Abstract
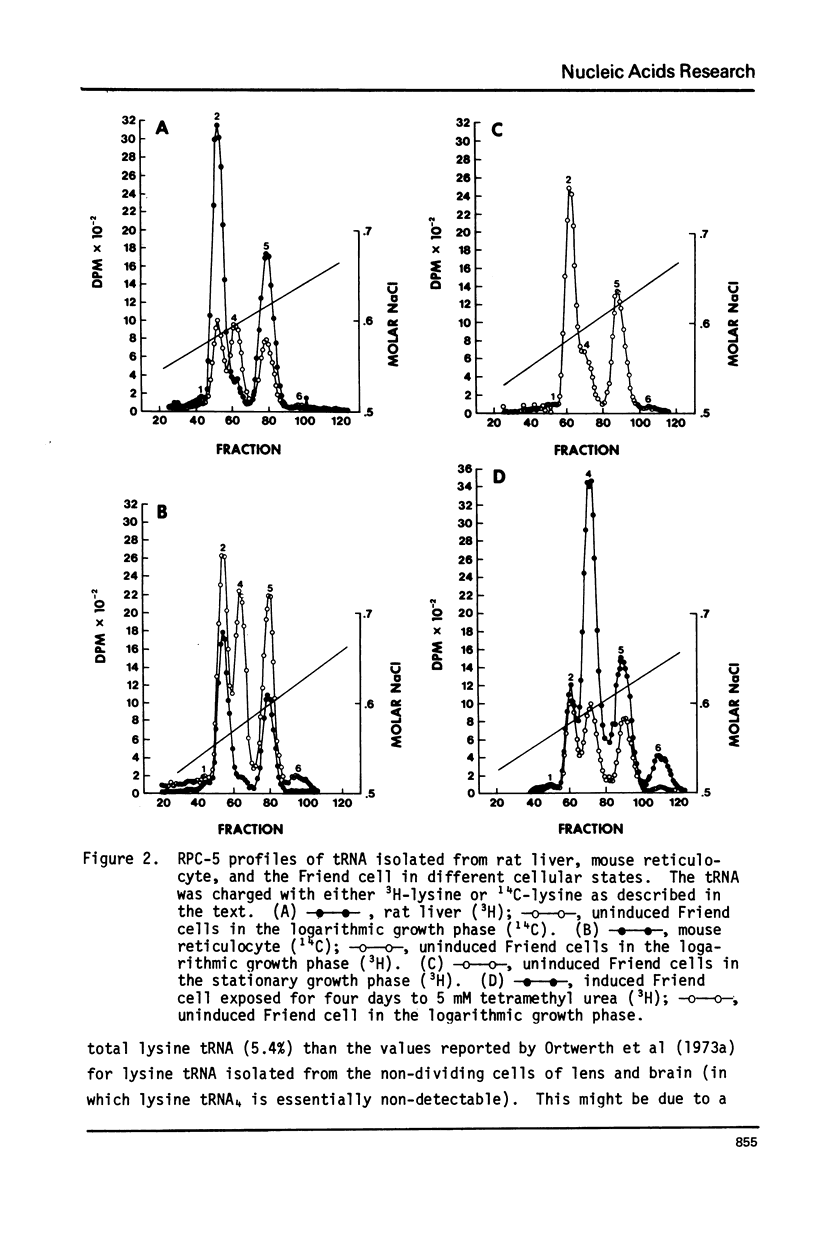
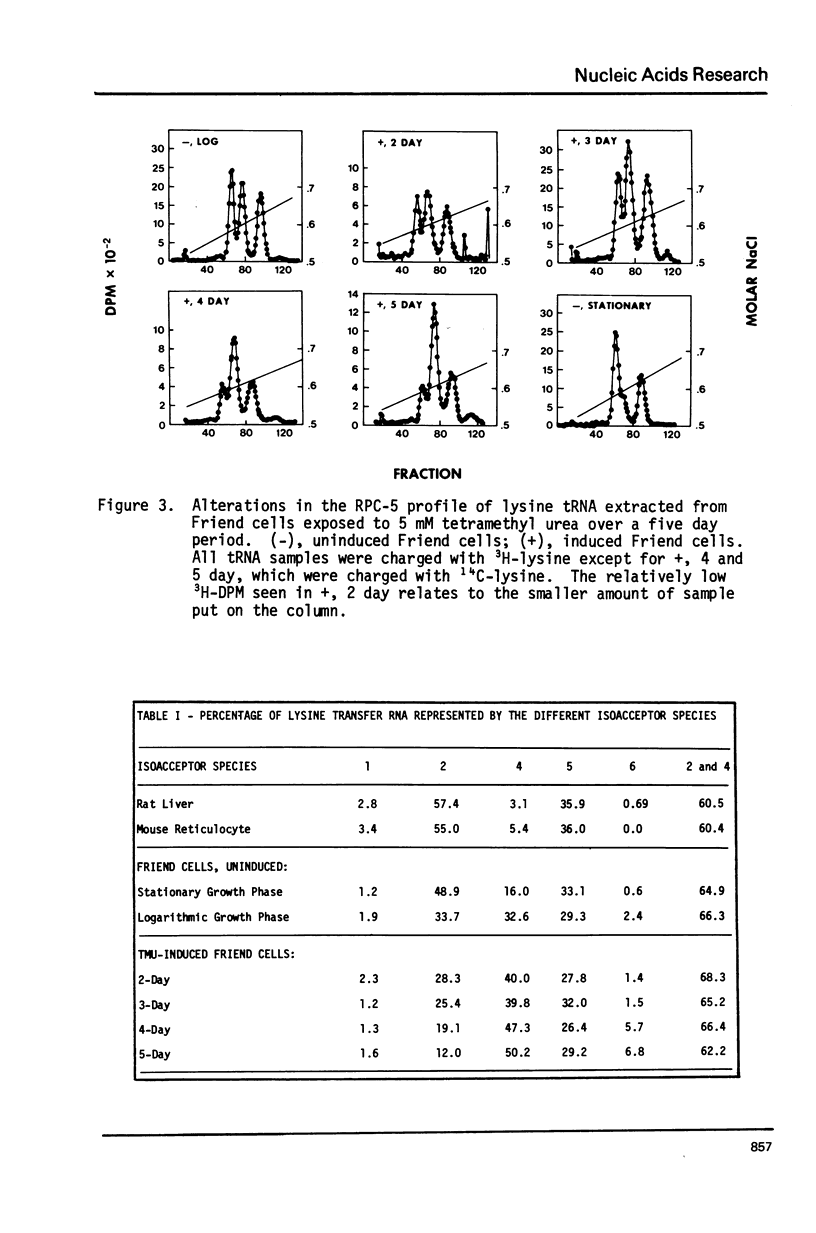
The proportion of lysine tRNA represented by the isoacceptor species lysine tRNA4 has previously been shown to be largest in cells with the greatest ability to proliferate. Using reverse phase chromatography (RPC-5), we have analyzed the changes in the relative quantities of lysine tRNA species which occur in different cellular states of the Friend cell, a transformed murine cell infected with Friend erythroleukemia virus complex. This cell undergoes erythroid differentiation when exposed to various chemicals. Lysine tRNA4 comprises 32% of the total lysine tRNA in rapidly dividing, uninduced Friend cells, but only 16% of the total lysine tRNA in uninducase. Friend cells undergoing erythroid differentiation divide more slowly than uninduced cells, and finally cease proliferation, but lysine tRNA4 becomes the major lysine tRNA species (greater than 50%). This does not appear to reflect erythroid properties of the cell, since the lysine tRNA of the mouse reticulocyte contains very little lysine tRNA4. The non-dividing erythroid Friend cell, therefore, represents an exception to the finding that non-dividing cells usually have little or no lysine tRNA4 present.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conkie D., Kleiman L., Harrison P. R., Paul J. Increase in the accumulation of globin mRNA in immature erythroblasts in response to erythropoietin in vivo or in vitro. Exp Cell Res. 1975 Jul;93(2):315–324. doi: 10.1016/0014-4827(75)90456-5. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Pragnell I. B., Kluge N., Gaedicke G., Steinheider G., Ostertag W. Induction of endogenous and of spleen focus-forming viruses during dimethylsulfoxide-induced differentiation of mouse erythroleukemia cells transformed by spleen focus-forming virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1863–1867. doi: 10.1073/pnas.72.