Abstract
The absence of specific diagnostic criteria, the urgency to begin plasma exchange treatment, and the risk for complications from plasma exchange make the initial evaluation of patients with suspected thrombotic thrombocytopenic purpura (TTP) difficult. Systemic infections may mimic the presenting clinical features of TTP. In the Oklahoma TTP-HUS (hemolytic-uremic syndrome) Registry, 1989–2010, 415 consecutive patients have been clinically diagnosed with their first episode of TTP; in 31 (7%) the presenting clinical features were subsequently attributed to a systemic infection. All 31 patients had diagnostic criteria for TTP; 16 (52%) had the complete “pentad” of microangiopathic hemolytic anemia, thrombocytopenia, neurologic abnormalities, renal failure and fever. Four (16%) of 25 patients who had ADAMTS13 measurements had <10% activity; three patients had a demonstrable ADAMTS13 inhibitor. Compared to 62 patients with severe ADAMTS13 deficiency (<10%) who had no recognized alternative disorders, patients with systemic infections had more frequent fever, coma, renal failure, and the complete “pentad” of clinical features. Seventeen different infectious etiologies were documented. A systematic literature review identified 67 additional patients with a diagnosis of TTP or HUS and also a systemic infection. Among all 98 patients, infections with 41 different bacteria, viruses, and fungi were documented, suggesting that many different systemic infections may mimic the presenting clinical features of TTP. Initial plasma exchange treatment is appropriate in critically ill patients with diagnostic features of TTP, even if a systemic infection is suspected. Continuing evaluation to document a systemic infection is essential to determine the appropriateness of continued plasma exchange.
Keywords: infection, thrombotic thrombocytopenic purpura, TTP, hemolytic uremic syndrome, HUS, ADAMTS13
INTRODUCTION
Thrombotic thrombocytopenic purpura (TTP) was first comprehensively described with a review of all published cases in 1966.(1) Based on this experience, a “pentad” of characteristic clinical features, anemia, thrombocytopenia, neurologic abnormalities, renal failure, and fever that occurred in 88–98% of the 271 patients, became the diagnostic criteria for TTP.(1) In that era, before the availability of effective treatment, only 10% of patients survived.(1) When plasma exchange was documented to increase survival to 78%,(2) initiation of treatment became urgent and therefore diagnostic criteria became less stringent. The diagnostic criteria for TTP developed for the clinical trial that established the efficacy of plasma exchange treatment(2) remain the current standard: only microangiopathic hemolytic anemia and thrombocytopenia with no apparent alternative etiology are required.(3) These less stringent diagnostic criteria resulted in an eight-fold increase in the number of patients treated with plasma exchange for TTP.(4) The apparent increased frequency of TTP may represent both improved recognition and inaccurate diagnosis. Multiple disorders, such as malignant hypertension, systemic malignancies, systemic lupus erythematosus, and systemic infections,(3;5–7) can mimic the diagnostic clinical features of TTP. The frequent uncertainty of the diagnosis of TTP, the urgency to initiate plasma exchange treatment in patients with suspected TTP, and the potential for serious complications of plasma exchange(8) can combine to create a management dilemma. The management dilemma is more difficult because patients may have both an acute inflammatory disorder, such as a systemic infection,(9;10) and TTP. In some patients, an infection may “trigger” the onset of an acute episode of TTP.(9;10)
The Oklahoma TTP-HUS (hemolytic-uremic syndrome) Registry has a unique perspective since patients are enrolled at the time of their initial diagnosis and request for plasma exchange treatment. All enrolled patients remain in the Registry, even if they are subsequently discovered to have an alternative disorder. Therefore the Registry reflects the intention to treat by physicians across our community, without retrospective judgment and patient selection. In this report we describe 31 patients who were diagnosed with TTP and treated with plasma exchange but in whom the presenting clinical features were subsequently attributed to a systemic infection. The goals of our report are: [1] to describe the presenting features and clinical course of these 31 patients, [2] to compare them to patients with severe ADAMTS13 deficiency who had no recognized alternative disorders, and [3] to document the variety of infectious etiologies in these 31 patients, supplemented by a systematic review of previous reports of patients who were diagnosed with both TTP and a systemic infection.
METHODS
The Oklahoma TTP-HUS Registry
The Registry includes all consecutive patients for whom the Oklahoma Blood Institute (OBI) has been requested to provide plasma exchange treatment for patients with a diagnosis of TTP or HUS since January 1, 1989.(3;7;11) The OBI is the sole provider of plasma exchange services for all hospitals in 58 of the 77 Oklahoma counties, a region with a population in 2000 of 2,310,000.(12) Therefore the Registry is an inception cohort of all consecutive patients of any age within a defined geographic region in whom the diagnosis of TTP or HUS was supported by a decision to initiate plasma exchange treatment. Because diagnostic criteria are not specific and TTP cannot be definitely excluded, all patients remain in the Registry with complete follow-up, even if the presenting clinical features are subsequently attributed to another etiology. The Oklahoma TTP-HUS Registry is approved by the institutional review boards of the University of Oklahoma Health Sciences Center and each participating hospital.
