Abstract
We present a rare case of left ventricular myxoma discovered incidentally in an asymptomatic 16-year old male. The patient underwent the appropriate work-up and a robotic-assisted excision of the mass. The patient had an uneventful recovery and was discharged home at postoperative day 3. To our knowledge, this is the first case of robotic-assisted left ventricular myxoma excision in the literature. Robotic-assisted surgery of left ventricular myxomas is a safe and feasible method of excision.
Keywords: Robotic surgery, Myxoma, Robotic-assisted cardiac surgery
INTRODUCTION
Primary cardiac tumours are rare; the majority of which are myxomas. The majority of myxomas are in the left atrium (75%) followed by the right atrium (20%). In rare cases, myxomas can be found in the ventricles, and has been reported at a rate of 2.5% in the left ventricle [1].
Clinical features of myxoma are determined by their location, size and mobility [1]. The most common symptoms include embolism, intracardiac obstruction and constitutional symptoms. Occasionally, patients are asymptomatic [1]. Electrocardiographic findings are non-specific. Chest X-rays may demonstrate an alteration of the cardiac contour or enlargement of a cardiac chamber or signs of pulmonary hypertension and congestion [1]. Coronary angiography may demonstrate opacification of an arterial branch ending in a tumour ‘blush’ [1, 2]. Diagnosis is primarily by transoesophageal echocardiogram (TEE) or magnetic resonance imaging (MRI), and treatment is surgical excision. We present a rare case of left ventricular myxoma which was excised with robotic assistance.
CASE REPORT
We present a case of a 16-year old male with an incidental finding of an asymptomatic left ventricular apical myxoma. The patient had been routinely followed by echocardiogram for congenital pulmonary stenosis which was not apparent on the most recent study. The myxoma was further evaluated by an MRI, after which he was referred to our service. He has a history of tonsillectomy, osteochondritis dissecans and migraines. There was no history of tobacco or alcohol abuse and the physical examination was normal.
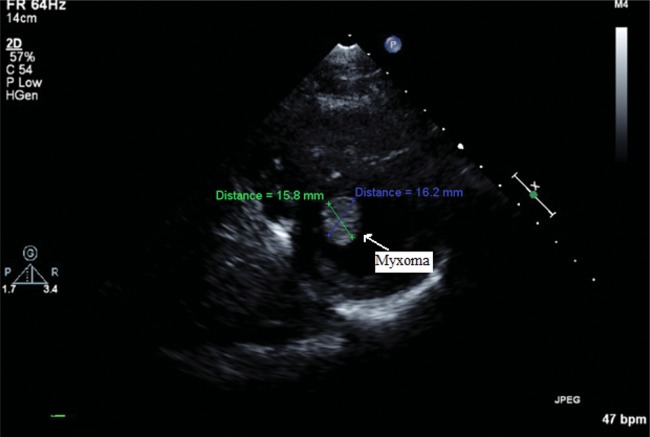
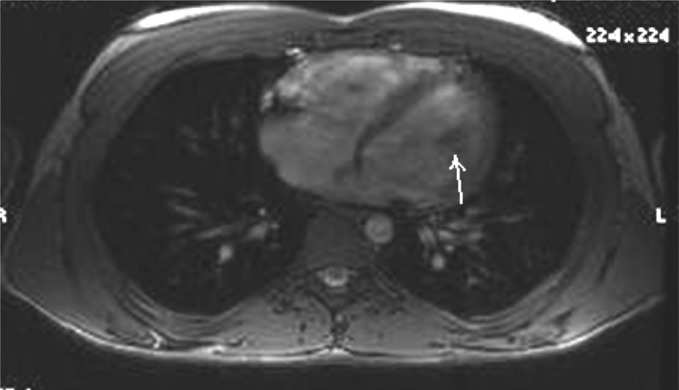
On review of the echocardiogram, there was a 15 × 16 mm mobile mass in the left ventricular apex, which was not adherent to the ventricular wall (see Fig 1). This was confirmed by MRI (Fig 2). Preoperative labs and CT angiogram of the chest, abdomen and pelvis were obtained. The aorta extending down to the femoral vessels was free of disease, and was suitable for femoral vessel cannulation. No intervention was needed for the pulmonary valve.
Figure 1:
Echocardiogram demonstrating a left ventricular mass.
Figure 2:
MRI with left ventricular mass (arrow).
