New thymus transplant experiments reveal that in the absence of competing bone marrow progenitors, existing thymocytes can self-renew, guaranteeing thymus cellularity and the rapid reconstitution of the peripheral T cell pools.
Abstract
Thymus transplants can correct deficiencies of the thymus epithelium caused by the complete DiGeorge syndrome or FOXN1 mutations. However, thymus transplants were never used to correct T cell–intrinsic deficiencies because it is generally believed that thymocytes have short intrinsic lifespans. This notion is based on thymus transplantation experiments where it was shown that thymus-resident cells were rapidly replaced by progenitors originating in the bone marrow. In contrast, here we show that neonatal thymi transplanted into interleukin 7 receptor–deficient hosts harbor populations with extensive capacity to self-renew, and maintain continuous thymocyte generation and export. These thymus transplants reconstitute the full diversity of peripheral T cell repertoires one month after surgery, which is the earliest time point studied. Moreover, transplantation experiments performed across major histocompatibility barriers show that allogeneic transplanted thymi are not rejected, and allogeneic cells do not induce graft-versus-host disease; transplants induced partial or total protection to infection. These results challenge the current dogma that thymocytes cannot self-renew, and indicate a potential use of neonatal thymus transplants to correct T cell–intrinsic deficiencies. Finally, as found with mature T cells, they show that thymocyte survival is determined by the competition between incoming progenitors and resident cells.
T lymphocytes are fundamental for the control of infection. In rare cases, T cell deficiencies are congenital, caused by mutations preventing the expression of any gene required for T cell generation. However, in most cases they are induced in the adult either by infections such as AIDS, by aggressive anticancer therapies, or by aging. In the current clinical practice, these situations have become frequent, rendering the reconstitution of the peripheral T cell pool an important clinical goal. In children who do not yet have competent thymus epithelia, T cell reconstitution may be achieved by the transplantation of a competent BM. However, because BM precursors must transit through the thymus to generate T cells, the peripheral T cell reconstitution is delayed by many months, during which time patients are very susceptible to infections (Parkman and Weinberg, 1997; Holländer, 2008; Cavazzana-Calvo et al., 2009; Reimann et al., 2010). In addition, BM transplantation cannot correct T cell deficiencies once the thymus atrophies in adults.
Thymus transplants could constitute an advantageous alternative or complementary therapy; these grafts could be a source of both a functional thymus epithelia and functional T cells, and thus might correct T cell deficiencies in both children and adults. They would not necessarily require the conditioning of the patient and should export mature T cells immediately, overcoming the long lag-time required for thymus T cell generation after BM transplantation. However, although thymus grafts were successful in correcting deficiencies of the thymus epithelium, as found in the complete DiGeorge syndrome (Markert et al., 2007) or in FOXN1 mutations (Markert et al., 2011), they were never used to correct intrinsic T cell deficiencies, as it is generally accepted that the thymus does not harbor precursors with self-renewal capacities. Indeed, in thymus transplants, resident thymocytes generate a single wave of mature T cells because precursors originating from the BM rapidly replace resident cells (Berzins et al., 1998). Moreover, this occurs even when the host cannot generate mature T cells. When WT thymi are transplanted into SCID or Rag2-deficient hosts, the competent thymocyte populations from the graft are rapidly replaced by the incompetent precursors from the host BM (Frey et al., 1992; Takeda et al., 1996), and mature T cell export fails by 3 wk after surgery. Based on these data, the current dogma postulates that all thymocyte subpopulations are short-lived, with their maintenance being strictly dependent on the continuous input of BM-derived progenitors.
In contrast, we describe that the neonatal thymus harbors populations with self-renewal capacity that maintain the thymocyte populations independently of any input from the BM, rapidly reconstitute the peripheral T cell pools, and the capacity to clear infections in T cell–deficient mice. Moreover, these transplants function across histocompatibility barriers, recalling early studies on the susceptibility of neonatal cells to become tolerant to alloantigens (Billingham et al., 1953). These results highlight a possible application of thymus transplants in correcting intrinsic T cell deficiencies.