Patients diagnosed with TTP are heterogeneous. To provide consistency for analysis, patients are assigned in a hierarchical, sequential order to one of six clinical categories related to associated conditions and potential etiologies at the time of their initial episode:(3;7;11) [1] following hematopoietic stem cell transplantation (HSCT), [2] pregnant/postpartum, [3] drug association, [4] bloody diarrhea prodrome, [5] presence of an additional or alternative disorder which may have caused the presenting features, and [6] idiopathic, if the criteria for the previous five clinical categories were not fulfilled. Assignments to categories [1] and [2] are clear but assignments to the other clinical categories may be less clear. Patients assigned to category [5] are also heterogeneous, including patients with additional autoimmune disorders and patients in whom the presenting clinical features were subsequently attributed to disorders such as malignant hypertension, systemic malignancies, systemic lupus erythematosus, and systemic infections. Therefore the designation of clinical categories was determined by consensus among three of the authors (DRT, SKV, JNG) based on clinical data from the first episode and before results of the ADAMTS13 measurements were available.
This report describes patients assigned to clinical category [5] in which a systemic infection was judged to be the probable cause of the presenting clinical features that had initially suggested the diagnosis of TTP. Patients following hematopoietic stem cell transplantation, clinical category [1], have been previously reported and are not included in this report although many of these patients had systemic infections in addition to other complications.(13) Patients who presented with bloody diarrhea and documented or suspected enteric infections with Escherichia coli O157:H7 or related Shiga toxin-producing bacteria are assigned to clinical category [4]; they have also been previously reported and are not included in this report because the role of Shiga toxin as an etiology of TTP-HUS is well described.(14;15) The seven Registry patients with HIV infection were assigned to their appropriate clinical categories:(7;16) one had idiopathic TTP and an incidental diagnosis of HIV infection; in four patients the clinical features were attributed to malignant hypertension caused by HIV-associated nephropathy; in two patients the clinical features were attributed to the HIV infection itself and these two patients are included in this report.
Prior to 2010, we did not systematically collect data on symptoms attributable to infectious diseases that may have occurred prior to the presentation with TTP. For example, some patients have had symptoms of a respiratory infection or influenza that were resolving by the time symptoms attributed to TTP initially occurred. These patients with infections that clearly preceded the onset of TTP were not previously documented in our Registry and are not included in this report. The patients with systemic infections who are included in this report had the onset of severe symptoms suggesting the diagnosis of TTP but in whom the clinical features were subsequently attributed to an infection.
Demographic, clinical, and laboratory data were collected prospectively.(11) Laboratory data reported here are the most abnormal values from the day of diagnosis, defined as the day of the first plasma exchange treatment, ± 7 days.(11) Determination of neurologic and renal abnormalities has been previously defined.(11) Death related to the acute episode is defined as death occurring within 30 days of stopping plasma exchange treatment.(11) Routine collection of serum samples immediately before the first plasma exchange procedure began on November 13, 1995. Samples are sent to Drs. Johanna A. Kremer Hovinga and Bernhard Lämmle (Department of Hematology, Inselspital, Berne, Switzerland) for ADAMTS13 measurements twice per year.(7) ADAMTS13 activity is measured by two methods, quantitative immunoblotting of proteolyzed von Willebrand factor multimers(17;18) and a fluorogenic assay using the FRETS-VWF73 substrate.(19;20) In patients with ADAMTS13 activity <20%, inhibitor activity was measured by the FRETS assay. Normal ADAMTS13 activity was defined as >50% with both assays; severe ADAMTS13 deficiency was defined as <10% activity identified by either assay.(7) Patients with severe ADAMTS13 deficiency who had no recognized alternative disorders and who therefore were considered to have characteristic clinical features of TTP were selected for comparison to the patients with systemic infections.
Systematic Literature Review
To compare the experience of the Oklahoma Registry to previous published experience, we searched for articles reporting individual patient data that described systemic infections in patients diagnosed with TTP or HUS.
