Abstract
The purpose of this study was to determine whether a clampless facilitating device (CFD) to perform proximal aortocoronary anastomoses would result in a lower incidence of cerebral embolic events compared with a partial clamping strategy during off-pump coronary artery bypass (OPCAB). After epiaortic ultrasound confirmed the mild aortic disease (Grades I and II), 57 patients were randomly assigned to have proximal anastomoses using a partial-occluding clamp (CL, n = 28) or a CFD [Heartstring (HS), n = 29] (Maquet Cardiovascular LLC, San Jose, CA). Solid and gaseous emboli in the middle cerebral arteries were detected using transcranial Doppler ultrasonography. The mean number of proximal anastomoses was similar between groups 1.93 ± 0.72 (CL) and 1.72 ± 0.70 (HS) (P = 0.28). The mean number of gaseous plus solid emboli was greater in the CL group than the HS group (90.0 ± 64.0 vs. 50.8 ± 36.6, P = 0.01). Emboli were fewest in patients undergoing HS anastomoses using the suction device. The number of intraoperative cerebral emboli was proportional to the number of proximal anastomoses in the HS groups, but independent of the number of proximal anastomoses in the CL groups. Among patients with a low burden of aortic atherosclerosis, partial clamping of the ascending aorta during OPCAB was associated with more cerebral embolic events compared with an anastomosis with a CFD.
Keywords: Coronary artery bypass grafting, Off-pump coronary artery bypass, Stroke, Neurologic events
INTRODUCTION
Intraoperative cerebral microemboli are associated with the development of post-operative stroke and neurocognitive decline in patients undergoing coronary artery bypass grafting (CABG) [1]. Atherosclerosis of the ascending aorta has been identified as one of the most important sources of emboli production after CABG. Manipulation of the atherosclerotic aorta by cannulation and clamping can be associated with intraoperative embolization into the cerebral circulation, resulting in persistent cognitive deficit or post-operative stroke [2]. Although aortic manipulation associated with cardiopulmonary bypass is responsible for the generation of cerebral emboli, the elimination of cardiopulmonary bypass alone has not been conclusively shown to improve the long-term neurologic outcomes [1].
The purpose of this study was to evaluate the intraoperative cerebral embolic events in patients undergoing off-pump coronary artery bypass (OPCAB) with proximal anastomoses performed with partial clamping compared with OPCAB patients with proximal anastomoses performed with clampless facilitating devices [CFDs; Heartstring (HS), Maquet Cardiovascular LLC].
METHODS
Patients
This study was conducted in compliance with the Health Insurance Portability and Accountability Act regulations. The Emory University Institutional Review Board approved the study. Patients older than 18 years treated at Emory University Midtown Hospital who were referred for CABG of one or more vessels and eligible for the OPCAB procedure were screened. Written informed consent was obtained from each patient according to the Helsinki declaration after the nature and purpose of the clinical trial were explained by the attending surgeon and the experienced research personnel. No patient was enrolled in any competing clinical study. Exclusion criteria included acute myocardial infarction within 24 h of planned surgery, urgent or emergent surgery, documented the presence of acute or suspected systemic infection, life expectancy of <1 year, recent (<4 weeks) history of cerebrovascular accident, inability to meet study requirements (travel and general health) and pregnancy. Screened patients had to meet the operative inclusion criteria of grade I or II ascending aorta (Table 1), as determined by intraoperative epiaortic ultrasound. A CONSORT 2010 checklist was utilized for this randomized trial. All study patients in all arms underwent the follow-up evaluations at 5 ± 1 weeks after surgery.
Table 1:
Epiaortic ultrasound grading system used at Emory University
| Epiaortic ultrasound grade of ascending aorta | Intimal thickness/severity of disease |
|---|---|
| 1 | Normal (<2 mm) |
| 2 | Mild (2–3 mm) |
| 3 | Moderate (3–5 mm) |
| 4 | Severe (>5 mm) |
| 5 | Mobile plaque, any thickness |
Sample
Following the screening and verification of eligibility criteria, each patient was randomized to one of four groups: (1) HS/suction, (2) HS/CO2 blower, (3) partial clamp (CL)/suction and (4) partial CL/CO2 blower. Three patients were excluded from the study after randomization; one converted intraoperatively to cardiopulmonary bypass, one had technical difficulties with the transcranial Doppler (TCD) machine and one required emergency CABG after initial enrolment. Patients were enrolled in this trial from 17 March 2009 to 12 November 2009.
