Sant and McMichael discuss new advances in detecting CD4+ T cells at the right time and place during viral infection.
Abstract
Protective immunity to chronic and acute viral infection relies on both the innate and adaptive immune response. Although neutralizing antibody production by B cells and cytotoxic activity of CD8+ T cells are well-accepted components of the adaptive immune response to viruses, identification of the specific role of CD4+ T cells in protection has been more challenging to establish. Delineating the contribution of CD4+ T cells has been complicated by their functional heterogeneity, breadth in antigen specificity, transient appearance in circulation, and sequestration in tissue sites of infection. In this minireview, we discuss recent progress in identifying the multiple roles of CD4+ T cells in orchestrating and mediating the immune responses against viral pathogens. We highlight several recent reports, including one published in this issue, that have employed comprehensive and sophisticated approaches to provide new evidence for CD4+ T cells as direct effectors in antiviral immunity.
What do we need to know about the effectors of the immune response to be able to manipulate the immune system to ensure protection from viral pathogens? We need both predictors and correlates of protection. We need to distinguish between those individuals whose immune systems are competent to withstand a challenge, and those who require boosting or de novo vaccination. When designing vaccines, we need to know what epitope sequences should be included to elicit the most useful specificities, and what form of antigen will elicit the most critical effector function. Durable immune responses are essential, and thus identifying correlates of persistence and continued functionality is critical. In the case of new pandemics such as influenza, rapid deployment and dose sparing of vaccines may be needed. Therefore, it will be critical to identify which individuals in a susceptible population will mount an adequately robust response to limiting doses of vaccine, thus preserving stocks for those whose immune status requires subsequent boosts or higher doses of the vaccine.
The first step in defining immune parameters of protection is identification of the full repertoire of cells that comprise the response to infection. Prediction and enhancing immune responses requires identification of the cellular components that limit the immune response and the cells responsible for delivery of effector function (Fig. 1). We need to identify the bottlenecks in specificity or function that limit protective immunity to virus infection or successful vaccination. The conventional wisdom has been that CD8+ T cell responses play a major role in antiviral immunity. Although this remains true for many viruses, recent papers show that CD4+ T cells are also important, and in some cases are the major T cell component in the antiviral response (Soghoian and Streeck, 2010; Porichis and Kaufmann, 2011; Thèze et al., 2011; Brown et al., 2012; Ranasinghe et al., 2012; Soghoian et al., 2012; Wilkinson et al., 2012). The complexity of CD4+ T cell function, coupled with their broad specificity, has made detection of their contribution to vaccine responses and protective immunity relatively difficult. Unlike CD8+ T cells, which have reasonably well defined function and narrow antigen specificity, CD4+ T cells are tremendously complex. Therefore, the development of assays that will reveal the presence and full quantification of the relevant epitope-specific CD4+ T cells is a major challenge. It will also be important to identify the mechanisms responsible for their antiviral activity in the response. Collectively, these challenges have hampered efforts to acquire definitive evidence for the role of CD4+ T cells in anti-viral immunity. However, recent studies, including one in this issue by Zhou et al., demonstrate a critical role for CD4 T cells in protection from viral infection.
