Abstract
We analysed the results of radical segmentectomy achieved through a hybrid video-assisted thoracic surgery (VATS) approach that used both direct vision and television monitor visualization at a median follow-up of over 5 years. Between April 2004 and October 2010, 102 consecutive patients able to tolerate lobectomy to treat clinical T1N0M0 non-small cell lung cancer (NSCLC) underwent hybrid VATS segmentectomy in which we used electrocautery without a stapler to divide the intersegmental plane detected by selective jet ventilation in addition to the path of the intersegmental veins. Curative resection was achieved in all patients. The median surgical duration and blood loss during the surgery were 129 min (range, 60–275 min) and 50 ml (range, 10–350 ml), respectively. The complication rate was 9.8% (10/102) with the most frequent being prolonged air leak, and there was no case of in-hospital death or 30-day mortality post procedure. Five and seven patients developed locoregional and distant recurrences, respectively. The overall and disease-free 5-year survival rates were 89.8% and 84.7%, respectively. Radical hybrid VATS segmentectomy including atypical resection of (sub)segments is a useful option for clinical stage-I NSCLC. The exact identification of anatomical intersegmental plane followed by dissection using electrocautery is critical from oncological and functional perspectives.
Keywords: Segmentectomy, Lung cancer, Video-assisted thoracic surgery, Sublobar resection
INTRODUCTION
Advances in radiographic devices such as high-resolution computed tomography (CT) and the widespread practice of low-dose helical CT for screening have resulted in an extraordinary increase in the early detection of ever smaller non-small cell lung cancers (NSCLCs), such as bronchioloalveolar carcinoma, that might possibly have more indolent biological behaviour. This trend has rapidly changed clinical practice in thoracic surgery, and thus concern has arisen over unified strategies that include whole lobectomy to treat small peripheral cancers. Removing a relatively large volume of healthy lung tissue could result in a poorer quality of postoperative life, a higher frequency of operative morbidity and a decreased likelihood of having a second or even a third NSCLC resected, for which such patients would survive long enough to become at risk. We have therefore actively performed radical segmentectomy with lymph node assessment not only for high-risk but also for good-risk patients with small clinical stage-I NSCLC [1–4]. The outcomes of the randomized study conducted by the Lung Cancer Study Group demonstrated that sublobar resections including wedge resections resulted in a higher rate of local recurrence compared with lobectomy in patients with clinical T1N0M0 NSCLC [5]. Thus, the incidence of non-anatomical stapled wedge resection has escalated and many recent residency programs in thoracic surgery do not cover segmentectomy as a mandatory procedure. However, we and other expert surgeons perceive segmentectomy as a crucial basic technique that should be mastered by all thoracic surgeons [6, 7].
We previously reported a novel segmentectomy in which the intersegmental plane was identified using selective jet ventilation under bronchofiberscopy [8]. Consequently, the segment to be removed can be inflated, whereas those to be preserved are maintained while deflated, which is contrary to the conventional procedure. However, this allows clear visualization of the anatomical intersegmental line between the segment to be resected and that to be preserved. The actual surgical margin in the inflated segment can be adequately grasped and a good surgical field can be obtained even through video-assisted thoracic surgery (VATS) without the need to physically suppress the other segments and lobes using an instrument. In addition, the anatomical intersegmental plane can be precisely dissected by electrocautery without any stapling. This allows the saved adjacent segments to remain completely expansive so that pulmonary function after surgery can be maximal.
Basically, complete VATS using only a monitor for visualization is limited to lung resections of minimal difficulty and cannot be applied to all cancer surgeries, including segmentectomy and bronchoplasty. We have applied hybrid VATS, an integrated surgical approach that combines a muscle-sparing minithoracotomy and a thoracoscopic hole with television monitoring and direct visualization to expand the use of minimally invasive techniques for treating various malignant pathologies [9]. The present study analyses the surgical results of radical hybrid VATS segmentectomy at a median postoperative follow-up of over 5 years.