RESULTS AND DISCUSSION
The fate of thymus transplants in IL-7R–deficient hosts
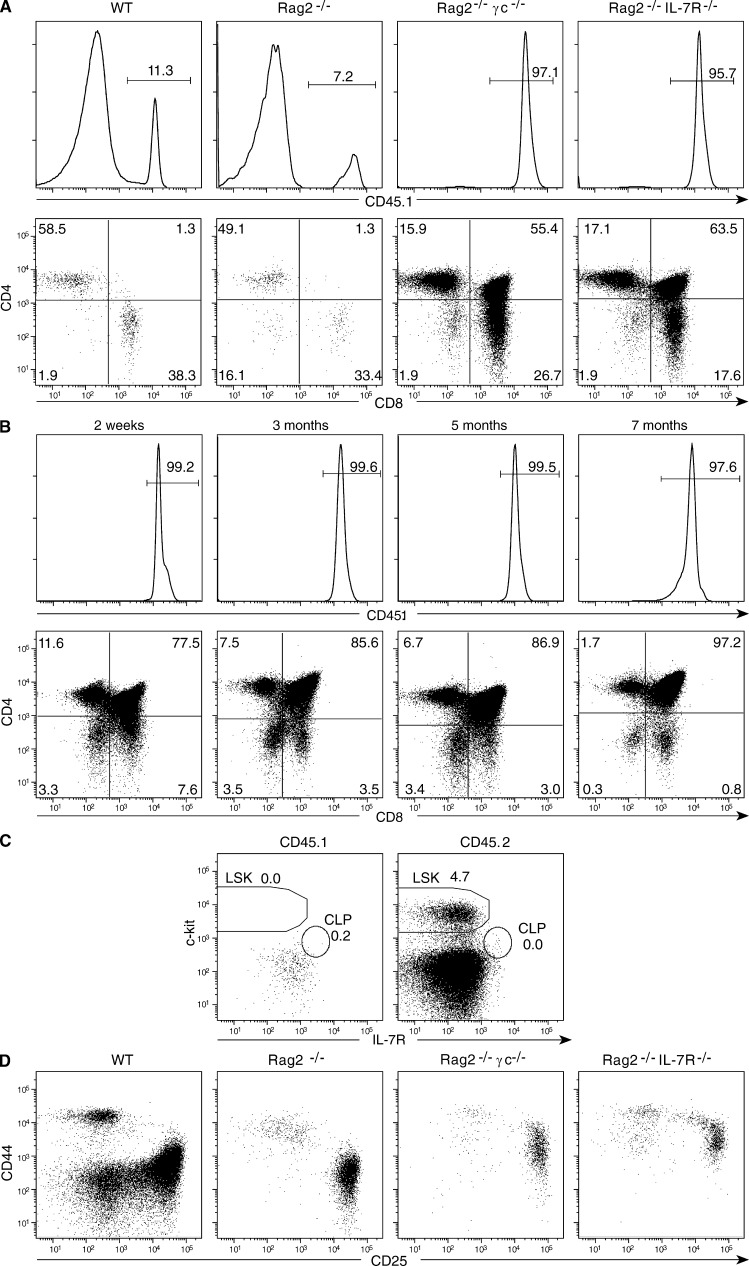
When searching for thymocyte populations able to generate gut intraepithelial lymphocytes (Lambolez et al., 2006; Peaudecerf et al., 2011) we found that thymus transplants behaved differently when transplanted into Ragγc− hosts. Indeed, as described previously (Berzins et al., 1998), when a single neonatal CD45.1+ thymus lobe is grafted into WT CD45.2+ hosts, the transplant is rapidly invaded by precursor cells derived from the host BM; 1 mo later, only rare mature T cells from the graft remain (Fig. 1 A). This substitution occurs even when the host cannot generate mature T cells (Frey et al., 1992; Takeda et al., 1996); when CD45.1+ thymi are transplanted into CD45.2+ Rag2- or CD3ε-deficient mice (not depicted) thymocytes of graft origin are also substituted by incompetent CD45.2+ precursors from the host BM (Fig. 1 A). In contrast, we were surprised to observe that this substitution did not occur when the hosts could not respond to IL-7; in CD45.2+ Rag2 IL-7R− or Rag2γc− hosts, the vast majority of thymocytes residing in the graft were CD45.1+ cells of graft origin (Fig. 1 A). These resident cells persisted and maintained a normal CD4/CD8αβ profile up to 7 mo after transplant, the latest time point studied (Fig. 1 B). However, from 4 mo after transplantation onwards, thymus cellularity usually declined, with 25% of the grafts undergoing full atrophy by 4 mo, and 50% by 7 mo after surgery. This decline may be caused by age-related hormonal influences because the host mice were 6–9 mo old. Alternatively, resident thymocytes might have exhausted self-renewal capacity.
Figure 1.
CD45.2+ B6 mice were transplanted with a single thymus lobe from 1-d-old CD45.1+ B6 mice. (A and B). Frequencies (histograms) and CD4/CD8β phenotypes (dot plots) of CD45.1+ donor thymocytes persisting in the thymus graft. (A). Hosts were WT, Rag2, Rag2γc, or Rag2 IL-7R–deficient, studied 1 mo after transplantation. (B) Hosts were Rag2γc-deficient, studied at different time points after transplantation. Similar results were obtained when hosts were Rag2 IL-7R–deficient. Results are from 1 experiment representative of 8 WT, 12 RagIL-7R–deficient, and 45 Rag2γc-deficient grafted mice, studied from 2 wk to 7 mo after grafting. (C) Stem cell precursors in the BM of host Rag2γc− transplanted mice, 3 mo after grafting. Results show Ter119−GR1−Mac1− cells of graft (CD45.1+) and the host (CD45.2+), and are representative of 5 individual mice studied in two experiments. (D) Repopulation of the transplanted thymi in different host mice. The CD44/CD25 phenotypes of CD45.2+ TN thymocytes derived from the host BM, 1 mo after grafting. They are representative of three experiments.
Mechanisms involved in autonomous thymocyte renewal
Several possibilities could explain the persistence of resident thymocytes in the thymus transplants grafted into IL-7R–deficient hosts. We excluded contamination by circulating hematopoietic stem cells, thereby ensuring continuous graft colonization. The host BM did not contain CD45.1+ precursors derived from the graft; the only precursors present were CD45.2+ ; LSK, Lineage− Sca-1+c-Kit+ BM precursors (LSKs) from the host, as it is characteristic for Rag IL-7R− or Ragγc− mice (Fig. 1 C). Thymocyte persistence was also not caused by any mechanism preventing the colonization of the graft by the host BM: all grafts were colonized by host BM-derived progenitors, which progressed through differentiation as characteristic of each set of host mice: WT BM generating all Lineage− CD4−CD8−CD3ε− triple-negative thymocytes (TN) precursor types, Rag2− BM-derived cells arresting their differentiation at the TN3, and IL-7R− or Ragγc− BM at the TN2 differentiation stage (Fig. 1 D; Egerton et al., 1990; Mombaerts et al., 1992; Di Santo et al., 1999). Finally, Ly5.1+ thymocytes of graft origin could have stopped dividing, but this was not the case (Fig. 2): we concluded that the persistence of a conserved CD4+/CD8αβ+ profile in the transplanted thymi indicated the presence of one or several populations with self-renewal capacity.