Data sources and search strategy
Ovid software was used to search the Medline database. Articles containing both a TTP or HUS-related key-word or Medical Subject Heading (MeSH) in the title or available text [thrombotic thrombocytopenic purpura, TTP, hemolyticuremic syndrome, HUS, thrombotic thrombocytopenic purpura-hemolytic uremic syndrome, TTP-HUS] and also a term for an infection were identified. The infection terms were both general [infection, mycoses, zoonoses, virus diseases, parasitic diseases, sepsis, bacterial infection, bacteremia, mycology] and specific. Throughout the process of article review, the list of key-words for specific infections was expanded with each new infectious disease that was identified as associated with TTP or HUS. The final key-word search terms for infectious diseases were: Acinetobacter anitratus, Actinomyces, Aeromonas hydrophila, anthrax, Bacillus anthracis, Bacteroides fragilis, Bordetella pertussis, brucellosis, Borrelia burgdorferi, Brucella melitensis, Campylobacter jejuni, Capnocytophaga canimorsus, Chlamydia pneumonia, Citrobacter, Clostridium, Clostridium difficile, Corynebacterium diphtheria, Q fever, Coxiella burnetti, ehrlichiosis, Ehrlichia, Ehrlichia chaffeenis, Ehrlichia equi, Enterococcus faecalis, Fusobacterium necrophoum, Helicobacter pylori, Legionaire, Legionaire's, Legionella pneumophila, leptospirosis, Leptospira, Leptospira bataviae, tuberculosis, Mycobacterium tuberculosis, Mycoplasma pneumoniae, Pseudomonas aeruginosa, rocky mountain spotted fever, rickettsial infection, Rickettsia rickettsii, Salmonella typhi, Salmonella typhimurium, Salmonella seftenberg, Staphylococcus, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus viridians, Yersinia enterocolitica, Yersinia pseudotuberculosis, adenovirus, coxsackie virus, cytomegalovirus, CMV, infectious mononucleosis, Epstein-Barr virus, EBV, hepatitis A virus, HAV, hepatitis B virus, HBV, hepatitis C virus, HCV, hepatitis D virus, HDV, hepatitis E virus, HEV, herpes simplex, human enterovirus, influenza virus, influenza A. influenza B, influenza C, parvovirus, parvovirus B19, herpes zoster, varicella zoster virus, Aspergillus, Aspergillus fumigatus, Candida albicans, Cryptococcus, Histoplasmosis, Entamoeba histolytica. Bibliographies of the articles selected for review and the files of the authors were also searched to identify additional articles.
Article and patient selection
Articles were selected for review if their title and abstract suggested that they reported data on individual patients with a diagnosis of TTP or HUS and a systemic infection. We accepted the authors' diagnosis of TTP or HUS and did not attempt to judge the validity of the diagnosis. Patient inclusion criteria were [1] explicit diagnosis of TTP or HUS, [2] age of 18 years or older, [3] documentation of the etiology of the infection, and [4] documentation that the infection preceded the diagnosis of TTP or HUS. Exclusion criteria were [1] infections with Escherichia coli O157:H7 or related Shiga toxin-producing bacteria, [2] HIV infection, [3] chronic infections of long duration, such as serologic evidence of hepatitis C, and [4] infections associated immunosuppressive treatment, HSCT or organ transplantation, or systemic malignancies.
Children and patients with Shiga toxin-related infections were excluded because a preliminary analysis documented that most articles reporting children described enteric infections that were assumed to cause diarrhea-associated HUS, a clinical category of Oklahoma Registry patients not included in this analysis. Also all 31 patients from the Oklahoma Registry were adults. Therefore articles published in pediatric journals were not retrieved. Patients whose infection occurred following the diagnosis of TTP or HUS and initiation of plasma exchange treatment were excluded because the goal of this review was to identify preceding infections that may have mimicked the diagnostic criteria for TTP. Also systemic infections occurring after plasma exchange treatment has begun may be a complication of the central venous catheter.(8) HIV infections were excluded because the association of HIV infection with TTP has been well described.(16) Patients with long-standing chronic infections were excluded because it was assumed that they may have been unrelated to the diagnostic decisions for an acute episode of TTP or HUS. Patients with infections associated immunosuppressive treatment, HSCT or organ transplantation, or systemic malignancies were excluded because interpretation of these reports on complex patients was often uncertain.(6;21)
Article review
All articles were reviewed independently by two of the authors (KKB, JNG). Disagreements of interpretation were resolved with discussion and re-review.
Statistical analysis
Two groups of patients were compared: [1] the 25 patients in whom presenting clinical features were subsequently attributed to systemic infection and who had ADAMTS13 activity measured and [2] the 62 patients with severe ADAMTS13 deficiency (activity <10%) in whom presenting clinical features were attributed to TTP. The nonparametric Mann-Whitney test was used to compare interval/ratio data. The chi-square test or Fisher's exact test were used to compare categorical data. The nonparametric Mann-Whitney test was used because the data were not normally distributed. The Fisher's exact test was used when the categorical data contained group subsets that had actual values of zero or very small numbers leading to expected values <5; otherwise the chi-square test was used. SAS software, version 9.2 (SAS Institute, Inc., Cary, North Carolina), was used to perform all analyses. A P value less than 0.05 was considered statistically significant.