METHODS
Hybrid VATS segmentectomy
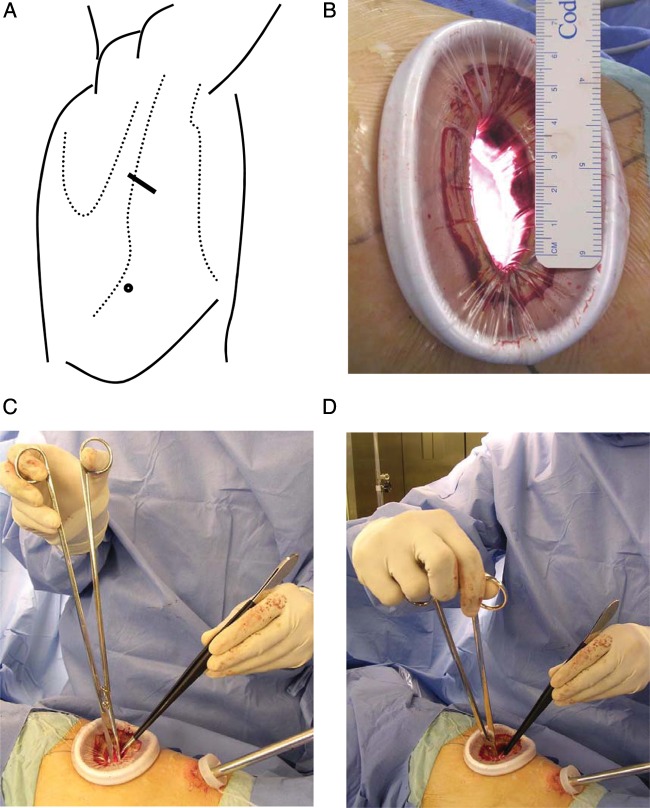
The hybrid VATS approach generally requires two skin incisions for access without cutting muscles or ribs [9]. One 4–5-cm incision is for manipulation and the other is an access port of 1-cm long for insertion of a thoracoscope (Fig. 1). The surgeon directly observes the hilum of the diseased lobe through the main access using a silicon rubber wound retractor with no rib spreading, and then individually isolates and severs all bronchi and vessels of the segment in question utilizing television monitor guidance when dissecting an area that is out of direct view. The skin incision was immediately extended when the surgeon has difficulty with the surgical view. Avoiding rib spreading is critical to the definition of a VATS approach. If the ribs were spread or a skin incision of ≥8 cm long was required, then the patient was excluded from the present study because it was considered a conversion to thoracotomy. We prefer a back hand grip to hold 30-cm-long scissors (model 101-8098-30; Mayo-Harrington; Stille, Sweden) for sharp dissection, long needle holders and forceps upside down (Fig. 1). Immediately after the insertion of a thoracoscope, subclinical dissemination of the tumour was checked by pleural lavage cytology [10].
Figure 1:
Hybrid VATS approach. (A) Skin is incised for one access port for a thoracoscope (circle) and for an access thoracotomy (solid line) over the mid-axillary line in the fourth interspace for upper or middle lobe tumours. Lower lobe tumours are approached through auscultatory triangle in the fifth interspace. (B) Operative exposure of about 4-cm wide is achieved using a wound retractor without rib spreading. (C and D) Sharp dissection in depths of an open thorax through direct vision is performed using an upside-down grip on 30-cm scissors, which allows manipulation with the thumb and index finger through the loops and flexible manoeuvring by turning up the wrist. The ulnar side of the hands rest comfortably alongside the margins of the incision, and awkward elevation of the forearms or elbows can be avoidable.
The procedure of radical segmentectomy has been outlined in a previously published article [8]. Identifying the intersegmental plane with jet ventilation and cutting the intersegmental parenchyma using cautery are unique features of the procedure. A 3.5-mm bronchofiberscope with a lighted tip that is visible at the surgical field is inserted into the orifice of the exposed targeted segmental bronchus. After selectively starting high-frequency oscillation (40 Hz, working pressure 2 kg/cm2, HFO JET VENTILATOR, MERA, Tokyo, Japan), the diseased segments are inflated with air while the preserved segments appear collapsed, and a line is quickly and clearly formed between the inflated and the deflated parenchyma, demonstrating the anatomical intersegmental plane. This method is contrary to conventional inflation–deflation. The target bronchus is tied and cut with a suture or stapler to keep the segment inflated following being filled by jet ventilation. The surgeon can selectively introduce the tip of the fiberscope into each segmental/subsegmental bronchus and sequentially inflate segments/subsegments when more than one is scheduled for removal. At the proximal portion around the hilum, the intersegmental plane is approached along the intersegmental vein, and the plane is divided along the inflation–deflation line at the peripheral site using electrocautery. The margin must be greater than the diameter of the tumour and comprise at least 2cm of unaffected lung tissue for controlling local recurrence. A few adjacent segments or subsegments should be removed unless the margin is sufficient. Air leakage at the bare surface of the preserved lung is controlled using an absorbable polyglycolic acid felt and a fibrin sealant consisting of fibrinogen and thrombin. Sampling or dissection of segmental, lobar, hilar and mediastinal lymph nodes followed by intraoperative frozen section analysis is mandatory to determine the applicability of segmentectomy. Standard lobectomy should proceed instead when the surgical margin is judged imperfect or any diseased lymph node is found. The chest is routinely drained using a single chest tube under water seal.