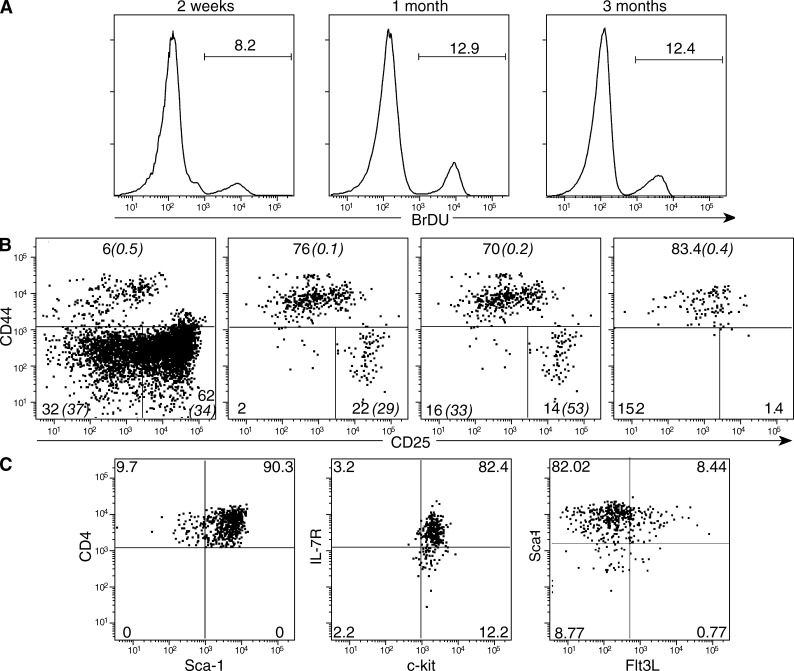
Figure 2.
The phenotype and division rates of the grafts’ thymocytes. Grafts were performed as in Fig. 1 B. At different time points, mice were injected with BrdU 1 h before sacrifice. (A) BrdU incorporation in DP cells in 1 out of 8 experiments with similar results. (B) The phenotype of CD45.1+ TN donor thymocytes at different time points after grafting. The percentage of cells incorporating BrdU in is italicized and in brackets. Results are from one out of 15 equivalent experiments (C) The phenotype of CD45.1+ TN1–TN2 thymocytes. Results are from one of five identical experiments.
We first considered that CD4+CD8αβ+ double-positive thymocytes(DP) cells might continuously renew in the absence of any intake from more immature precursors, although their BrdU incorporation was similar to that of DP cells from a normal thymus (Fig. 2 A). To address this possibility, we investigated DP cells’ TCRA repertoires, as the continuous division of the same cohort of DP cells should reduce diversity and/or switch TCRA repertoires to the preferential usage of 5′ Vα and 3′ Jα genes (Guo et al., 2002; Pasqual et al., 2002; Krangel et al., 2004). However, the high-throughput analysis of ∼107 TCRA chains expressed by normal DP thymocytes and 8 × 106 TCRA chains expressed by DP cells from transplanted thymi showed no modifications of TCRA repertoires (Fig. S1). Diversity was maintained because samples had equivalent number of unique in-frame CDR3s (Fig. S1 A), and VA and JA usage were also comparable (Fig. S1 B). Therefore, DP differentiation was not modified, indicating that maintenance of DP cells in these grafts should be ensured by more immature progenitors.
Analysis of CD45.1+ TN cells revealed the persistence of T cell progenitors of graft origin. Resident ETPs and TN2s declined rapidly (Fig. 2 B), likely substituted by the hosts’ TN precursors (Fig. 1 D). CD45.1+ TNs were progressively enriched in CD44+CD25low TN1–TN2 transition cells, with a CD44+ Sca-1+ c-kit+ IL-7Rlow, Fl3L+/− phenotype (Fig. 2, B and C). We previously studied this population in detail in the WT thymus and showed that it is more abundant in neonatal than in adult thymi (Peaudecerf et al., 2011) and is mostly T cell but not αβ/γδ lineage committed, yet may generate few NK cells. Moreover, these cells are capable of considerable expansion, generating all thymocyte sets as well as the gut nonconventional TCRαβ/γδ gut intraepithelial lymphocytes (Lambolez et al., 2006; Peaudecerf et al., 2011). Similar to neonatal TN1–TN2 cells, resident progenitors expressed all the molecular markers of T cell–committed progenitors (Notch1, Gata3, Bcl11b, and Rag1) and a constant small fraction divided; however, differentiation potential was restricted to T cell lineages because we could not visualize other hematopoietic populations of thymus graft origin in transplanted mice (unpublished data). Up to 5 mo after transplantation, TN3 and TN4 populations were also present, with TN3 division rates increasing by 3 mo after surgery. Coinciding with transplant atrophy, the TN3 and TN4 populations eventually disappeared (Fig. 2 B). The characteristics of such precursors explain the overall aspects of our data. They ensure that TCR rearrangements will follow relatively normal kinetics, justifying the transplants’ DP unbiased TCRA repertoires (Fig. S1), and the unbiased TCRB and TCRA repertoires found in the periphery (Fig. S2). They also explain why thymocytes from the transplanted thymi are only able to persist in hosts that cannot respond to IL-7. In other hosts, the BM will have the capacity to generate such cells, and thus substitute resident TN1–TN2 populations. In contrast, IL-7R– and γc-deficient hosts cannot generate these cells, explaining why TN1–TN2 transitional cells from the transplanted thymi persist in these hosts. Overall, these results showed that T cell production in the thymus is not necessarily dependent of a continuous input from the BM. In certain conditions, the thymus may be self-sufficient, continuously generating mature T cells.
