative to replacement of the dilated ascending aorta? Interact CardioVasc Thorac Surg. 2009;9:693–7. doi: 10.1510/icvts.2009.213405. [DOI] [PubMed] [Google Scholar]
- 4.Robicsek F, Thubrikar MJ. Conservative operation in the management of annular dilatation and ascending aortic aneurysm. Ann Thorac Surg. 1994;57:1672–4. doi: 10.1016/0003-4975(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 5.Harrison LH, Jr, Heck HA., Jr Shawl lapel aortoplasty. Ann Thorac Surg. 1996;62:1867. doi: 10.1016/s0003-4975(96)00686-8. [DOI] [PubMed] [Google Scholar]
- 6.Cotrufo M, Della Corte A, De Santo LS, De Feo M, Covino FE, Dialetto G. Asymmetric medial degeneration of the ascending aorta in aortic valve disease: a pilot study of surgical management. J Heart Valve Dis. 2003;12:127–33. [PubMed] [Google Scholar]
- 7.Sundt T., III Invited commentary on: Walker T, Bail DH, Gruler M, Vonthein R, Steger V, Ziemer G. Unsupported reduction ascending aortoplasty: fate of diameter and of Windkessel function. Ann Thorac Surg. 2007;83:1053–4. doi: 10.1016/j.athoracsur.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Ogus NT, Ciçek S, Isik O. Selective management of high risk patients with an ascending aortic dilatation during aortic valve replacement. J CardioVasc Surg (Torino) 2002;43:609–15. [PubMed] [Google Scholar]
- 9.Walker T, Bail DH, Gruler M, Vonthein R, Steger V, Ziemer G. Unsupported reduction ascending aortoplasty: fate of diameter and of Windkessel function. Ann Thorac Surg. 2007;83:1047–53. doi: 10.1016/j.athoracsur.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Arsan S, Akgun S, Kurtoglu N, Yildirim T, Tekinsoy B. Reduction aortoplasty and external wrapping for moderately sized tubular ascending aortic aneurysm with concomitant operations. Ann Thorac Surg. 2004;78:858–61. doi: 10.1016/j.athoracsur.2004.03.101. [DOI] [PubMed] [Google Scholar]
- 11.Conaglen P, Luthra S, Skillington P. Comparison of reduction ascending aortoplasty and ascending aortic replacement for bicuspid valve related aortopathy in young adult patients undergoing aortic valve replacement: long-term follow-up. Heart Lung Circ. 2009;18:337–42. doi: 10.1016/j.hlc.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Kuralay E, Demirkilic U, Ozal E, Oz BS, Cingöz F, Günay C, et al. Surgical approach to ascending aorta in bicuspid aortic valve. J Card Surg. 2003;18:173–80. doi: 10.1046/j.1540-8191.2003.02025.x. [DOI] [PubMed] [Google Scholar]
- 13.Bauer M, Pasic M, Schaffarzyk R, Siniawski H, Knollmann F, Meyer R, et al. Reduction aortoplasty for dilatation of the ascending aorta in patients with bicuspid aortic valve. Ann Thorac Surg. 2002;73:720–3. doi: 10.1016/s0003-4975(01)03455-5. [DOI] [PubMed] [Google Scholar]
- 14.Cotrufo M, De Santo LS, Esposito S, Renzulli A, Della Corte A, De Feo M, et al. Asymmetric medial degeneration of the intrapericardial aorta in aortic valve disease. Int J Cardiol. 2001;81:37–41. doi: 10.1016/s0167-5273(01)00526-5. [DOI] [PubMed] [Google Scholar]
- 15.Bauer M, Gliech V, Siniawski H, Hetzer R. Configuration of the ascending aorta in patients with bicuspid and tricuspid aortic valve disease undergoing aortic valve replacement with or without reduction aortoplasty. J Heart Valve Dis. 2006;15:594–600. [PubMed] [Google Scholar]
- 16.Cotrufo M, Della Corte A. The association of bicuspid aortic valve disease with asymmetric dilatation of the tubular ascending aorta: identification of a definite syndrome. J CardioVasc Med (Hagerstown) 2009;10:291–7. doi: 10.2459/JCM.0b013e3283217e29. [DOI] [PubMed] [Google Scholar]
- 17.Della Corte A, Bancone C, Quarto C, Dialetto G, Covino FE, Scardone M, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007;31:397–404. doi: 10.1016/j.ejcts.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Hwang HY, Shim MS, Park EA, Ahn H. Reduction aortoplasty for the ascending aortic aneurysm with aortic valve disease. Does bicuspid valve matter? Circ J. 2011;75:322–8. doi: 10.1253/circj.cj-10-0792. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Lu F, Qu D, Han L, Xu J, Ji G, et al. Treatment of fusiform ascending aortic aneurysms: a comparative study with 2 options. J Thorac CardioVasc Surg. 2011;141:738–43. doi: 10.1016/j.jtcvs.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, Hinkamp TJ, Jacobs WR, Lichtenberg RC, Posniak H, Pifarre R. Effect of an inelastic aortic synthetic vascular graft on exercise hemodynamics. Ann Thorac Surg. 1995;59:981–9. doi: 10.1016/0003-4975(95)00068-v. [DOI] [PubMed] [Google Scholar]
- 21.Conti CA, Della Corte A, Votta E, Del Viscovo L, Bancone C, De Santo LS, et al. Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data. J Thorac CardioVasc Surg. 2010;140:890–6. doi: 10.1016/j.jtcvs.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011;39:809–14. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Bauer M, Grauhan O, Hetzer R. Dislocated wrap after previous reduction aortoplasty causes erosion of the ascending aorta. Ann Thorac Surg. 2003;75:583–4. doi: 10.1016/s0003-4975(02)04338-2. [DOI] [PubMed] [Google Scholar]
- 24.Barker AJ, Lanning C, Shandas R. Quantification of haemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann Biomed Eng. 2010;38:788–800. doi: 10.1007/s10439-009-9854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polvani G, Barilli F, Topkara VK, Cheema FH, Penza E, Ferrarese S, et al. Reduction ascending aortoplasty: midterm follow-up and predictors of redilatation. Ann Thorac Surg. 2006;82:586–91. doi: 10.1016/j.athoracsur.2006.03.025. [DOI] [PubMed] [Google Scholar]