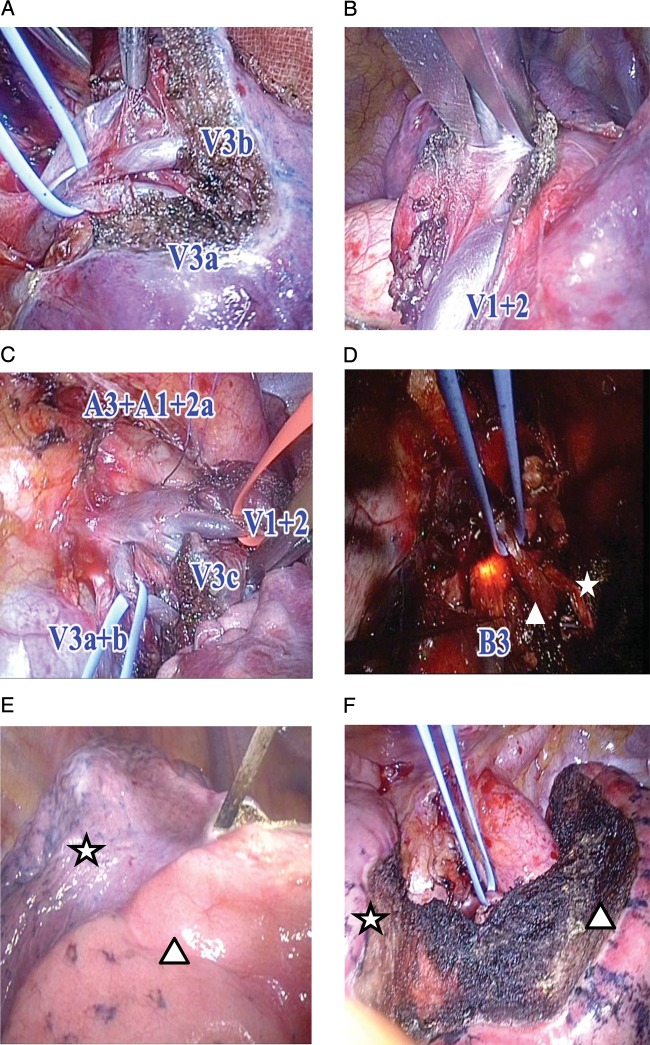
Although the simplest segments in order of easiest to hardest to remove are the upper division (S1-3) and lingular segments (S4+5) of the left upper lobe, the superior (S6) and the basilar (right S7-10 or left S8-10) segments of either lower lobe, we have aggressively performed complicated resection of segments/subsegments such as the left S3 segment + S1+2a subsegment (Fig. 2).
Figure 2:
Left S3 +S1+2a segmentectomy. (A) V3a and V3b branches of the vein running between upper division and lingular segment are identified and sufficiently exposed to distal portion. (B) Vein branch V1+2 is also exposed to distal site, and V1+2a, V1+2b + c and V1+2 superior can be identified. V1+2a runs between S3c and S1+2a, and V1+2b + c runs between S1+2a and S1+2b + c. (C) First two arterial branches (A3 and A1+2a) and vein branches (V1+2a, V1+2b + c, V3a, V3b and V3c) are adequately exposed. Intersegmental branches of vein (V1+2b + c, V3b) are saved for venous return from preserved adjacent segments. (D) Bronchofiberscope through the double-lumen tube inserted into the orifice of the targeted segmental bronchus (B3) in addition to B1+2a (star), where high-frequency oscillation is applied. V1+2b + c (triangle) is preserved. (E) Inflation–deflation line between and inflated (resected) S1+2a subsegment (triangle) and deflated (preserved) S1+2b + c subsegment (star) along which anatomical intersubsegmental plane is dissected with cautery. (F) Saved parenchyma of S1+2b + c (triangle) and S4 (star) is fully inflated after removal of S3 +S1+2a.