The capacity of thymus transplants to reconstitute the peripheral T cell pools
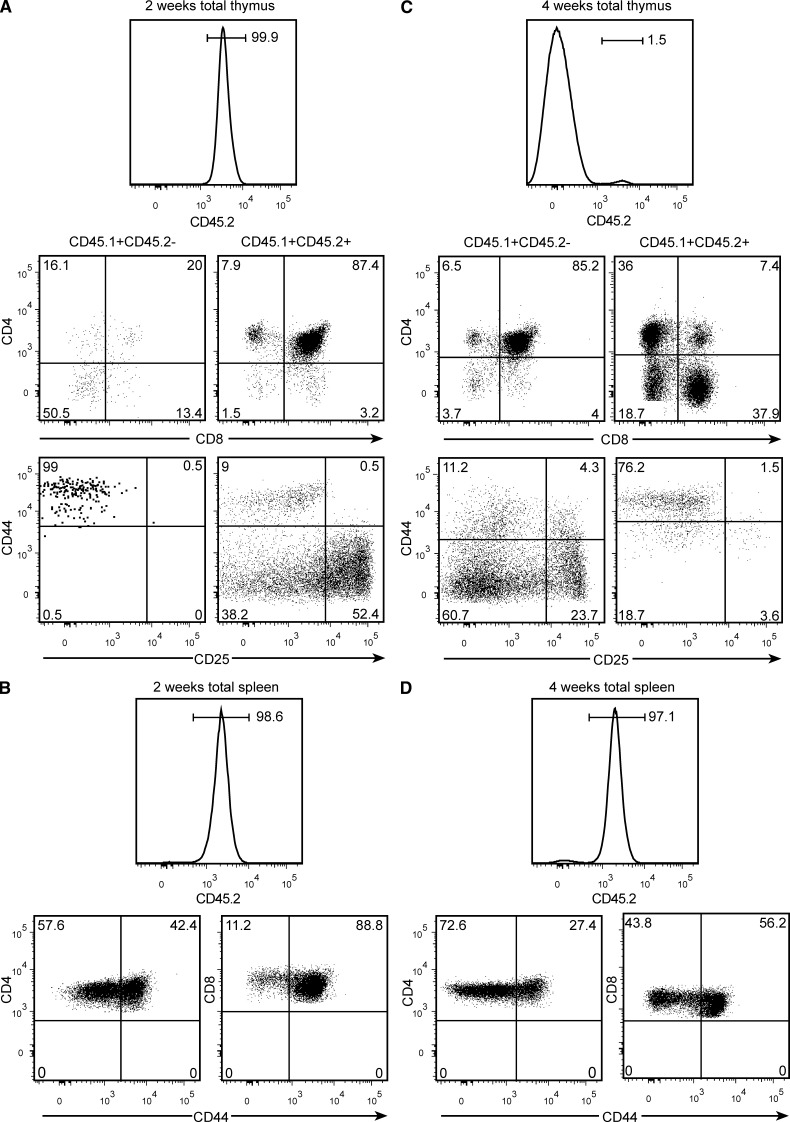
After BM transplantation in the mouse, mature T cells were reported to start leaving the thymus by 4 wk, and peripheral pools were found to be fully reconstituted only 2.5–3 mo later (Almeida et al., 2001). To directly compare the kinetics of peripheral reconstitution after BM or thymus transplants, sublethally irradiated CD45.2+ Ragγc− mice were injected simultaneously with 2 × 104 CD45.1+ LSKs and transplanted with one CD45.1+x CD45.2+ neonatal thymus lobe. 2 wk later, the progeny of the injected LSKs had barely reached the graft, most having the CD44+CD25− TN1 phenotype, whereas resident thymocytes maintained a normal CD4+CD8αβ+ profile (Fig. 3 A). The spleen of these mice, however, already harbored a substantial pool of naive T cells of graft origin, although CD44+s were enriched (Fig. 3 B). In contrast, 1 mo later, LSK-derived cells had replaced most resident thymocytes in the graft (Fig. 3 C), but had not yet exported mature T cells. Virtually all T lymphocytes present in the spleen were still of grafted thymus origin (Fig. 3 D). Compared to the CD4 and CD8 naive cells found in normal mice, the frequency of the naive CD4+ pool was equivalent and the frequency of CD8+ naive pool was slightly lower in mice receiving thymus transplants (Fig. 4, A and B), supporting the observation that peripheral reconstitution is caused by the export of naive T cells (see Martins et al. in this issue). These results formally demonstrate that thymus transplants reconstitute the peripheral T cell pools much earlier than BM-derived precursors. Moreover, they also show that the kinetics of BM reconstitution is not modified by the presence of a thymus graft, and that when competent BM precursors are present, resident thymocytes are substituted even in IL-7R–deficient hosts.
Figure 3.
The kinetics of peripheral reconstitution after BM or thymus grafts. CD45.2+ B6 Rag2γc− mice were sublethally irradiated (600 rads) and grafted simultaneously with 2 × 104 WT CD45.1+ LSKs, and a single thymus lobe from CD45.2+xCD45.1+ WT B6 neonatal mice and studied for 2 wk (A and B) and 1 mo later (C and D). (A and C) Percentages (histograms) and the phenotypes (dot plots) of thymus graft–derived (right) and BM-derived (left) cells in the grafted thymus. Top dot plots showing the CD4/CD8β profile, and bottom dot plots show TN cells. (B and D) Percentages (histograms) and CD44 expression (dot plots) of CD45.2+ T cells of thymus graft origin in the spleen. Results are from one mouse at each time point representative for the four mice studied.