Epiaortic ultrasound scanning
Epiaortic ultrasound scanning is a rapid, non-invasive and sensitive technique that provides information about the ascending aortic wall in its entire length and circumference. The ascending aorta was scanned directly by the surgeon using an ultrasound probe connected to an echocardiography ultrasound scanner. The probe uses 8.5 MHz in a linear array fashion and is placed inside a sterile sleeve filled with saline to act as a medium between the probe and the surface of the aorta.
Interventions
OPCAB was performed via median sternotomy utilizing commercially available positioners and stabilizers for performing coronary anastomoses. All incisions and closure techniques were the same for all four treatment arms. The HS device (Maquet Cardiovascular LLC) allows for the construction of proximal aortocoronary anastomoses without the use of a partial-occluding CL. The punch device creates the aortotomy. The cup is then inserted into the aortotomy and then deployed which creates a seal to provide a relatively bloodless field during the construction of the proximal anastomosis. Once completed, the cup can be unwound and removed from the aortotomy and the suture can then be tied. Anastomoses created with a partial CL or a HS device are both performed manually using a 5-0 or 6-0 Surgipro suture.
Measurements
Intraoperative TCD monitoring was performed in each patient using QL software and Doppler Box (Compumedics, Germany) with the ability to distinguish between gaseous and solid emboli. Two 2.5 MHz probes are placed on each patient, fixed on a customized headset and connected to the Doppler Box machine to detect the Doppler signals from the middle cerebral arteries bilaterally with a sample volume of 10–14 mm3 and an average depth of 55 mm used as initial setting. Various stages of the operation are examined and recorded with particular attention devoted to the time of clamping, anastomosis construction and unclamping in cases performed with the partial CL. Similarly, special attention was paid to the time of aortotomy, device deployment, anastomosis construction and string removal in cases performed with the HS device. These signals were recorded continuously on a dedicated hard drive for off-line analysis. The number of gaseous and solid emboli (high-intensity transient signals, HITS) that were present throughout each case was counted and totalled for each patient.
Study design
This study was a randomized, complete factorial experiment designed to evaluate the effect of two treatment factors—the presence of partial clamping and the type of device used to clear the proximal anastomotic field of blood—on the number of solid and gaseous emboli emitted during the construction of proximal anastomoses during OPCAB. The randomization scheme allocated 60 patients into four groups (15 per group) based on the combination of the two factors (Fig. 1). Randomization assignments were prepared in secured randomization envelopes using a four-block randomization scheme. The experienced clinical research personnel generated the random allocation sequence, enrolled participants and assigned participants to interventions.
Figure 1:
Consort Flowchart of randomization and enrolment scheme.
Statistical analysis
To determine whether either factor affected the number of HITS, or to determine whether the factors interacted, a two-way analysis of variance (ANOVA, a general linear model) was fit and assumptions checked. The assumption of equal variances among each treatment combination was violated so a non-parametric two-way ANOVA was performed to test whether any of the factors influenced HITS. In this analysis, the number of HITS was ranked from smallest to largest and these ranks were treated as the dependent variable in the analysis, and the assumptions related to normality and equal variances were no longer necessary. Interaction between clamping strategy (HS vs. clamping) and clearance device (CO2 blower vs. suction) was checked for and was determined to be statistically insignificant.
Comparisons of preoperative risk factors and post-operative outcomes were determined using χ2 and ANOVA methods. Because the patients were randomly assigned to treatment groups, no risk adjustment for covariates was performed. All tests were evaluated at the 0.05 α-level and were performed using SAS version 9.2 (Cary, NC, USA).
RESULTS
Group characteristics
The demographic profile of patients is provided in Table 2. No significant differences were found between subgroups.