In the operating room, after double-lumen intubation, bilateral radial arterial lines were inserted. The right common femoral vessels were dissected for bypass and the port sites were made in the right lateral chest wall. The patient was heparinized and a 25 Fr Quickdraw venous cannula (Edwards LifeSciences, CA, USA) was inserted over guidewire and confirmed to be in the cavoatrial junction by TEE. A 23 Fr EndoReturn arterial cannula (Edwards LifeSciences) was then inserted in the common femoral artery followed by insertion of the EndoClamp (Edwards LifeSciences) through the side arm and positioned in the ascending aorta by TEE. The da Vinci system (Intuitive Surgical, CA, USA) was docked and cardiopulmonary bypass was started. The pericardium was opened with direct visualization of the phrenic nerve. The EndoClamp was then inflated and the heart was arrested with a 2 l dose of cardioplegia which was the only dose given during the surgery. The left atrium was then opened and the robotic fourth arm retractor was positioned through the mitral valve to expose the left ventricle. The mass was easily visualized and there was no difficulty in identifying the stalk. The mass was attached by filamentous chords to the ventricular wall. Those chords were excised, including the base of the stalks, and the specimen was retrieved using an EndoPouch. The left atrium was then closed. A dose of warm blood (800 ml hot shot) was then given, and the EndoClamp was deflated after thorough de-airing. A 19 Blake drain was left in the pericardium and a 32 Fr chest tube was left in the right pleural space. Cross-clamp time was 25 min, while cardiopulmonary bypass time was 56 min. The recovery was uneventful and he was discharged at postoperative day 3. The pathology report confirmed a cardiac myxoma. The patient was scheduled for a follow-up echocardiogram to rule out recurrence.
DISCUSSION
Cardiac myxoma is a benign neoplasm of endocardial origin affecting primarily ages 11–82 years with a female predominance [1]. The tumour projects from the endocardium to the cardiac chamber. Myxomas are sporadic in 86% of the cases and familial in 7% of the cases. Familial cases have atypical features such as multicentricity, atypical location, recurrence after excision (12–22%) and association with Carney complex (20%) [1]. The growth rate of myxomas has been cited as reaching 0.15 cm per month [1]. Right atrial and ventricular myxomas tend to be multicentric.
During surgical removal, every effort should be made to avoid a left ventriculotomy and its potential complications [3]. Video-assisted exposure and excisions have been reported by going through an aortotomy and the aortic valve [3]. The removal via a small right submammary incision has also been described [3, 4, 5]. Robotic-assisted excision of a left ventricular thrombus has also been described [4]. The dynamic robotic atrial retractor greatly enhances exposure and visualization deep in the left ventricle. The magnification provided by the robotic optical system also affords a detailed view of the left ventricular apex. Even though the operation is carried out under a confined space, the robotic instruments allow ease of manoeuvrability. We present a patient with a left ventricular myxoma which was successfully excised with robotic assistance with a short cross-clamping time and hospital stay. In the reported case, the robot afforded great visualization and ease of resection. Robotic-assisted myxoma excision of the left ventricle is a feasible and safe approach and, to our knowledge, one which has not been previously described in the literature.
Conflict of interest: none declared.
REFERENCES
- 1.Rendón F, Agosti J, Llorente A, Rodrigo D, Montes K. Intramural cardiac myxoma in left ventricular wall: an unusual location. Asian CardioVasc Thorac Ann. 2002;10:170–2. doi: 10.1177/021849230201000220. [DOI] [PubMed] [Google Scholar]
- 2.Singh RN, Burkholder JA, Magovern GJ. Coronary arteriography as an aid in left atrial myxoma diagnosis. CardioVasc Intervent Radiol. 1984;7:40–3. doi: 10.1007/BF02552676. doi:10.1007/BF02552676. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko Y, Kobayashia J, Saitoh F, Ono M. Thoracoscopic removal of a papillary fibroelastoma in the left ventricular apex. Interact CardioVasc Thorac Surg. 2006;5:640–2. doi: 10.1510/icvts.2006.131326. doi:10.1510/icvts.2006.131326. [DOI] [PubMed] [Google Scholar]
- 4.Lutz CJ, Bhamidipati CM, Ford B, Swartz M, Hauser M, Kyobe M, et al. Robotic-assisted excision of a left ventricular thrombus. Innovations. 2007;2:251–3. doi: 10.1097/IMI.0b013e31815cea73. [DOI] [PubMed] [Google Scholar]
- 5.Gulbins H, Reichenspurner H, Wintersperger BJ, Reichart B. Minimally invasive extirpation of a left-ventricular myxoma. Thorac CardioVasc Surg. 1999;47:129–30. doi: 10.1055/s-2007-1013126. doi:10.1055/s-2007-1013126. [DOI] [PubMed] [Google Scholar]