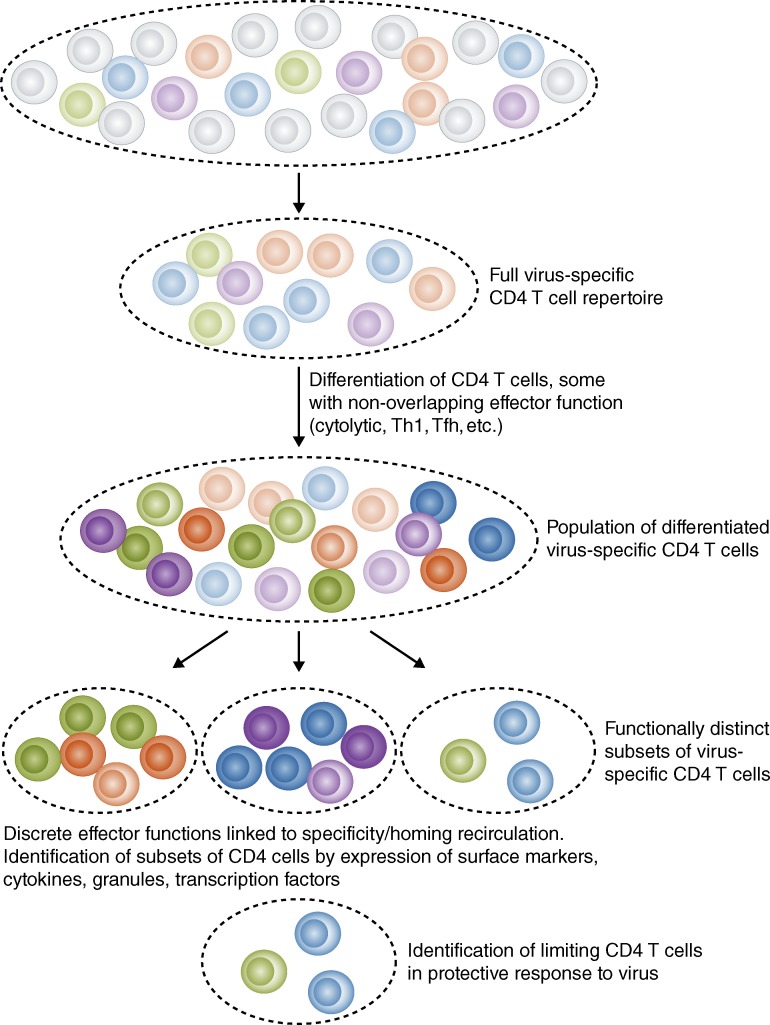
Figure 1.
Many CD4 T cells expand in parallel in response to virus infection. Recent advances now allow a full and unbiased assessment of this initial CD4 T cell repertoire to viral pathogens, typically composed of many peptide specificities, indicated by different colored symbols. As CD4 T cells expand in response to infection, they differentiate into subsets that possess distinct effector functions, including cytolysis, help for antibody responses, and cytokine production (three examples of subsets from the same original CD4 T cell precursor are shown here in alternate depths of color). Some of these functional subsets may be enriched for particular epitope specificities and others may be sequestered in infected tissues. Identification of the CD4 T cell population that limits protective immunity requires robust and sophisticated assays to isolate, quantify, and distinguish virus-specific CD4 T cells.
Known activities of CD4+ T cells in antiviral immunity
CD4+ T cells contribute a myriad of activities in protective immunity against viruses that are initiated by infection or by vaccination. These activities can be broadly separated into distinct categories that include recruitment of key lymphoid cell populations into secondary lymphoid tissue or sites of pathogen infection, provision of help for expansion or function of other effector cells, or offering direct effector function through production of cytokines or cell-mediated cytotoxicity. One key activity of CD4+ T cells is recruitment of other lymphoid cells: CD4+ T cells can promote engagement of CD8+ T cells with dendritic cells (DCs) in secondary lymphoid tissue (Beuneu et al., 2006; Castellino et al., 2006), cause influx of lymphoid cells into draining lymph node (Kumamoto et al., 2011), and recruit innate or antigen-specific effectors to the site of viral replication (Nakanishi et al., 2009; Strutt et al., 2010; Teijaro et al., 2010). Whether these CD4+ T cells are ever limiting in response to infection, and can thus serve as predictors of disease susceptibility, is not yet known.
The role of CD4+ T cell help in CD8+ T cell priming, effector function, and memory has been extensively studied in recent years (Wiesel and Oxenius, 2012). Although such help may not be as critical for viruses that offer many CD8 epitopes (Sette and Rappuoli, 2010) and/or generate potent activating signals from DCs through strong Toll-like receptor engagement, it may be critical for pathogens that antagonize the immune response by down-regulating the activity of proinflammatory mediators. It is also likely to be essential for the development of memory CD8+ T cells that can be recalled upon challenge (Williams et al., 2006). With chronic viral infections, the role and importance of CD4+ T cell help is even more profound. Under these conditions, CD8+ T cells rely on continued rounds of expansion for which CD4+ T cell cytokine production is critical (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009; Aubert et al., 2011).