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Juarez H., Juarez D., Hedgcoth C., Ortwerth B. J. Amounts of isoaccepting lysine tRNAs change with the proliferative state of cells. Nature. 1975 Mar 27;254(5498):359–360. doi: 10.1038/254359a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortwerth B. J. Isoaccepting transfer ribonucleic acids in specialized mammalian tissues. Biochemistry. 1971 Nov;10(23):4190–4197. doi: 10.1021/bi00799a006. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Pragnell I. B., Ostertag W., Paul J. The expression of viral and globin genes during differentiation of the Friend cell. Exp Cell Res. 1977 Sep;108(2):269–278. doi: 10.1016/s0014-4827(77)80034-7. [DOI] [PubMed] [Google Scholar]
- Preisler H. D., Christoff G., Taylor E. Cryoprotective agents as inducers of erythroleukemic cell differentiation in vitro. Blood. 1976 Mar;47(3):363–368. [PubMed] [Google Scholar]
- Sherton C. C., Evans L. H., Polonoff E., Kabat D. Relationship of Friend murine leukemia virus production to growth and hemoglobin synthesis in cultured erythroleukemia cells. J Virol. 1976 Jul;19(1):118–125. doi: 10.1128/jvi.19.1.118-125.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Ho T., Yang W. K. Ability of tryptophan tRNA to hybridize with 35S RNA of avian myeloblastosis virus and to prime reverse transcription in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2155–2159. doi: 10.1073/pnas.72.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Hellman A., Martin D. H., Hellman K. B., Novelli G. D. Isoaccepting transfer RNA's of L-M cells in culture and after tumor induction in C3H mice. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1411–1418. doi: 10.1073/pnas.64.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]