RESULTS
Presenting features and clinical course of Oklahoma TTP-HUS Registry patients in whom clinical features were attributed to a systemic infection
During 22 years, 1989–2010, the Registry enrolled 415 consecutive patients with an initial episode of clinically diagnosed TTP. In 31 (7%) patients the presenting clinical features were subsequently attributed to a systemic infection (Tables 1, 2). All 31 patients had microangiopathic hemolytic anemia and thrombocytopenia; 27 (87%) had neurologic abnormalities which were major in 20 (65%) patients; 28 (90%) had abnormal renal function; 21 (68%) had fever; 16 (52%) had the complete “pentad” of these clinical features. Although coagulation parameters (prothrombin time, activated partial thromboplastin time, and fibrinogen concentration) were not systematically collected, data from the initial hospitalization were available for 29 patients, including fibrinogen measurements on 20 patients. Results of one or more of the coagulation parameters were abnormal in 14 (48%) of patients; abnormalities were attributed to disseminated intravascular coagulation (DIC) in 10 patients and to severe liver disease in four. Twenty-two (71%) patients had preceding health problems that may have contributed to risk for infection. TTP was diagnosed and plasma exchange ordered by 23 different physicians across 15 of the 22 years of the Registry, from 1991 to 2010. Eighteen of the 23 physicians managed only one patient. One of the authors (JNG) examined 23 (74%) of these patients during their initial hospitalization and was responsible for the initial diagnosis of TTP and initiation of plasma exchange treatment in two of the patients.
Table 1.
Presenting features of the 31 patients in whom TTP was initially diagnosed and plasma exchange treatment begun, and in whom the clinical abnormalities were subsequently attributed to a systemic infection. Year indicates year of diagnosis. Major neurologic abnormalities were coma, stroke, seizures, and focal signs. Minor neurologic abnormalities in these 31 patients were confusion and headache.
| No. | Age | Year | Presenting clinical features | Laboratory data | Pentad | Preceding disorders | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Neuro | Other | Hct | Plt | Cr | LDH | ADAMTS13 | |||||
| 1 | 70 | 1991 | + | Major | Cough, pulmonary infiltrates, | 23 | 9 | 8.0 | 5120 | NA | + | None |
| 2 | 49 | 1995 | + | Major | Multiple cerebral, renal infarcts | 20 | 21 | 1.7 | 2150 | NA | + | None |
| 3 | 71 | 1996 | + | Major | Rigid neck, + Brudzinski, Kernig signs, CSF: 90 WBC, 68% neutrophils, negative cultures | 24 | 14 | 4.1 | 1904 | 40% | + | Chronic kidney disease |
| 4 | 57 | 1996 | 0 | Major | Abdominal pain, acral cyanosis, | 17 | 7 | 3.5 | 2458 | 70% | 0 | None |
| 5 | 80 | 1996 | + | Major | pulmonary infiltrates, respiratory arrest | 22 | 20 | 0.6 | 1201 | NA | 0 | Resection of lung cancer 3 mo previously |
| 6 | 48 | 1997 | + | Major | Chills, myalgias | 16 | 9 | 0.8 | 1884 | 100% | 0 | Sarcoidosis, steroids azathrioprine |
| 7 | 39 | 1997 | + | Major | Jaundice, anuria, Dx hepatitis A before TTP considered | 15 | 2 | 6.6 | 1845 | 9% (1.3 BU) | + | None |
| 8 | 47 | 1998 | + | Major | Chills, pulmonary infiltrate, hypotensive | 25 | 13 | 4.3 | 1373 | 9% (0 BU) | + | Diabetes, Alcoholism |
| 9 | 37 | 1999 | + | Major | Chills, muscle tenderness, purpuric cyanotic extremities | 25 | 10 | 4.1 | 777 | 25% | + | None |
| 10 | 78 | 2000 | + | Minor | Chills, blood cultures reported + for S. viridans, E. aerogenes, mitral valve vegetation, before TTP considered | 25 | 8 | 2.8 | 1006 | 30% | + | Pancreatitis 4 weeks previously |
| 11 | 79 | 2000 | 0 | Major | Suddenly unresponsive, hypotensive, digital ischemia and gangrene | 26 | 15 | 1.8 | 309 | 15% (0 BU) | 0 | Cholecystectomy 4 weeks previously |
| 12 | 27 | 2001 | + | Major | HIV Dx 4 weeks previously, suddenly unresponsive | 18 | 11 | 11.1 | 3108 | 12% (1.7 BU) | + | HIV infection |
| 13 | 39 | 2001 | + | Major | Chills, abdominal pain | 40 | 22 | 5.4 | 1078 | 40% | + | None |
| 14 | 72 | 2001 | 0 | Major | GI bleeding, hypotension, pneumonia, CMV antigen + reported before TTP considered | 29 | 9 | 3.7 | 513 | 20% | 0 | Focal segmental glomerulosclerosis, cyclophosphamide, steroid |
| 15 | 65 | 2001 | 0 | Minor | Cough, bilateral pneumonia, confusion, coma | 26 | 7 | 2 | 256 | 8% (1.2 BU) | 0 | Hepatitis C, liver transplant 4 years previously; tacrolimus |