That CD4+ T cell help is needed for high-affinity, neutralizing antibody responses by B cells has been known for decades, but more recent work has identified the follicular helper CD4+ T cells (Tfh) as the key subset that mediates this function (Crotty, 2011; King, 2011). Recent identification of cells with Tfh lineage markers and functional activity in circulation of human subjects (Chevalier et al., 2011; Morita et al., 2011) raises the possibility that quantifying this subset may be useful as a biomarker for future vaccine responses, particularly if coupled with analyses of CD4 specificity. Finally, increasing evidence supports the view that CD4+ T cells have direct roles as effectors in antiviral immunity either through provision of key antiviral cytokines or through direct cytotoxicity (Swain et al., 2012). Defining the complexity of CD4+ T cells has provided a pallet to dissect protective immunity in different human populations for distinct viral pathogens, but improved and more sophisticated assays are needed to reveal if the activities of CD4 T cells ever truly limit protective responses.
Complexity in assessing contribution of CD4+ T cells
There are several major difficulties in assessing the contributions of CD4+ T cells to the control of virus infections. There is the breadth of the CD4+ T cell repertoire, which for influenza virus can include up to 100 distinct peptides specificities (Chaves et al., 2012). A recent study of human responses to vaccinia virus using a whole-proteome approach demonstrated that a mean of 39 open reading frames, each of which likely contain multiple peptide epitopes, were recognized by vaccinees (Jing et al., 2008). Limited epitope sampling through use of a few favored peptides or limited antigens may prevent successful identification of the true correlates of protection as only some subsets of CD4+ T cells may participate in particular responses. For example, in HIV, subsets of lymphoid cells distinguish those that control infection versus those that do not (Porichis and Kaufmann, 2011; Thèze et al., 2011). Recent comprehensive screening of a large cohort of infected subjects using peptides representing the entire proteome has revealed that differences in the CD4+ T cell immunodominance profile distinguished those who control the virus compared with those that progress (Ranasinghe et al., 2012). The overall breadth of the response was inversely correlated with viral load, and the ratios of Gag-specific to Env-specific CD4+ T cells determined virus progression.
CD4+ T cell help for antibody responses is another situation that links CD4+ T cell specificity and functionality, because delivery of help for antibody responses normally requires that the B cell and CD4+ T cell recognize epitopes from the same antigen. Such help for antibody responses is restricted primarily to follicular helper cells (Crotty, 2011; King, 2011). Tracking cells with irrelevant specificities or the wrong function may only add noise to assessments of CD4+ T cell functionality. Additionally, in humans, some CD4+ T cells may be invisible to experimentation because of restricted recirculation, and thus low abundance in peripheral blood. Finally, rapid and transient kinetics of the responses detected in peripheral blood after pathogen infection or vaccination (Weinfurter et al., 2011; Li et al., 2012; Riou et al., 2012) may diminish detection and characterization of the responding CD4+ T cells in humans if sampled at the wrong time. Incomplete or inadequate consideration of these factors can lead to false-negative assessments of the role of CD4+ T cell responses to infection and vaccination, particularly in human subjects, in whom there are significant barriers to tissue sampling and response assessment.
In this issue, Zhou et al. (2012) track CD4+ and CD8+ T cell responses over time during acute resolving hepatitis A virus (HAV) infection in chimpanzees. The studies were revealing because of the detailed and comprehensive nature of the study design. Frequent sampling of peripheral blood allowed simultaneous tracking of responses and viral gene expression, both during the acute phase (weeks 1–6) and long term. Sampling of serum, fecal material, and liver for HAV RNA allowed assessment of virus expansion, control, and rebound. The authors also sampled liver tissue biweekly for HAV-specific CD4+ and CD8+ T cells. CD4+ T cell specificity and functionality was probed using pools of HAV peptides representing the entire HAV genome in conjunction with intracytoplasmic staining for the key cytokines IL-2, TNF, IL-21, and IFN-γ. Surprisingly, these studies revealed few kinetic correlates between CD8+ T cell activity and control of viremia. Rather, early expansion of multifunctional CD4+ T cells that produced IL-2 and IL-21 IFN-γ and TNF was observed, with contraction after clearance of the virus. The highly differentiated phenotype of CD4+ T cells early in the response, lacking in the CD8 compartment, was noteworthy because of the role IFN-γ and TNF in inhibiting HAV replication. These coordinate kinetics of expansion of virus and CD4+ T cells in this primate model of infection suggested that the virus-specific CD4+ T cells contributed to ultimate control of virus in vivo.