Patients
We performed radical hybrid VATS segmentectomy in which the intersegmental plane was divided using electrocautery without any stapling between April 2004 and October 2010 in 102 consecutive patients with clinical T1N0M0 NSCLC who could tolerate lobectomy. Surgical-pathologic staging was performed according to the New International Staging System for Lung Cancer [11]. Operative mortality was defined as death during hospitalization for pulmonary resection or within 30 days of the procedure, whichever was longer. Pathological cancer staging proceeded in accordance with the guidelines established by the American Joint Committee on Cancer [12]. Each patient provided his/her informed written consent based on the protocol approved by the institute's review board before surgery. After surgery, every patient was essentially evaluated every 3 months for the first 2 years and at 6-month intervals thereafter.
Statistical analysis
Overall and disease-free survival rates were estimated using the Kaplan–Meier method. Overall survival was defined as elapsed time from surgery until death from any cause or last follow-up. Disease-free survival was defined as elapsed time from surgery until the first diagnosis of local, regional or distant recurrent disease or until the last follow-up. We performed multivariate analyses using the Cox proportional hazards model to identify predictors of postoperative recurrence. Data were statistically analysed using SPSS software (version 10.5, SPSS Inc., Chicago, IL, USA).
RESULTS
Table 1 lists the characteristics of the 102 patients (52 women and 50 men; median age, 67 years; range, 34–89 years). Tumour size ranged from 8 to 29 mm (median; 18 mm). Most of the patients had an adenocarcinoma (91/102, 89.2%), of which a high proportion contained a bronchioloalveolar carcinoma component (58/91, 63.7%). Pathological assessments showed that curative resections were achieved with free surgical margins in all patients. Of all 102 patients with cT1N0M0 (stage-IA) disease, 84 (82.4%) were clinically staged as having T1a disease. Final pathological examination demonstrated that 92 (90.2%), 8 (7.8%), and 1 (1%) each had pathological stage IA, IB, IIA and IIIA disease, respectively. Table 2 shows the locations of the burdened segments. Actually, segmentectomy can be performed in any lobe except in the middle lobe, and no conversions to incisions longer than 8 cm were required in this series.
Table 1:
Patient characteristics (n = 102)
| Age (years) | |
| Median | 67 |
| Range | 34–89 |
| Sex | |
| Female | 52 |
| Male | 50 |
| Size of tumour on HR-CT (mm) | |
| Median | 18 |
| Range | 8–29 |
| Histology | |
| Adenocarcinoma | 91 |
| Squamous cell | 7 |
| Adenosquamous | 2 |
| Large cell | 1 |
| Carcinoid | 1 |
| Serum CEA | |
| ≤5.0 ng/ml | 89 |
| >5.0 ng/ml | 13 |
| Clinical stage | |
| IA | 102 (T1a: 84, T1b: 18) |
| Pathologic stage | |
| IA | 92 |
| IB | 8 |
| IIA | 1 |
| IIIA | 1 |
CEA, carcinoembryonic antigen.
Table 2:
Location of burdened lung (n = 102)
| Right upper lobe: 30 |
| S1: 8, S1 + S2a: 1, S2: 9, S2b + S3a: 6, S2 + S3a: 1, S3: 5 |
| Right lower lobe: 26 |
| S6: 16, S6 + S8: 1, S7 + S8: 1, S8: 3, S8 + S9: 2, S9 + S10: 1, S7–S10: 2 |
| Left upper lobe: 22 |
| S1+2 + S3: 10, S1+2a: 1, S1+2: 3, S3 + S1+2a: 1, S3a + b + S4 + S5: 1, S4 + S5: 6 |
| Left Lower lobe: 24 |
| S6: 16, S8: 2, S9: 2, S9 + S10: 3, S8–S10: 1 |
a, posterior subsegment; b, anterior subsegment; S1, apical; S2, posterior; S1+2, apicoposterior; S3, anterior; S4, superior; S5, inferior; S6, superior; S7, medial basal; S8, anterior basal; S9, lateral basal; S10, posterior basal.