| 16 | 74 | 2002 | + | Major | Chills, cough, pneumonia | 30 | 37 | 5.1 | 1587 | NA | + | Coronary artery bypass reoperation 6 weeks previously. |
| 17 | 82 | 2002 | + | Major | abdominal pain, jaundice | 26 | 14 | 6.1 | 467 | 90% | + | Diabetes |
| 18 | 51 | 2002 | + | Major | Weakness, diarrhea | 23 | 18 | 1.4 | 298 | 60% | + | Back surgery 4 mo previously |
| 19 | 63 | 2002 | + | Minor | Nausea, vomiting, confusion, lung mass | 31 | 21 | 7.5 | 451 | 85% | + | Renal transplant 11 mo previously, on rapamycin, mycophenolate mofetil, prednisone |
| 20 | 43 | 2003 | + | Major | Chills, seizure, cyanosis, necrosis of hands, feet | 33 | 14 | 6.4 | 2198 | NA | + | Alcoholism |
| 21 | 48 | 2003 | + | Minor | Chills, pneumonia. BAL + Cryptococcus, Aspergillus; blood cultures + Cryptococcus, Acinetobacter, Enterococcus; CMV antigenemia. All reported before TTP considered. | 21 | 9 | 7.4 | 897 | 45% | + | Renal transplant 6 mo previously, mycophenolate mofetil, rapamune, tacrolimus, prednisone |
| 22 | 50 | 2003 | 0 | Minor | Pneumonia, hypotension | 27 | 41 | 8.8 | 354 | 45% | 0 | Alcoholism, aortic valve replacement 3 years previously |
| 23 | 83 | 2005 | 0 | Minor | Pulmonary infiltrates, intestinal perforation. | 23 | 13 | 4.3 | 553 | 40% | 0 | None |
| 24 | 62 | 2005 | + | 0 | Chills, dyspnea, jaundice, hypotension | 24 | 19 | 3.2 | 3459 | 65% | 0 | Cardiac pacemaker placed 6 weeks previously |
| 25 | 60 | 2007 | 0 | Minor | Shoulder pain, hypotension, respiratory distress | 25 | 9 | 1.8 | 632 | 67% | 0 | Traumatic osteoarthritis (5 sites) requiring multiple surgeries |
| 26 | 60 | 2007 | + | 0 | Weakness, dyspnea. Blood cultures + for S. viridans, tricuspid valve vegetation reported before TTP considered | 22 | 13 | 2.1 | 316 | 47% | 0 | Alcoholism, IV drug abuse |
| 27 | 55 | 2008 | 0 | Major | Dyspnea, chest pain, myocardial infarction, stroke | 33 | 33 | 1.8 | 1084 | <5 (0BU) | 0 | None |
| 28 | 85 | 2008 | 0 | Major | Dyspnea, abdominal pain | 24 | 71 | 2.6 | 2633 | 40% | 0 | COPD, steroid |
| 29 | 57 | 2008 | + | 0 | Renal graft rejection, hypotension, blood cultures + for Enterobacter cloacae reported before TTP considered | 12 | 56 | 4.1 | 4851 | Artifact | 0 | Liver/kidney transplant 2 weeks previously, mycophenolate mofetil |
| 30 | 61 | 2009 | + | Major | Chills, petechiae, cyanosis of hands and feet, 1 week after tick bite. | 27 | 36 | 1.9 | 1922 | 25% | + | None |
| 31 | 45 | 2010 | 0 | 0 | Dizziness, myalgias, abdominal pain | 26 | 10 | 6.7 | 2563 | 38% | 0 | HIV infection |
(11) NA, not available; CSF, cerebrospinal fluid; Dx, diagnosis; BU, Bethesda Units; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease.
Table 2.
Clinical course and outcomes of the 31 patients in whom TTP was initially diagnosed and plasma exchange treatment begun, and in whom the clinical abnormalities were subsequently attributed to a systemic infection. Recovery is indicated if the patient survived for more than 30 days following discontinuation of plasma exchange treatment. PEX, plasma exchange.
| No. | PEX | Clinical course following diagnosis of TTP | Infection etiology documented | Outcome (day of death) |
|---|---|---|---|---|
| 1 | 6 | PEX stopped, no response. Dx at autopsy: systemic Aspergillus fumigatus, no evidence of TTP | Aspergillus fumigatus | Died (11) |
| 2 | 1 | PEX stopped, death. Dx at autopsy: mitral valve vegetation, no bacteria seen, multi-organ infarcts, no evidence of TTP. Clinical impression, infectious endocarditis | None documented | Died (2) |
| 3 | 3 | PEX stopped, death. Clinical impression, infectious meningitis | None documented | Died (4) |
| 4 | 9 | PEX stopped, death. Rickettsia rickettsii serology + from before PEX, reported after PEX began | Rickettsia rickettsii | Died (9) |
| 5 | 11 | Blood, sputum, urine + Enterococcus faecalis day 6, assumed to be etiology of pneumonia at admission. PEX continued after Dx of infection, stopped at death | Enterococcus faecalis | Died (12) |
| 6 | 2 | PEX stopped when CMV antigen + reported, day 2 | CMV | Died (7) |
| 7 | 3 | PEX stopped, no response. Autopsy: hepatic necrosis, no evidence for TTP | Hepatitis A | Died (6) |
| 8 | 2 | PEX stopped when blood cultures from before PEX reported + for S. pyogenes | Streptococcus pyogenes | Survived |
| 9 | 2 | PEX stopped, death. Rickettsia rickettsii serology + on day 3. Dx at autopsy: myocardial necrosis, systemic vasculitis, no evidence for TTP | Rickettsia rickettsii | Died (3) |