This work echoes the conclusion of Soghoian et al. (2012) in a recent broad survey of acutely HIV-infected subjects, in which HIV CD4+ and CD8+ T cells were tracked over time using peptide pools representing the major virus proteins, in parallel with virus progression. Strikingly, early expansion and phenotype of CD4+ T cells, but not CD8+ T cells, specific for HIV were most closely associated with virus control. Moreover, expansion of granule-positive, HIV-specific cytotoxic CD4+ T cells was most tightly correlated with a positive outcome. Similarly, a recently reported study by Wilkinson et al. (2012) explored the importance of CD4 T cells in protection from influenza in a challenge model in human subjects. As in the study by Zhou et al. (2012), and those of Soghoian et al. (2012), multiple parameters were tracked over time, including viral titers in nasal washes, symptom scores, serological conversion to neutralizing antibody, and T cell responses that were elicited by large pools of synthetic peptides representing the whole virus proteome. Strikingly, the strongest association was a correlation between increased levels of preexisting influenza-specific CD4+ T cells with increased virus clearance and reduced symptom score. Additionally, early CD4+ T cell responses to virus infection were characterized by production of IFN-γ and cytotoxic activity. These results collectively suggest that preexisting memory CD4+ T cells can be rapidly mobilized for protection against novel virus strains, and that these T cells exert direct antiviral activity. This result is in agreement with mouse models of sequential infection for protection against pandemic challenge (Alam and Sant, 2011) and surveys of human populations (Greenbaum et al., 2009; Ge et al., 2010). The protection observed in Wilkinson et al. (2012) can also be related to recent findings in animal models, showing that memory CD4+ T cells can promote rapid recruitment of innate effectors to the lung to establish an accelerated antiviral state (Strutt et al., 2010; Teijaro et al., 2010). Additionally, recent studies in both murine influenza (Brown et al., 2012), human cytomegalovirus, and HIV (Soghoian and Streeck, 2010; Swain et al., 2012) suggest that CD4 T cell–mediated cytotoxicity of infected cells can play a critical function in viral clearance (Appay, 2004; Casazza et al., 2006; Soghoian et al., 2012). Thus, although the balance between CD4+ and CD8+ T cell–mediated effects are different for individual viruses, accumulating evidence points to the direct role of CD4+ T cells in protective immunity to many viruses.
The comprehensive analysis of CD4+ T cell responses by Zhou et al. (2012), as well as the other recent studies highlighted in this minireview, provides provocative evidence of the essential role of CD4+ T cells in protection from virus infection in humans and primates. Coupled with mechanistic studies in animal models, advances in discovery (Koelle, 2003; Sette and Rappuoli, 2010; Chaves et al., 2012) and organization (Vita et al., 2010) of T cell epitopes, increasingly sophisticated T cell phenotyping (Nepom, 2012; Newell et al., 2012), and gene expression profiling (Chaussabel et al., 2010; Nakaya et al., 2011) all provide roadmaps to select optimal vaccination strategies that elicit the preferred CD4+ T cells for the most effective protective immunity to virus infection.