The operative results are shown in Table 3. The median operative time measured from skin incision to skin closure and median bleeding during surgery were 129 min (range, 60–274 min) and 50 ml (range, 10–350 ml), respectively. The median length of the skin incision for utility access was 50 mm (range, 40–80 mm). Postoperative complications developed in 10 patients (9.8%), with the most common being prolonged air leak in 4 patients (3.9%). Three patients (2.9%) had a late alveolopleural fistula requiring tube drainage, which is characteristic of our procedure as the intersegmental plane is divided without using a stapler. In 66 patients (64.7%), no air leak was observed at the time of surgery. The median postoperative duration of chest tube drainage was 1 day, that is, the drain was usually removed on the day following the procedure. None of the patients died while in hospital or during the 30 days after the procedure. Five deaths from cancer and four from other causes occurred during a median follow-up of 61 months (range, 8–84 months). Pleural dissemination/malignant effusion and mediastinal lymph node metastasis developed in two patients each and distant metastasis occurred in seven. Local margin recurrence that developed in one patient after lingulectomy was treated by completion left upper lobectomy, who has since remained recurrence free.
Table 3:
Surgical results (n = 102)
| Operation time (min) | |
| Median | 129 |
| Range | 60–275 |
| Bleeding (ml) | |
| Median | 50 |
| Range | 10–350 |
| Utility access incision (mm) | |
| Median | 50 |
| Range | 40–80 |