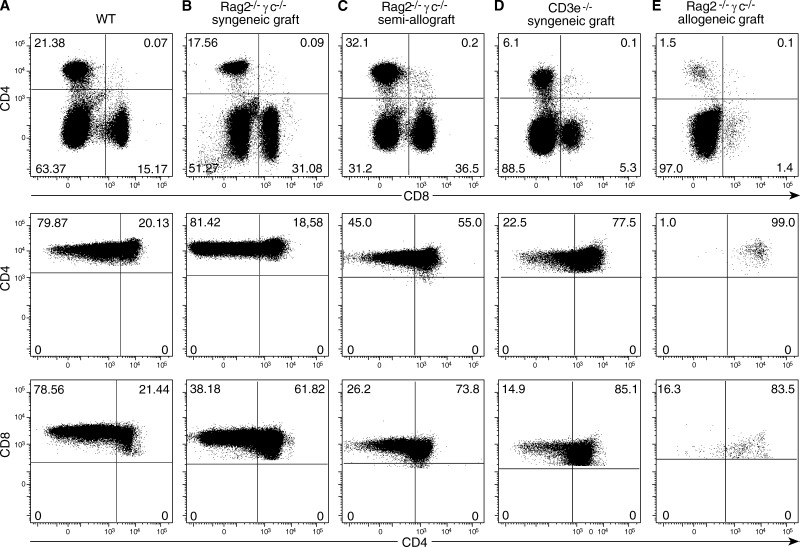
Figure 4.
Peripheral reconstitution after thymus transplantation in different conditions. Different host mice were transplanted with neonatal thymi and studied 1 mo later. Top dot plots show the distribution of CD4/CD8 T cells, and bottom dot blots show the ratio of naive and CD44+ activated cells in the spleen, in one out of five equivalent experiments.
To further evaluate the quality of the mature T cell repertoires generated by thymus transplantation, we analyzed the spleen T lymphocytes of Ragγc−-transplanted mice. High-throughput sequencing of ∼107 TCRB and TCRA chains showed the same number of unique CDR3s as found in normal mice (Fig. S2 A). VB/JB and VA/JA usage was also very similar (Fig. S2 B). In contrast to Martins et al. (2012), we did not find clonal expansions. In the rare cases when some V genes were used at slightly higher frequency (for example TCRB V12-2 or TCRA V12D-3 in the grafted spleen; Fig. S2 C) the analysis of these apparent “expansion peaks” showed that each corresponded to multiple T cells using the same V gene but otherwise differing in both TCRBJ usage and CDR3 composition. We conclude that thymus transplants already reconstitute the diversity of the peripheral T cell repertoires 1 mo after grafting.
Peripheral pools generated by thymus transplants in different conditions
IL-7R–deficient hosts are but a small minority of potential candidates for thymus transplantation, and fully histocompatible fetal thymi would not likely always be available for transplantation. For a wider therapeutic use, thymus grafts should also be beneficial in other conditions, i.e., partial histocompatibility match between the host and the donor thymi, and in hosts able to respond to IL-7, where thymus substitution by BM precursors occurs. We found a substantial reconstitution of the peripheral pools in Ragγc− B6 mice transplanted with (BALB/c x B6) F1 thymi, although the frequency of CD8+ naive T cells but not of naive CD4+ cells was slightly reduced when compared with syngeneic transplants (Fig. 4, B and C). When a single thymus lobe was transplanted into IL-7-competent, CD3ε-deficient mice a substantial peripheral T cell pool was generated and naive T cells were present, although, as expected, CD44+ cells were enriched by homeostatic proliferation (Fig. 4 D). These results recall early studies reporting that 10% of a normal thymus export is sufficient to reconstitute the peripheral T cell pools (Almeida et al., 2001).
Thymus transplants in children lacking thymus epithelium were reported to ensure peripheral reconstitution across full histocompatibility barriers, T cells selected in these thymi neither inducing graft-versus-host (GVH) reactions nor rejecting the transplanted tissue, and conferring protection to infections (Markert et al., 2007, 2011). We investigated if the same phenomena would occur when Ragγc− B6 hosts were transplanted with a fully allogeneic BALB/c neonatal thymus. As expected, these grafts were less efficient in reconstituting the periphery: naive cells, which require the recognition of the same MHC that induced their positive selection in the thymus to survive (Tanchot et al., 1997), were absent in these mice (Fig. 4 E). However, these mice were healthy, not showing any signs of GVH, indicating that the neonatal T cells exported by these thymi became tolerant to the host MHC. These results recall early studies (Billingham et al., 1953) reporting that neonatal T cells were easily tolerized. This was attributed to the neonatal environment, peculiar circulatory characteristics of neonatal mice promoting the extensive migration of T cells throughout nonlymphoid tissues (Arnold et al., 2005), or/and a putative immaturity of neonatal dendritic cells (Ridge et al., 1996) favoring tolerance induction. Because we transplanted adult mice, both of these mechanisms can be excluded, indicating that the properties of neonatal cells (do Canto et al., 2008) rather than their environment are responsible for tolerance induction. Besides the induction of T cell tolerance, early studies also described tolerance to fetal thymus tissue. When nude mice were transplanted into each kidney capsule with fetal thymi expressing different MHCs, neither thymus was rejected (Zamoyska et al., 1989). To investigate if a neonatal thymus epithelium would be tolerated, sublethally irradiated immunocompetent B6 mice were grafted with neonatal (B6xBALB/c) thymus and followed up for 2 mo after surgery. These transplants were invaded by BM-derived precursors from the host, but the thymus tissue was not rejected (unpublished data). The capacity of the thymus tissue to be tolerated, and the relative “indifference” of neonatal T lymphocytes issuing from transplanted thymi to MHC mismatches contrasts with the frequent GVH reactions induced by mismatched BM transplantation. It is tempting to speculate that this behavior may have evolved to prevent the possibly ill effects of MHC mismatches during pregnancy/delivery and that the use of fetal hematopoietic precursors for BM transplantation may minimize/prevent GVH reactions.