References
- Alam S., Sant A.J. 2011. Infection with seasonal influenza virus elicits CD4 T cells specific for genetically conserved epitopes that can be rapidly mobilized for protective immunity to pandemic H1N1 influenza virus. J. Virol. 85:13310–13321 10.1128/JVI.05728-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V. 2004. The physiological role of cytotoxic CD4(+) T-cells: the holy grail? Clin. Exp. Immunol. 138:10–13 10.1111/j.1365-2249.2004.02605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert R.D., Kamphorst A.O., Sarkar S., Vezys V., Ha S.J., Barber D.L., Ye L., Sharpe A.H., Freeman G.J., Ahmed R. 2011. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc. Natl. Acad. Sci. USA. 108:21182–21187 10.1073/pnas.1118450109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuneu H., Garcia Z., Bousso P. 2006. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J. Immunol. 177:1406–1410 [DOI] [PubMed] [Google Scholar]
- Brown D.M., Lee S., Garcia-Hernandez Mde.L., Swain S.L. 2012. Multifunctional CD4 Cells Expressing Gamma Interferon and Perforin Mediate Protection against Lethal Influenza Virus Infection. J. Virol. 86:6792–6803 10.1128/JVI.07172-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza J.P., Betts M.R., Price D.A., Precopio M.L., Ruff L.E., Brenchley J.M., Hill B.J., Roederer M., Douek D.C., Koup R.A. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865–2877 10.1084/jem.20052246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F., Huang A.Y., Altan-Bonnet G., Stoll S., Scheinecker C., Germain R.N. 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 440:890–895 10.1038/nature04651 [DOI] [PubMed] [Google Scholar]
- Chaussabel D., Pascual V., Banchereau J. 2010. Assessing the human immune system through blood transcriptomics. BMC Biol. 8:84 10.1186/1741-7007-8-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves F.A., Lee A.H., Nayak J.L., Richards K.A., Sant A.J. 2012. The utility and limitations of current Web-available algorithms to predict peptides recognized by CD4 T cells in response to pathogen infection. J. Immunol. 188:4235–4248 10.4049/jimmunol.1103640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N., Jarrossay D., Ho E., Avery D.T., Ma C.S., Yu D., Sallusto F., Tangye S.G., Mackay C.R. 2011. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 186:5556–5568 10.4049/jimmunol.1002828 [DOI] [PubMed] [Google Scholar]
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621–663 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- Elsaesser H., Sauer K., Brooks D.G. 2009. IL-21 is required to control chronic viral infection. Science. 324:1569–1572 10.1126/science.1174182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich A., Kisielow J., Schmitz I., Freigang S., Shamshiev A.T., Weber J., Marsland B.J., Oxenius A., Kopf M. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 324:1576–1580 10.1126/science.1172815 [DOI] [PubMed] [Google Scholar]
- Ge X., Tan V., Bollyky P.L., Standifer N.E., James E.A., Kwok W.W. 2010. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J. Virol. 84:3312–3319 10.1128/JVI.02226-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum J.A., Kotturi M.F., Kim Y., Oseroff C., Vaughan K., Salimi N., Vita R., Ponomarenko J., Scheuermann R.H., Sette A., Peters B. 2009. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. USA. 106:20365–20370 10.1073/pnas.0911580106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L., Davies D.H., Chong T.M., Chun S., McClurkan C.L., Huang J., Story B.T., Molina D.M., Hirst S., Felgner P.L., Koelle D.M. 2008. An extremely diverse CD4 response to vaccinia virus in humans is revealed by proteome-wide T-cell profiling. J. Virol. 82:7120–7134 10.1128/JVI.00453-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. 2011. A fine romance: T follicular helper cells and B cells. Immunity. 34:827–829 10.1016/j.immuni.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Koelle D.M. 2003. Expression cloning for the discovery of viral antigens and epitopes recognized by T cells. Methods. 29:213–226 10.1016/S1046-2023(02)00344-4 [DOI] [PubMed] [Google Scholar]