Table 2:
Clinical and demographic factors by treatment type
| Variable | Partial occluding clamp |
HS |
P-value clamp vs. HS | ||||
|---|---|---|---|---|---|---|---|
| Suction (N = 13) | Blower (N = 15) | P-value | Suction (N = 14) | Blower (N = 15) | P-value | ||
| Age (SD) | 64.7 (10.3) | 61.2 (12.0) | 0.42 | 63.7 (9.9) | 60.9 (13.5) | 0.53 | 0.85 |
| Female gender (%) | 4 (30.8) | 3 (33.3) | 0.88 | 7 (50.0) | 3 (20.0) | 0.09 | 0.85 |
| Body mass index (SD) | 29.4 (5.3) | 30.1 (7.6) | 0.79 | 35.9 (7.0) | 31.5 (5.6) | 0.08 | 0.031 |
| Smoker (%) | 3 (23.1) | 6 (40.0) | 0.34 | 4 (28.6) | 4 (26.7) | 0.91 | 0.71 |
| Dyslipidemia (%) | 1 (7.7) | 1 (6.7) | 0.92 | 12 (85.7) | 15 (100.0) | 0.13 | 0.97 |
| Cerebrovascular Disease (%) | 7 (53.9) | 7 (46.7) | 0.70 | 3 (21.4) | 2 (13.3) | 0.56 | 0.009 |
| CVA (%) | 1 (7.7) | 3 (20.0) | 0.35 | 2 (14.3) | 1 (6.7) | 0.50 | 0.65 |
| Diabetes (%) | 6 (46.2) | 9 (60.0) | 0.46 | 7 (50.0) | 6 (40.0) | 0.59 | 0.51 |
| Hypertension (%) | 13 (100.0) | 15 (100.0) | 1.0 | 14 (100.0) | 14 (93.3) | 0.33 | 0.32 |
| Renal failure (%) | 0 (0.0) | 0 (0.0) | 1.0 | 2 (14.3) | 1 (6.7) | 0.50 | 0.08 |
| MI (%) | 5 (38.5) | 6 (40.0) | 0.93 | 7 (50.0) | 9 (60.0) | 0.59 | 0.23 |
| Ejection fraction (SD) | 58.1 (8.7) | 52.7 (11.0) | 0.17 | 56.0 (6.9) | 50.1 (9.9) | 0.08 | 0.35 |
| Number of disease and vessels (SD) | 2.46 (0.52) | 2.67 (0.62) | 0.35 | 2.64 (0.63) | 2.67 (0.49) | 0.91 | 0.58 |
CVA, cerebrovascular accident; HS, Heartstring; MI, myocardial infarction; SD, standard deviation.
Fifty-seven patients were randomly assigned to have proximal anastomoses using a partial-occluding CL (n = 28) or HS CFD (n = 29). Patients were further randomized to the use of a humidified CO2 blower (n = 30) or suction (n = 27) to visualize the anastomotic site. The mean number of proximal anastomoses was similar between groups 1.93 ± 0.72 (CL) and 1.72 ± 0.70 (HS) (P = 0.28).
Clinical outcomes
There were no in-hospital deaths in either group. Two patients in the CL group did develop delayed (36 and 48 h) post-operative strokes which resulted in a prolonged hospital stay. During a mean follow-up of 5 ± 1 weeks after surgery, there were no procedure-related rehospitalizations or deaths in any group.
Intraoperative cerebral microembolic counts
The frequency of intraoperative embolic events during specific time periods was analysed. In the HS group, 90% of observed microembolic events occurred during the sutureless anastomotic device insertion and deployment and only 10% during the removal. In the CL group, 30.0% of the observed microemboli were recorded during the aortic partial CL application and 70% recorded during the removal. There was no difference between embolic events detected in the right vs. the left middle cerebral artery.