The capacity of thymus transplants to confer protection
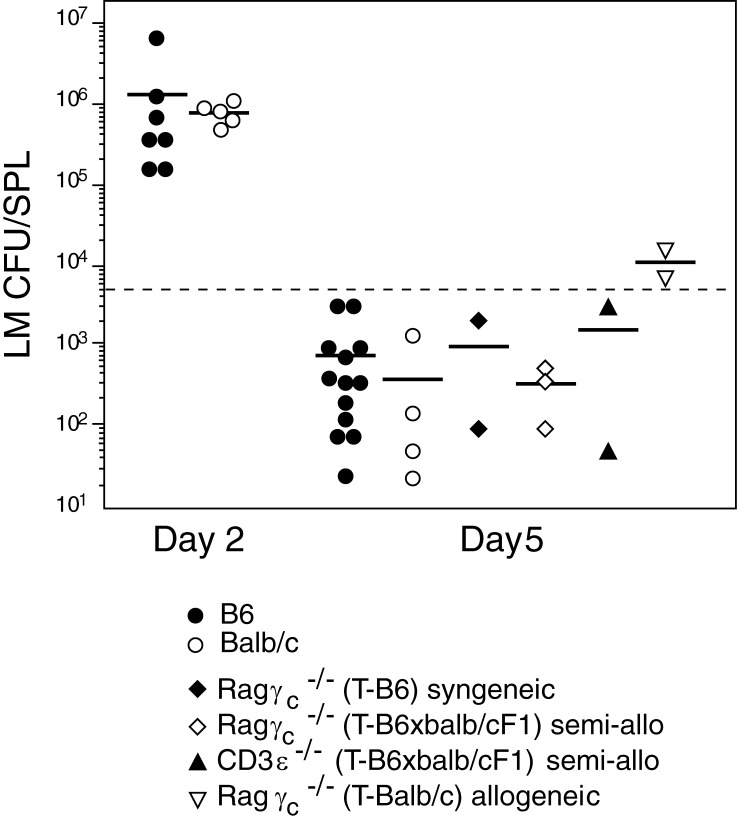
To determine the capacity of different types of transplanted mice to deal with infection, we studied the T cell–dependent response to Listeria monocytogenes (LM). We first determined the kinetics of LM elimination in WT mice in the conditions we used. We found that bacterial loads reached the highest levels by 2 d after infection. Bacteria elimination varied in individual mice, but by day 5, LM was still detectable in all mice (Fig. 5), whereas by day 6 several infected mice had already fully eliminated LM (not depicted). We thus selected day 5 after infection as the best time point to compare bacterial clearance between different transplanted mice. We found that transplants conferred protection to infection in all mice: LM was cleared with similar kinetics in normal mice, Ragγc− mice transplanted with syngeneic or semiallogeneic thymi, or even in IL-7R–competent CD3ε− mice transplanted with semiallogeneic thymi, where besides a partial MHC mismatch the thymus transplants originated a single wave of T cell export. These results indicate that thymus transplants may have wide applications as they are capable of precocious T cell generation and confer the capacity to clear infections even in IL-7R+ adult mice. Bacterial clearance was less efficient in mice transplanted with fully allogeneic BALB/c thymi, but even these mice were able to reduce bacterial loads (Fig. 5).
Figure 5.
The capacity of thymus transplants to protect from infection. WT B6 and BALB/c controls and the transplanted mice described in Fig. 4 were injected i.v with live LM. The dotted line shows the number of injected LM. Results show bacteria loads evaluated as LM CFU/spleen at day 2 and 5 after infection, each point showing an individual mouse. T denotes the genotype of donor thymus.
To summarize, the present data and also those from Martins et al. (2012) show that in contrast to the current dogma, maintenance of thymocyte populations does not depend on the continuous input of BM-derived progenitors. The thymus harbors populations with self-renewal capacities that are capable of maintaining apparently normal CD4/CD8αβ profiles for several months. However, our combined data show that autonomous thymocyte renewal may be achieved by different mechanisms. In our experiments, the unbiased TCRA repertoires of DP cells indicate a major contribution of early TN thymocyte progenitors, which are indeed present and persist for long time periods. In contrast, Martins et al. (2012) did not detect these progenitors, with the TCRA repertoires generated having reduced diversity and showing several clonal expansions even at early points after transplantation, indicating that autonomous thymocyte renewal in their conditions is instead ensured by the continuous division of the same cohort of DP cells. Importantly, the outcomes of these two types of autonomous renewal are also very different. While our mice survive, are protected from infection, and transplanted thymi eventually atrophy, the continuous division of the same DP cells likely favors genetic instability because a large fraction of them develop T cell lymphomas later in life. The reasons behind these differences are yet unclear, but our combined data provide an unbiased and complete perspective on the advantages and possible dangers of thymus transplantation. Thymus transplants may pose a risk when used alone in the therapy of congenital IL-7R deficiencies. However, thymus transplants rapidly export T cells, function across histocompatibility barriers, and confer rapid protection to infection even in IL-7R–competent hosts where the thymocytes from the graft are substituted by precursors originated in the host BM. Therefore, thymus transplantation may be of major therapeutic value for the rapid correction of other T cell deficiencies, either when used alone or in combination with simultaneous BM transplantation.
MATERIALS AND METHODS
Mice, transplantation procedures, and infection with LM.