| 10 | 2 | PEX stopped, death | Streptococcus viridans, Enterobacter aerogenes | Died (3) |
| 11 | 4 | PEX stopped, death. Cultures negative. Clinical impression, sepsis possibly related to recent cholecystitis | None documented | Died (5) |
| 12 | 7 | PEX stopped, no response. Continued treatment for HIV infection. No other infectious etiology discovered | HIV | Survived |
| 13 | 1 | PEX stopped, death. Dx at autopsy: tissue immunohistochemical and PCR + for Rickettsia rickettsii; no evidence for TTP | Rickettsia rickettsii | Died (2) |
| 14 | 2 | PEX stopped, death. | CMV | Died (3) |
| 15 | 7 | PEX stopped, death. Sputum culture + for Candida albicans PEX continued 2 days after Dx of infection | Candida albicans | Died (7) |
| 16 | 0 | Progressive respiratory failure, cultures negative. Family declined PEX. Clinical impression, bacterial pneumonia. | None documented | Died (1) |
| 17 | 18 | Blood cultures before PEX begun + for Pseudomonas and Enterococcus, reported after PEX begun, no infection source identified. PEX continued after Dx of sepsis; stopped, no response. | Pseudomonas aeruginosa, Enterococcus faecalis | Survived |
| 18 | 2 | Blood cultures before PEX begun + for S. aureus. PEX stopped when + blood cultures reported | Staphylococcus aureus | Survived |
| 19 | 14 | PEX stopped, no response. Developed skin nodules after PEX stopped, biopsy + for Blastomyces dermatitidis. | Blastomyces dermatitidis | Survived |
| 20 | 0 | Blood cultures before PEX begun + for Streptococcus pneumoniae. Died before PEX begun. | Streptococcus pneumoniae | Died (1) |
| 21 | 3 | PEX stopped, death. | Cryptococcus neoformans, Aspergillus fumigatus, Acinetobacter baumanii, Enterococcus faecalis, CMV | Died (4) |
| 22 | 3 | Blood cultures before PEX begun + for S. pneumoniae; PEX stopped when + blood cultures reported | Streptococcus pneumoniae | Survived |
| 23 | 4 | PEX stopped, death. Cultures negative. Clinical impression, bacterial pneumonia, sepsis. | None documented | Died (5) |
| 24 | 10 | PEX stopped, no response. Dx at autopsy: intracardiac thrombus around pacemaker wires infected with Aspergillus fumigatus causing pulmonary infarcts; no evidence for TTP | Aspergillus fumigatus | Died (12) |
| 25 | 19 | PEX stopped when S. pyogenes osteomyelitis Dx by shoulder surgery | Streptococcus pyogenes | Survived |
| 26 | 5 | PEX stopped, no response. | Streptococcus viridans | Died (28) |
| 27 | 12 | Blood cultures before PEX begun + for S. epidermidis; aortic valve vegetation. PEX continued after Dx of endocarditis until platelet count normal | Staphylococcus epidermidis | Survived |
| 28 | 4 | PEX stopped, no response. Cholecystitis Dx by ultrasound, surgery contraindicated, blood cultures negative. Day 4, repeat ultrasound demonstrated gangrenous gall bladder. | None documented | Died (6) |
| 29 | 4 | PEX stopped, no response. | Enterobacter cloacae | Survived |
| 30 | 1 | Died during 1st PEX. Clinical impression, Rickettsia rickettsii; serologic tests negative | None documented | Died (1) |
| 31 | 6 | PEX stopped when HIV infection diagnosed. No other etiologies for clinical features determined. | HIV | Survived |
In six (19%) patients, the physician considered TTP as an additional diagnosis after a systemic infection had been previously diagnosed. In three of these patients the plasma exchange treatment was subsequently stopped because of no response; in the other three patients, plasma exchange was continued for 2–3 days until death. In the other 25 (81%) patients, although a systemic infection may have been suspected when plasma exchange treatment was begun, the diagnosis remained uncertain. Plasma exchange treatment was then stopped when the systemic infection was diagnosed or because of no response or death in 21 patients. In four patients, plasma exchange treatment was continued after the etiology of the preceding systemic infection was identified because of continuing concern about TTP as an additional diagnosis. In all 31 patients, the final clinical impression was that the systemic infection had caused the presenting clinical features. This impression was confirmed in all six patients who had autopsy examinations: the diagnosis of systemic infection was documented and no evidence for TTP was identified. Twenty-one (68%) patients died during their acute episode.