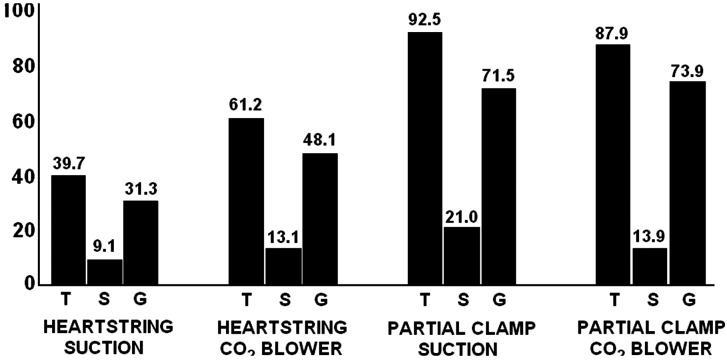
The mean number of total emboli was greater in the CL group than the HS group (90.0 ± 64.0 vs. 50.8 ± 36.6, P < 0.001; Table 3). Total emboli were fewest in patients undergoing HS anastomoses using the suction device (Fig. 2).
Table 3:
Gaseous, solid and total microemboli compared between groups
| Event type | CL (N = 28) | HS (N = 29) | P-value: CL vs. HS | CL/suction (N = 13) | HS/suction (N = 14) | P-value, suction: CL vs. HS | CL/CO2 (N = 15) | HS/CO2 (N = 15) | P-value CO2: CL vs. HS |
|---|---|---|---|---|---|---|---|---|---|
| Total (SD) | 90.0 (64.0) | 50.8 (36.6) | 0.007 | 92.5 (49.5) | 39.7 (24.6) | 0.003 | 87.9 (76.1) | 61.2 (43.3) | 0.25 |
| Solid (SD) | 17.2 (10.6) | 11.2 (10.1) | 0.033 | 21 (9.8) | 9.1 (8.2) | 0.002 | 13.9 (10.4) | 13.1 (11.5) | 0.84 |
| Gas (SD) | 72.8 (62.9) | 40.0 (28.3) | 0.016 | 71.5 (46.3) | 31.3 (19.4) | 0.01 | 73.9 (76.0) | 48.1 (33.2) | 0.24 |
CL, clamp; CO2, carbon dioxide blower; HS, Heartstring; SD, standard deviation.
Figure 2:
Mean number of emboli by type (total, solid and gas), clamping strategy (partial CL or HS) and clearance device (CO2 blower or suction).
The number of intraoperative cerebral emboli was proportional to the number of proximal anastomoses in the HS groups, but independent of the number of proximal anastomoses in the CL groups. This coincides with the number of device deployments needed and utilized to construct each proximal anastomosis with an HS device. In the CL group, only a single application of a partial-occluding CL was utilized regardless of the number of proximal anastomoses performed. In patients with a single proximal anastomosis, there were more total TCD-detected embolic events in the CL group compared with the HS group (104.3 ± 62.6 vs. 37.0 ± 28.1, P = 0.03; Table 4). In patients with more than one proximal anastomosis, the difference in total embolic events between groups was no longer statistically significant (Table 5).
Table 4:
Number of embolic events in each group among patients with a single proximal anastomosis
| Event type | CL (N = 7) | HS (N = 12) | P-value: CL vs. HS | CL/suction (N = 4) | HS/suction (N = 4) | P-value, suction: CL vs. HS | CL/CO2 (N = 3) | HS/CO2 (N = 8) | P-value CO2: CL vs. HS |
|---|---|---|---|---|---|---|---|---|---|
| Total (SD) | 104.3 (62.6) | 37.0 (28.1) | 0.029 | 109.0 (83.8) | 17.8 (7.7) | 0.12 | 98.0 (33.3) | 46.6 (30.0) | 0.036 |
| Solid (SD) | 17.7 (8.1) | 9.6 (7.9) | 0.046 | 17.0 (7.7) | 4.0 (2.6) | 0.019 | 18.7 (10.1) | 12.4 (8.3) | 0.31 |
| Gas (SD) | 86.6 (60.4) | 27.4 (20.7) | 0.041 | 92.0 (81.1) | 13.8 (7.5) | 0.15 | 79.3 (30.6) | 34.3 (22.1) | 0.023 |
CL, clamp; CO2, carbon dioxide blower; HS, Heartstring; SD, standard deviation.