BALB/c mice were purchased from Charles River, and all other mice were obtained from our breeding colonies. Host mice were 6–8-wk-old CD45.2+ and were transplanted under the kidney capsule with a single thymus lobe from 1-d-old mice. CD45.1+ B6, Rag2−, Rag2− IL7R−, or CD3ε− mice received a lobe from CD45.1+ or CD45.1xCD45.2 donor mice. For allogeneic transfers, CD45.1+ Rag2γc− mice were transplanted with a lobe from CD45.2+ Balb/c donors. Fully allogeneic transplants were performed in Rag2γc− CD45.1+ hosts, grafted with one thymus lobe from 1-d-old CD45.2+ BALB/c mice. When mentioned mice were injected with 5 × 103 live LM. Bacterial loads were evaluated at different time points after infection as CFU per spleen. Experiments were approved by Comitié d’ethique pour l’experimentation animale, licence # CEEA34.BR.020.12.
Cytofluorometry analysis.
For surface staining, the following mAbs obtained from BD were used: anti-CD45.1/Ly5.1 (A20-1.7), anti-CD45.2/Ly5.2 (104–2.1), anti-CD3 (145-2C11), anti-CD11b/Mac1 (M1/70), anti-CD25 (PC-61), anti-CD117/c-kit (2B8), anti-TCRb (H57-597), anti-GR1 (8C5), anti-erythroid cells (TER-119), and anti–Sca-1 (E13-161.7). Anti-CD4 (GK1.5), anti-CD8β (H35-172), and anti-CD127/IL7-Rα (A7R34, a gift from Dr S.-I. Nishikawa, Kyoto University, and Institute of Physical and Chemical Research Center, Kyoto, Japan) were purified and conjugated in our laboratory. Anti-CD44 (1M781) was obtained from eBioscience. All of the aforementioned mAbs were directly coupled to FITC, PE, PerCP-Cy5.5, PECy7, APC, APC-Alexa Fluor 750, and Pacific blue or conjugated with biotin. Biotinylated mAbs were revealed with PECy7-streptavidin (BD), PE-Alexa Fluor 750-streptavidin (Invitrogen), Pacific blue–streptavidin (Invitrogen), or Pacific orange–streptavidin (Invitrogen). Cells were analyzed in a FACSCanto and sorted in a FACSAria (BD). For the determination of cell division, each mouse received a 1-h pulse of 1 mg of BrDU i.p., and BrDU incorporation was determined using a BrdU Flow kit (BD).
High-throughput sequence analysis.
The populations analyzed were sorted from the thymus or the spleen of transplanted mice or age-matched controls. Deep sequence analysis of the TCRA and TCRB repertoires was performed as described previously (Wang et al., 2010).
Analysis of gene expression in the progenitors persisting in the thymus graft.
B6 CD45.2 Ragγc-deficient mice were transplanted with a single neonatal thymus lobe from CD45.1 syngeneic WT mice. 1 mo after surgery, TN1–TN2 populations were sorted as 10 or single cells into individual wells and tested for the expression of Gata3, Notch1, Bcl11b, Rag1, and a housekeeping gene by single-cell seminested RT-PCR, using the approach we described and validated previously (Peixoto et al., 2004). In total, we studied 23 wells recovered from three different transplanted mice, with similar results. The primers pairs used are listed in Table S1.
Online supplemental material.
Fig. S1 shows the CD4+CD8αβ+ (DP) populations persisting in the grafts. Figure S2 displays the repertoire of the peripheral T cell pools 1 mo after grafting. The primer sequences used for single-cell genetic profiling are listed in Table S1. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20120845/DC1.
Supplementary Material
Acknowledgments
The Institut National de la Santé et de la Recherche Médicale (INSERM) Unit 1020 receives core money from the INSERM. L. Peaudecerf., A. Galgano, and G. Krenn were supported by the European Research Council, and S. Lemos by the Fundaçao de Ciencia e Tecnologia, Portugal. This work was supported by a grant from the European Research Council.
The authors declare no conflicts of interests.
Footnotes
Abbreviations used:
- DP
- CD4+CD8αβ+ double-positive thymocytes
- GVH
- graft-versus-host
- LM
- Listeria monocytogenes
- LSK
- Lineage− Sca-1+c-Kit+ BM precursors
- TN
- Lineage− CD4−CD8−CD3ε− triple-negative thymocyte
References
- Almeida A.R., Borghans J.A., Freitas A.A. 2001. T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J. Exp. Med. 194:591–599 10.1084/jem.194.5.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B., Schüler T., Hämmerling G.J. 2005. Control of peripheral T-lymphocyte tolerance in neonates and adults. Trends Immunol. 26:406–411 10.1016/j.it.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Berzins S.P., Boyd R.L., Miller J.F. 1998. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J. Exp. Med. 187:1839–1848 10.1084/jem.187.11.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham R.E., Brent L., Medawar P.B. 1953. Actively acquired tolerance of foreign cells. Nature. 172:603–606 10.1038/172603a0 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., André-Schmutz I., Dal Cortivo L., Neven B., Hacein-Bey-Abina S., Fischer A. 2009. Immune reconstitution after haematopoietic stem cell transplantation: obstacles and anticipated progress. Curr. Opin. Immunol. 21:544–548 10.1016/j.coi.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Di Santo J.P., Aifantis I., Rosmaraki E., Garcia C., Feinberg J., Fehling H.J., Fischer A., von Boehmer H., Rocha B. 1999. The common cytokine receptor gamma chain and the pre-T cell receptor provide independent but critically overlapping signals in early alpha/beta T cell development. J. Exp. Med. 189:563–574 10.1084/jem.189.3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Canto F.B., Lima Junior C., Teixeira I.A., Bellio M., Nóbrega A., Fucs R. 2008. Susceptibility of neonatal T cells and adult thymocytes to peripheral tolerance to allogeneic stimuli. Immunology. 125:387–396 10.1111/j.1365-2567.2008.02855.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton M., Shortman K., Scollay R. 1990. The kinetics of immature murine thymocyte development in vivo. Int. Immunol. 2:501–507 10.1093/intimm/2.6.501 [DOI] [PubMed] [Google Scholar]