| Complication | 10 |
| Prolonged air leak (>7 days) | 4 |
| Late alveolopleural fistula | 3 |
| Supraventricular arrhythmia | 2 |
| Interstitial pneumonia | 1 |
| Operative mortality | 0 |
| Mortality during follow-up | 9 |
| Cancer death | 5 |
| Other | 4 |
| Recurrence | 12 |
| Locoregional | |
| Dissemination | 2 |
| Mediastinum | 2 |
| Margin | 1 |
| Distant | |
| Lung | 4 |
| Brain | 2 |
| Meningitis | 1 |
Follow-up of alive patients (month) median: 61 (range: 8–84).
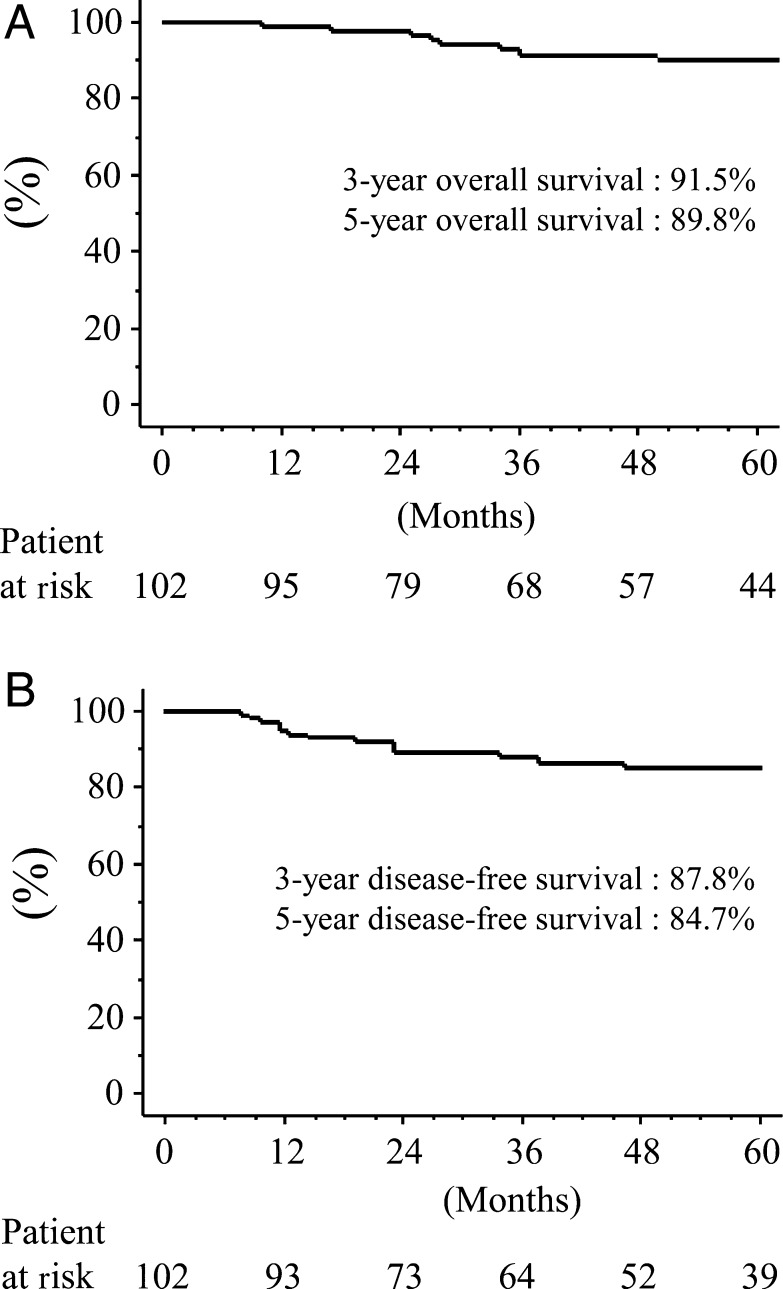
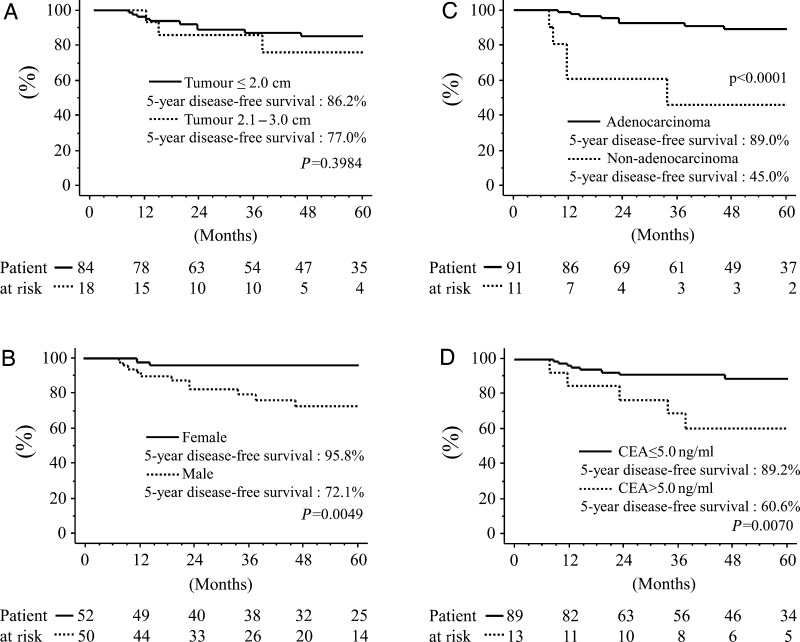
The overall and disease-free 5-year survivals of all patients were 89.8 and 84.7%, respectively (Fig. 3). Univariate analyses demonstrated that being male (P = 0.0049), having cancer other than adenocarcinoma (P < 0.0001) or a high CEA value (P = 0.0070) significantly and negatively affected disease-free survival (Fig. 4). Multivariate analysis showed that histological type (P = 0.0133) significantly correlated with risk for recurrence, whereas age (P = 0.9880), tumour size (P = 0.4827), skin incision (P = 0.5623), gender (P = 0.0847), side (P = 0.9681), lobe (P = 0.4679) and CEA value (P = 0.6217) did not (Table 4). Adenocarcinoma was therefore a significantly better independent prognostic determinant of recurrence.
Figure 3:
Postoperative Kaplan–Meier curves following radical hybrid VATS segmentectomy. (A) Overall survival; (B) disease-free survival.
Figure 4:
Disease-free survival following radical hybrid VATS segmentectomy, according to (A) tumour size, (B) gender, (C) histology and (D) serum CEA value.