Table 5:
Number of embolic events among patients with more than one proximal anastomosis
| Event type | CL (N = 21) | HS (N = 17) | P-value: CL vs. HS | CL/suction (N = 9) | HS/suction (N = 10) | P-value, suction: CL vs. HS | CL/CO2 (N = 12) | HS/CO2 (N = 7) | P-value CO2: CL vs. HS |
|---|---|---|---|---|---|---|---|---|---|
| Total (SD) | 85.3 (65.3) | 60.6 (39.4) | 0.16 | 85.2 (29.0) | 48.5 (23.5) | 0.007 | 85.3 (84.5) | 77.9 (52.2) | 0.84 |
| Solid (SD) | 17.0 (11.5) | 12.4 (11.5) | 0.22 | 22.8 (10.4) | 11.2 (8.9) | 0.018 | 12.8 (10.6) | 14.0 (15.1) | 0.83 |
| Gas (SD) | 68.2 (64.4) | 48.8 (30.1) | 0.23 | 62.4 (21.2) | 38.3 (18.3) | 0.016 | 72.6 (18.8) | 63.9 (38.2) | 0.80 |
CL, clamp; CO2, carbon dioxide blower; HS, Heartstring; SD, standard deviation.
DISCUSSION
Atherosclerosis of the ascending aorta has been reported to be one of the strongest predictors of stroke after CABG [3,4]. It is therefore intuitive that the manipulation of a diseased ascending aorta is a major source of cerebral embolization [5–7]. Although avoiding cardiopulmonary bypass may reduce aortic manipulation associated with cannulation and extracorporeal circulation, embolization may still occur during the partial clamping of the aorta for proximal anastomoses during OPCAB [8]. Pre- or intraoperative diagnosis of atherosclerosis of the ascending aorta is essential to guide surgical decision-making to avoid aortic clamping in such patients, as intraoperative manoeuvres may mitigate the risk of perioperative stroke [6]. Combining off-pump techniques with anastomotic devices that permit proximal anastomoses without the need for aortic clamping is a reasonable approach to minimize the manipulation of the ascending aorta and therefore reduce the risk for neurologic complications [9]. The HS device allows the construction of a hand-sewn proximal aortic anastomosis without the need for an aortic CL [9, 10]. Cost limits its routine use and its impact on cerebral emboli has not been tested in patients with less severe degrees of ascending aortic atherosclerosis. Moreover, while an anastomotic device reduces aortic manipulation, it does not eliminate it completely [5, 9, 11].
The present study demonstrated that even in patients with minimal aortic disease (grades I and II) cerebral emboli are generated during the construction of proximal anastomoses and this number is significantly reduced when the HS device is used compared with a partial-occluding CL. This was statistically significant when one proximal anastomosis was performed but not significantly different for patients requiring more than one proximal anastomosis. This can be explained by the need for two or more HS devices for multiple proximals and the cumulative effect of multiple manipulations on the aorta. On the contrary, a single application of a partial-occluding CL is used regardless of the number of proximal anastomoses.
Our previous practice has been to reserve the use of this device for those patients who have moderate or severe atherosclerosis of the ascending aorta (grades III and IV). This study suggests that minimizing aortic manipulation with the use of facilitating proximal anastomotic devices combined with the off-pump techniques may reduce the cerebral emboli even in patients without overt aortic atherosclerosis.
In a small series of 19 patients with a calcified aorta, Biancari et al. [12] reported one post-operative stroke in patients in which the HS device was utilized for the construction of aortocoronary proximal anastomoses. In our study, we adopted a different approach by applying the HS device to patients with minimal aortic disease according to epiaortic ultrasound.
Limitations
There are several important limitations to our study. Because this study was powered to detect differences in TCD detected cerebral emboli and not clinical outcomes, the correlation of these findings and their implications on post-operative stroke and post-operative neurocognitive dysfunction can only be postulated. Only a larger randomized trial powered to detect differences in post-operative stroke or neurocognitive dysfunction would be able to adequately address this question.
In summary, avoidance of aortic clamping by using a facilitating device is associated with fewer intraoperative cerebral emboli during OPCAB, even in patients without significant atherosclerosis of the ascending aorta.
Funding
This study was sponsored and funded by Maquet Cardiovascular LLC, San Jose, CA.
Conflict of interest: J.D.P. served as a consultant for Maquet Cardiovascular LLC, San Jose, CA. None of the other authors have any conflict of interest.