ADAMTS13 activity was measured in 25 (81%) of the 31 patients. Two patients presented before routine serum sample collection began in 1995; two patients died before a serum sample could be collected; the sample for one patient was lost; in one patient extreme hemoglobinemia made the assay invalid.(7) ADAMTS13 activity was normal (60–100%) in both assays in seven (28%) patients; activity was low (12–47%) in 14 (56%) patients, and severely deficient (<5–9%) in four (16%) patients in at least one assay. Three of the six patients with ADAMTS13 activity <20% had demonstrable ADAMTS13 inhibitors (1.2–1.7 Bethesda Units [BU]). Three of the four patients with ADAMTS13 activity <10% had discrepant values by the two assays: patient 7: 9% by FRETS, 20% by immunoblot (IB); patient 8: 12% by FRETS, 9% by IB; patient 15: 28% by FRETS, 8% by IB.(7) Patients 7 and 15 had demonstrable ADAMTS13 inhibitors. Although the magnitude of these discrepancies is small, the significance is that each of these three patients had one value for ADAMTS13 activity that was less than 10% and therefore was designated as having severe ADAMTS13 deficiency.(7) Patient 27 had <5% ADAMTS13 activity by both assays but no demonstrable inhibitor. She is the only patient who has had repeat ADAMTS13 activity measurements during remission: one year after recovery, in 2009, her ADAMTS13 activity was 92% by FRETS and 100% by IB; in 2010 her ADAMTS13 activity was <5% by FRETS, without a demonstrable inhibitor, and 50% by IB. Although we attributed her acute illness to bacterial endocarditis and not TTP, we acknowledge that she could have had both bacterial endocarditis and TTP.
Comparison of patients with systemic infections to patients with severe ADAMTS13 deficiency who had no recognized alternative disorders
From November 13, 1995 to December 31, 2010, 68 consecutive patients had ADAMTS13 activity <10%, including the four patients described in Table 1 in whom the clinical features were attributed to systemic infection and two other patients who were subsequently diagnosed with alternative disorders (one following HSCT with acute graft-vs.-host disease and infection, one with systemic malignancy(7)). These six patients were excluded from the comparison group of ADAMTS13-deficient patients because the goal for this group was to include patients who clinical features were attributable only to TTP. ADAMTS13 activity was measured in 25 (81%) of the patients with systemic infections. The four patients with a systemic infection who had ADAMTS13 activity <10% are included in the group of patients with systemic infections. Therefore Table 3 compares the demographic, clinical, and laboratory features and outcomes of the 25 patients with systemic infections who also had ADAMTS13 measurements to the 62 patients with severe ADAMTS13 deficiency who did not have an alternative etiology for their clinical features.
Table 3.
25 patients in whom presenting clinical features were subsequently attributed to systemic infection and who had ADAMTS13 activity measured are compared to 62 patients with severe ADAMTS13 deficiency (activity <10%) in whom presenting clinical features were attributed to TTP.
| Clinical features and outcome | Patients with systemic infection (N=25) | Patients with ADAMTS13 <10% (N=62) | P |
|---|---|---|---|
| Demographic features | |||
| age (median, range) | 60 (26–85) | 39 (9–71) | <0.001 |
| race (% black) | 2 (8%) | 23 (37%) | 0.007 |
| gender (% women) | 11 (44%) | 51 (82%) | <0.001 |
| Clinical features | |||
| fever (%) | 15 (60%) | 13 (21%) | 0.001 |
| neurologic abnormalities (%) | |||
| major | 15 (60%) | 31 (50%) | 0.183 |
| minor | 7 (28%) | 12 (19%) | |
| none | 3 (12%) | 19 (31%) | |
| major neurologic abnormalities (%) | |||
| coma | 13 (52%) | 5 (8%) | <0.001 |
| stroke | 2 (8%) | 8 (13%) | 0.717 |
| seizure | 3 (12%) | 10 (16%) | 0.749 |
| focal deficits | 3 (12%) | 23 (37%) | 0.021 |
| Laboratory data (median, range) | |||
| hematocrit (%) | 25 (15–40) | 21 (13–30) | 0.005 |
| platelet (μL × 10–3) | 13 (2–71) | 11 (2–101) | 0.043 |
| LDH (U/L) | 1006 (256–3459) | 1378 (274–3909) | 0.087 |
| creatinine (mg/dL) | 3.7 (0.8–11.1) | 1.3 (0.7–6.5) | <0.001 |
| Pentad of clinical features (%) | 11 (44%) | 2 (3%) | <0.001 |
| Outcome | |||
| death (%) | 16 (64%) | 9 (15%) | <0.001 |
| relapse (% of survivors) | 0/9 (0%) | 18/53 (34%) | <0.001 |
Six of the 31 patients with systemic infections were excluded from this analysis because they did not have ADAMTS13 measurements. Six of all 68 consecutive patients who had ADAMTS13 activity <10% were excluded from this analysis because their clinical features were attributed to other disorders. Four of the six patients had systemic infection and are included in the infection group; in 2 additional patients the presenting clinical features were subsequently attributed to systemic malignancy and graft-vs.-host disease together with infection. Laboratory data are the most abnormal values from the day of diagnosis ± 7 days. Major neurologic abnormalities were coma, stroke, seizures, or focal neurologic signs; minor abnormalities in these 87 patients included headache, ataxia, blurred vision, and mental status changes with transient confusion.(11) The pentad of clinical features includes microangiopathic hemolytic anemia, thrombocytopenia, neurologic abnormalities (major or minor), renal function abnormalities (any serum creatinine value ≥1.5 mg/dL), and fever. For categorical data, the chisquare test was used except for the comparisons of stroke, seizure, the pentad of clinical features, and relapse. For these four clinical features the Fisher's exact test was used. Although the median values and ranges for the hematocrits and platelet counts are similar and overlapping, there was more difference in the interquartile ranges. The middle 50% of values, quartiles 1 to 3, for the patient groups for hematocrit were: Infection, 23–26%; ADAMTS13<10%, 18–24% and for platelet count: Infection 9000–19,000/μL; ADAMTS13<10%, 6000–15,000/μL
The patients with systemic infections were older and they did not have the racial and gender disparities associated with severe ADAMTS13 deficiency.(22) All clinical features of the patients with systemic infections, with the exception of the serum LDH level, were different from patients with severe ADAMTS13 deficiency. Fever, coma, renal failure and the complete “pentad” of diagnostic features were more common and mortality was greater in patients with systemic infections. The severity of anemia and thrombocytopenia was slightly greater among patients with severe ADAMTS13 deficiency. Although the occurrence of neurologic abnormalities was not different between these two groups, the types of major neurologic abnormalities were different: patients with systemic infections had a greater frequency of coma while patients with ADAMTS13 deficiency had a greater frequency of focal neurologic abnormalities which were typically transient. None of the nine survivors with systemic infections have relapsed; five have subsequently died.