Table 4:
Multivariate analysis of factors predicting recurrence
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Continuous factors | |||
| Age | 0.999 | 0.926–1.078 | 0.9880 |
| Tumour size | 1.069 | 0.887–1.288 | 0.4827 |
| Skin incision | 1.011 | 0.974–1.050 | 0.5623 |
| Categorized factors | |||
| Gender | |||
| Male | Reference | ||
| Female | 0.240 | 0.047–1.216 | 0.0847 |
| Side | |||
| Right | Reference | ||
| Left | 1.028 | 0.269–3.919 | 0.9681 |
| Lobe | |||
| Upper | Reference | ||
| Lower | 0.599 | 0.151–2.387 | 0.4679 |
| Histology | |||
| AD | Reference | ||
| Non-AD | 6.775 | 1.488–30.839 | 0.0133 |
| CEA | |||
| ≥5.0 ng/ml | Reference | ||
| >5.0 ng/ml | 1.452 | 0.330–6.383 | 0.6217 |
AD, adenocarcinoma; CEA, carcinoembryonic antigen.
DISCUSSION
The present study examined the relatively long-term outcomes of patients undergoing hybrid VATS segmentectomy, in which the intersegmental plane identified by the path of the intersegmental veins and by selective inflation of jet ventilation was divided by electrocautery without using a stapler. Because of the unavoidable selection bias in retrospective study, we could not compare the results between VATS segmentectomy and VATS lobectomy, which should be investigated in a prospective trial. The present study nevertheless showed that hybrid VATS segmentectomy is safe for treating patients with small clinical stage-I NSCLC and the results are excellent.
The approach via two skin incisions including a median minithoracotomy incision of 5 cm and a thoracoscope hole of 1 cm was minimally invasive, and none of our patients needed conversion to thoracotomy requiring an incision of ≥8 cm in length. The perioperative complication rates were very low (9.8%, 10/102) and no operative mortality occurred. Since the selection of patients was not random in this series, which may have produced a bias in the selection of patients for specific procedures, we cannot describe the prognosis in detail even at a median follow-up of more than 5 years. The locoregional and distant recurrence rates were low at 4.9% (5/102) and 6.9% (7/102), respectively. The overall and disease-free 5-year survival rates of 89.8 and 84.7%, respectively, were comparable to previously published data [4, 13]. The outcomes of recent studies including ours indicate that VATS segmentectomy for recent smaller and more indolent tumours should be satisfactory from an oncological viewpoint [13, 14]. The ongoing clinical trial comparing the surgical results between lobectomy and sublobar resection conducted by the Cancer and Leukemia Group B (CALGB 140503) and by the Japan Clinical Oncology Group/West Japan Oncology Group (JCOG0802/WJOG4607L) [15] will demonstrate the role of segmentectomy for patients with NSCLC 2 cm or smaller. The former randomly assigns patients to undergo segmentectomy or wedge resection and lobectomy, but the latter cannot permit wedge resection as a sublobar resection although surgeons in both trials can freely select the access technique by VATS or by thoracotomy.
The primary target of ideal function-saving surgery is to preserve the pulmonary parenchyma, and the secondary aim is to reduce trauma through selecting the appropriate surgical approach. As you know from accumulated experience, complete VATS approach can be used in simple lobectomy for lung cancer. However, we have applied a hybrid VATS approach to more technically demanding surgical procedures for treating lung cancer, such as bronchoplasty, angioplasty and atypical segmentectomy [9]. Segmentectomy allows optimal resection of small deep non-palpable lesions with suspected malignancy, which helps preserve lung function. Under these conditions, the hybrid VATS approach can provide safe surgical margins, although lobectomy even using a complete VATS approach must not be an option for any small undiagnosed tumour. The three-dimensional view provided by direct vision through hybrid VATS enables surgeons to understand the appropriate margins, thus minimizing the likelihood of missing diseased lung tissue or removing an excessive volume of healthy tissue. The advantages of treating lung cancer with VATS using only monitor visualization are arguable for the patient, although the surgeon seems to get a great sense of achievement. Whether or not direct vision is applied in VATS is in essence a peripheral matter. Thoracic surgeons need to balance the benefits and disadvantages of complete VATS, for example, to avoid lobectomy thoughtlessly performed for a tiny undiagnosed tumour. The most important aspects of cancer surgery are radicality and the preservation of functionality for the patients.