REFERENCES
- 1.Scarborough JE, White W, Derilus FE, Mathew JP, Newman MF, Landolfo KP. Combined use of off-pump techniques and a suturless proximal aortic anastomotic device reduces cerebral microemboli generation during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;126:1561–7. doi: 10.1016/s0022-5223(03)01039-0. doi:10.1016/S0022-5223(03)01039-0. [DOI] [PubMed] [Google Scholar]
- 2.Mark DB, Newman MF. Protecting the brain in coronary artery bypass graft surgery. JAMA. 2002;287:1448–50. doi: 10.1001/jama.287.11.1448. doi:10.1001/jama.287.11.1448. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi A, Adachi H, Tanaka M, Ino T. Efficacy of intraoperative epiaortic ultrasound scanning for preventing stroke after coronary artery bypass surgery. Ann Thorac Cardiovasc Surg. 2009;15:98–104. [PubMed] [Google Scholar]
- 4.Puskas JD, Winston AD, Wright CE, Gott JP, Brown WM, 3rd, Craver JM, et al. Stroke after coronary artery operation: incidence, correlates, outcome, and cost. Ann Thorac Surg. 2000;69:1053–6. doi: 10.1016/s0003-4975(99)01569-6. doi:10.1016/S0003-4975(99)01569-6. [DOI] [PubMed] [Google Scholar]
- 5.Khatibzadeh M, Mitusch R, Stierle U, Gromoll B, Sheikhzadeh A. Aortic atherosclerotic plaques as a source of systemic embolism. J Am Coll Cardiol. 1996;27:664–9. doi: 10.1016/0735-1097(95)00526-9. doi:10.1016/0735-1097(95)00526-9. [DOI] [PubMed] [Google Scholar]
- 6.Hilker M, Arlt M, Keyser A, Schopka S, Klose A, Diez C, et al. Minimizing the risk of perioperative stroke by clampless off-pump bypass surgery: a retrospective observational analysis. J Cardiothorac Surg. 2010;5:14. doi: 10.1186/1749-8090-5-14. doi:10.1186/1749-8090-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbut D, Hinton RB, Szatrowski TP, Hartman GS, Bruefach M, Williams-Russo P, et al. Cerebral emboli detected during bypass surgery are associated with clamp removal. Stroke. 1994;25:2398–402. doi: 10.1161/01.str.25.12.2398. doi:10.1161/01.STR.25.12.2398. [DOI] [PubMed] [Google Scholar]
- 8.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter study of perioperative ischemia research group and the ischemia research and education foundation investigators. N Engl J Med. 1996;335:1857–63. doi: 10.1056/NEJM199612193352501. doi:10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 9.Kanemitsu S, Tanaka K, Suzuki H, Tokui T, Kinoshita T. The HEARTSTRING proximal seal system is a possible source of atheroembolism. Circ J. 2006;70:638–40. doi: 10.1253/circj.70.638. doi:10.1253/circj.70.638. [DOI] [PubMed] [Google Scholar]
- 10.Antona C, Scrofani R, Lemma M, Vanelli P, Mangini A, Danna P, et al. Assessment of an aortosaphenous vein graft anastomotic device in coronary surgery: clinical experience and early angiographic results. Ann Thorac Surg. 2002;74:2101–5. doi: 10.1016/s0003-4975(02)04039-0. doi:10.1016/S0003-4975(02)04039-0. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein FS, Bonilla LF, Englberger L, Immer FF, Berg TA, Schmidli J, et al. The St Jude medical symmetry aortic connector system for proximal vein graft anastomoses in coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2002;123:777–82. doi: 10.1067/mtc.2002.119695. doi:10.1067/mtc.2002.119695. [DOI] [PubMed] [Google Scholar]
- 12.Biancari F, Mosorin M, Lahtinen J, Heikkinen J, Rasinaho E, Anttila V, et al. Results with the Heartstring anastomotic device in patients with diseased ascending aorta. Scand Cardiovasc J. 2006;40:238–9. doi: 10.1080/14017430600849211. doi:10.1080/14017430600849211. [DOI] [PubMed] [Google Scholar]