- Frey J.R., Ernst B., Surh C.D., Sprent J. 1992. Thymus-grafted SCID mice show transient thymopoiesis and limited depletion of V beta 11+ T cells. J. Exp. Med. 175:1067–1071 10.1084/jem.175.4.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Hawwari A., Li H., Sun Z., Mahanta S.K., Littman D.R., Krangel M.S., He Y.W. 2002. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat. Immunol. 3:469–476 10.1038/ni791 [DOI] [PubMed] [Google Scholar]
- Holländer G.A. 2008. Lymphoid reconstitution following hematopoietic stem cell transplantation. Of mice and men: progress made in HSCT immunobiology. Semin. Immunopathol. 30:369–370 10.1007/s00281-008-0139-y [DOI] [PubMed] [Google Scholar]
- Krangel M.S., Carabana J., Abbarategui I., Schlimgen R., Hawwari A. 2004. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol. Rev. 200:224–232 10.1111/j.0105-2896.2004.00155.x [DOI] [PubMed] [Google Scholar]
- Lambolez F., Arcangeli M.L., Joret A.M., Pasqualetto V., Cordier C., Di Santo J.P., Rocha B., Ezine S. 2006. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat. Immunol. 7:76–82 10.1038/ni1293 [DOI] [PubMed] [Google Scholar]
- Markert M.L., Devlin B.H., Alexieff M.J., Li J., McCarthy E.A., Gupton S.E., Chinn I.K., Hale L.P., Kepler T.B., He M., et al. 2007. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 109:4539–4547 10.1182/blood-2006-10-048652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert M.L., Marques J.G., Neven B., Devlin B.H., McCarthy E.A., Chinn I.K., Albuquerque A.S., Silva S.L., Pignata C., de Saint Basile G., et al. 2011. First use of thymus transplantation therapy for FOXN1 deficiency (nude/SCID): a report of 2 cases. Blood. 117:688–696 10.1182/blood-2010-06-292490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V.C., Ruggiero E., Schlenner S.M., Madan V., Schmidt M., Fink P.J., von Kalle C., Rodewald H.-R. 2012. Thymus-autonomous T cell development in the absence of progenitor import. J. Exp. Med. 209:1409–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 68:869–877 10.1016/0092-8674(92)90030-G [DOI] [PubMed] [Google Scholar]
- Parkman R., Weinberg K.I. 1997. Immunological reconstitution following bone marrow transplantation. Immunol. Rev. 157:73–78 10.1111/j.1600-065X.1997.tb00975.x [DOI] [PubMed] [Google Scholar]
- Pasqual N., Gallagher M., Aude-Garcia C., Loiodice M., Thuderoz F., Demongeot J., Ceredig R., Marche P.N., Jouvin-Marche E. 2002. Quantitative and qualitative changes in V-J α rearrangements during mouse thymocytes differentiation: implication for a limited T cell receptor alpha chain repertoire. J. Exp. Med. 196:1163–1173 10.1084/jem.20021074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaudecerf L., dos Santos P.R., Boudil A., Ezine S., Pardigon N., Rocha B. 2011. The role of the gut as a primary lymphoid organ: CD8αα intraepithelial T lymphocytes in euthymic mice derive from very immature CD44+ thymocyte precursors. Mucosal Immunol. 4:93–101 10.1038/mi.2010.47 [DOI] [PubMed] [Google Scholar]
- Peixoto A., Monteiro M., Rocha B., Veiga-Fernandes H. 2004. Quantification of multiple gene expression in individual cells. Genome Res. 14:1938–1947 10.1101/gr.2890204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann C., Dal Cortivo L., Hacein-Bey-Abina S., Fischer A., André-Schmutz I., Cavazzana-Calvo M. 2010. Advances in adoptive immunotherapy to accelerate T-cellular immune reconstitution after HLA-incompatible hematopoietic stem cell transplantation. Immunotherapy. 2:481–496 10.2217/imt.10.36 [DOI] [PubMed] [Google Scholar]
- Ridge J.P., Fuchs E.J., Matzinger P. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 271:1723–1726 10.1126/science.271.5256.1723 [DOI] [PubMed] [Google Scholar]
- Takeda S., Rodewald H.R., Arakawa H., Bluethmann H., Shimizu T. 1996. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 5:217–228 10.1016/S1074-7613(00)80317-9 [DOI] [PubMed] [Google Scholar]
- Tanchot C., Lemonnier F.A., Pérarnau B., Freitas A.A., Rocha B. 1997. Differential requirements for survival and proliferation of CD8 naïve or memory T cells. Science. 276:2057–2062 10.1126/science.276.5321.2057 [DOI] [PubMed] [Google Scholar]
- Wang C., Sanders C.M., Yang Q., Schroeder H.W., Jr, Wang E., Babrzadeh F., Gharizadeh B., Myers R.M., Hudson J.R., Jr, Davis R.W., Han J. 2010. High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc. Natl. Acad. Sci. USA. 107:1518–1523 10.1073/pnas.0913939107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoyska R., Waldmann H., Matzinger P. 1989. Peripheral tolerance mechanisms prevent the development of autoreactive T cells in chimeras grafted with two minor incompatible thymuses. Eur. J. Immunol. 19:111–117 10.1002/eji.1830190118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.