- Kumamoto Y., Mattei L.M., Sellers S., Payne G.W., Iwasaki A. 2011. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc. Natl. Acad. Sci. USA. 108:8749–8754 10.1073/pnas.1100567108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Miao H., Henn A., Topham D.J., Wu H., Zand M.S., Mosmann T.R. 2012. Ki-67 expression reveals strong, transient influenza specific CD4 T cell responses after adult vaccination. Vaccine. 30:4581–4584 10.1016/j.vaccine.2012.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G., Foucat E., Dullaers M., Oh S., Sabzghabaei N., et al. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 34:108–121 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Lu B., Gerard C., Iwasaki A. 2009. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 462:510–513 10.1038/nature08511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya H.I., Wrammert J., Lee E.K., Racioppi L., Marie-Kunze S., Haining W.N., Means A.R., Kasturi S.P., Khan N., Li G.M., et al. 2011. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 12:786–795 10.1038/ni.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G.T. 2012. MHC class II tetramers. J. Immunol. 188:2477–2482 10.4049/jimmunol.1102398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell E.W., Sigal N., Bendall S.C., Nolan G.P., Davis M.M. 2012. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 36:142–152 10.1016/j.immuni.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porichis F., Kaufmann D.E. 2011. HIV-specific CD4 T cells and immune control of viral replication. Curr Opin HIV AIDS. 6:174–180 10.1097/COH.0b013e3283454058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe S., Flanders M., Cutler S., Soghoian D.Z., Ghebremichael M., Davis I., Lindqvist M., Pereyra F., Walker B.D., Heckerman D., Streeck H. 2012. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J. Virol. 86:277–283 10.1128/JVI.05577-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou C., Ganusov V.V., Campion S., Mlotshwa M., Liu M.K., Whale V.E., Goonetilleke N., Borrow P., Ferrari G., Betts M.R., et al. 2012. Distinct kinetics of Gag-specific CD4+ and CD8+ T cell responses during acute HIV-1 infection. J. Immunol. 188:2198–2206 10.4049/jimmunol.1102813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Rappuoli R. 2010. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 33:530–541 10.1016/j.immuni.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian D.Z., Streeck H. 2010. Cytolytic CD4(+) T cells in viral immunity. Expert Rev. Vaccines. 9:1453–1463 10.1586/erv.10.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian D.Z., Jessen H., Flanders M., Sierra-Davidson K., Cutler S., Pertel T., Ranasinghe S., Lindqvist M., Davis I., Lane K., et al. 2012. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci. Transl. Med. 4:123ra25 10.1126/scitranslmed.3003165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt T.M., McKinstry K.K., Dibble J.P., Winchell C., Kuang Y., Curtis J.D., Huston G., Dutton R.W., Swain S.L. 2010. Memory CD4+ T cells induce innate responses independently of pathogen. Nat. Med. 16:558–564: 1p: 564 10.1038/nm.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.L., McKinstry K.K., Strutt T.M. 2012. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 12:136–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Verhoeven D., Page C.A., Turner D., Farber D.L. 2010. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J. Virol. 84:9217–9226 10.1128/JVI.01069-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Chakrabarti L.A., Vingert B., Porichis F., Kaufmann D.E. 2011. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin. Immunol. 141:15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita R., Zarebski L., Greenbaum J.A., Emami H., Hoof I., Salimi N., Damle R., Sette A., Peters B. 2010. The immune epitope database 2.0. Nucleic Acids Res. 38(Database issue):D854–D862 10.1093/nar/gkp1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfurter J.T., Brunner K., Capuano S.V., III, Li C., Broman K.W., Kawaoka Y., Friedrich T.C. 2011. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog. 7:e1002381 10.1371/journal.ppat.1002381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel M., Oxenius A. 2012. From crucial to negligible: functional CD8+ T-cell responses and their dependence on CD4+ T-cell help. Eur. J. Immunol. 42:1080–1088 10.1002/eji.201142205 [DOI] [PubMed] [Google Scholar]
- Wilkinson T.M., Li C.K., Chui C.S., Huang A.K., Perkins M., Liebner J.C., Lambkin-Williams R., Gilbert A., Oxford J., Nicholas B., et al. 2012. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18:274–280 10.1038/nm.2612 [DOI] [PubMed] [Google Scholar]
- Williams M.A., Holmes B.J., Sun J.C., Bevan M.J. 2006. Developing and maintaining protective CD8+ memory T cells. Immunol. Rev. 211:146–153 10.1111/j.0105-2896.2006.00389.x [DOI] [PubMed] [Google Scholar]
- Yi J.S., Du M., Zajac A.J. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 324:1572–1576 10.1126/science.1175194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Callendret B., Xu D., Brasky K.M., Feng Z., Hensley L.L., Guedj J., Perelson A.S., Lemon S.M., Lanford R.E., Walker C.M. 2012. Dominance of the CD4+ T helper cell response during acute resolving hepatitis A virus infection.J. Exp. Med. 209:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]