The key to successful high-quality procedures using the hybrid VATS approach is the upside-down backhand grip on long scissors for incisive dissection, and the long needle holder for bronchoplasty or angioplasty developed by Belsey (Frenchay Hospital; Bristol, UK) and Pearson (Toronto General Hospital; Toronto, ON, Canada) to facilitate deep manoeuvring. This skill was advanced before the introduction of VATS and we consider it suitable for manoeuvring through a small access thoracotomy in this heyday of VATS. In general, segmentectomy is technically more demanding than lobectomy because it requires a thorough three-dimensional knowledge of all relevant bronchoarterial relationships and possible anomalies of the arterial branches. Sharp dissection with scissors that can accurately and rapidly expose the segmental hilar structures is important for radical hybrid VATS segmentectomy.
We concluded that hybrid VATS segmentectomy is a safe and useful option for patients with small N0 NSCLC who can tolerate lobectomy. The accurate identification of the intersegmental plane and dissection along an adequate cutting line by electrocautery prevents local failure and maintains lung function that are crucial during radical segmentectomy even through the VATS approach. Minimally invasive strategies will be applied to ever more challenging segmentectomies such as radical atypical resection of segments and/or subsegments, and thus become a standard approach for treating lung cancer.
Conflict of interest: none declared.
REFERENCES
- 1.Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71:956–61. doi: 10.1016/s0003-4975(00)02223-2. doi:10.1016/S0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa K, Tsubota N, Kodama K, Ayabe H, Taki T, Mori T. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg. 2002;73:1055–8. doi: 10.1016/s0003-4975(01)03466-x. doi:10.1016/S0003-4975(01)03466-X. [DOI] [PubMed] [Google Scholar]
- 3.Tsubota N, Ayabe K, Doi O, Mori T, Namikawa S, Taki T, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg. 1998;66:1787–90. doi: 10.1016/s0003-4975(98)00819-4. doi:10.1016/S0003-4975(98)00819-4. [DOI] [PubMed] [Google Scholar]
- 4.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–75. doi: 10.1016/j.jtcvs.2006.02.063. doi:10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg RJ, Rubenstein LV Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. doi:10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 6.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg. 1994;107:1087–93. [PubMed] [Google Scholar]
- 7.Jones DR, Stiles BM, Denlinger CE, Antippa P, Daniel TM. Pulmonary segmentectomy: results and complications. Ann Thorac Surg. 2003;76:343–9. doi: 10.1016/s0003-4975(03)00437-5. doi:10.1016/S0003-4975(03)00437-5. [DOI] [PubMed] [Google Scholar]
- 8.Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg. 2007;133:753–8. doi: 10.1016/j.jtcvs.2006.11.005. doi:10.1016/j.jtcvs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Okada M, Sakamoto T, Yuki T, Mimura T, Miyoshi K, Tsubota N. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest. 2005;128:2696–701. doi: 10.1378/chest.128.4.2696. doi:10.1378/chest.128.4.2696. [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Sakamoto T, Nishio W, Uchino K, Tsuboshima K, Tsubota N. Pleural lavage cytology in non-small cell lung cancer: lessons from 1000 consecutive resections. J Thorac Cardiovasc Surg. 2003;126:1911–5. doi: 10.1016/s0022-5223(03)00715-3. doi:10.1016/S0022-5223(03)00715-3. [DOI] [PubMed] [Google Scholar]
- 11.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. doi:10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. pp. 12–44. [Google Scholar]
- 13.Shapiro M, Weiser TS, Wisnivesky JP, Chin C, Arustamyan M, Swanson SJ. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg. 2009;137:1388–93. doi: 10.1016/j.jtcvs.2009.02.009. doi:10.1016/j.jtcvs.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Oizumi H, Kanauchi N, Kato H, Endoh M, Suzuki J, Fukaya K, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: A report of 52 consecutive cases. J Thorac Cardiovasc Surg. 2011;141:678–82. doi: 10.1016/j.jtcvs.2010.08.027. doi:10.1016/j.jtcvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Saji H, Nakajima R, Okada M, Asamura H, Shibata T, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (J COG0802/WJOG4607L) Jpn J Clin Oncol. 2010;40:271–4. doi: 10.1093/jjco/hyp156. doi:10.1093/jjco/hyp156. [DOI] [PubMed] [Google Scholar]