Etiologies of the systemic infections
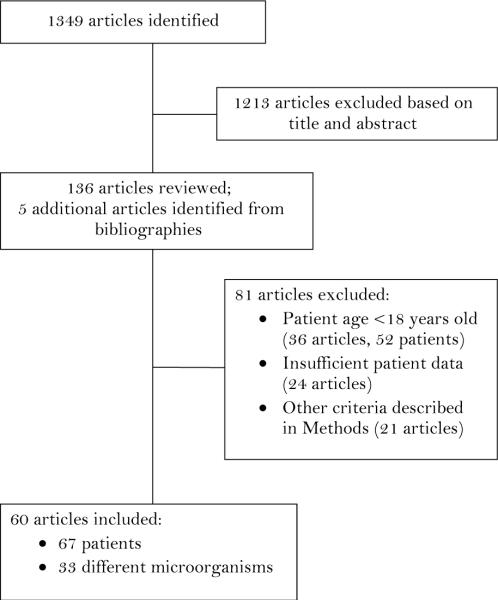
In 24 (77%) patients, the etiology of the infection was documented by positive cultures or serologic tests. Seventeen different microorganisms were identified as the etiology of the systemic infections; in three patients multiple microorganisms apparently contributed to the etiology. To supplement our experience, we searched for previously published reports of patients with TTP or HUS who had a preceding systemic infection. The literature search, completed on November 30, 2010, identified 1349 articles; 1213 articles were excluded by review of titles and abstracts; 136 articles from the literature search plus five additional articles identified from their bibliographies were reviewed (Figure 1). Eighty-one of these 141 articles were excluded, most often because of the patient's age and because there was insufficient or no primary patient data. The 60 articles selected for analysis described 67 patients reported from 20 countries; 20 patients were reported from the US, from 12 different states; 19 other countries reported 1–5 patients each. The articles were published from 1968 to 2010, 21 before 1990 when plasma exchange treatment was not yet established as the standard of care(2) and 22 from 2000 to 2010, when it was recognized that severe ADAMTS13 deficiency was associated with TTP.(17;82) The patients' median age was 45 years (range, 18–83 years); 36 (54%) of the patients were men. The description of the presenting clinical features was not complete in all case reports. Only two articles reported ADAMTS13 activity: 55% in one patient in whom cytomegalovirus (CMV) infection was interpreted as a mimic of TTP(70) and <5% in one patient in whom influenza A infection was interpreted as a trigger event precipitating an acute episode of TTP.(75)
Figure 1.
The sequence of article and patient selection for our systematic review is illustrated.
The diversity of infectious etiologies was great: 33 different bacterial, viral, and fungal infections were reported (Table 4). In only 19 (28%) patients was the infection considered to be a mimic of TTP; in the other 48 patients, the authors' interpretation was that the patient had both an infection and TTP. In most of these 48 patients, the infection was described as a cause of the TTP or a contributor to the onset of the acute episode. Thirty (45%) patients were treated with plasma exchange; 17 (25%) died; eight of these 17 patients had not been treated with plasma exchange. Twelve of the 17 patients who died had autopsy examinations; autopsies on seven patients in whom the infection was considered to be a mimic of TTP documented only the infection; four of the five autopsies on patients in whom the authors interpreted that both a systemic infection and TTP were present documented microvascular thrombosis consistent with TTP.
Table 4.
Infectious etiologies documented in patients diagnosed with TTP or HUS in published reports and from Oklahoma Registry patients.
| Infectious Organisms | Published reports | Oklahoma Registry Patients | |
|---|---|---|---|
| Patients | Reference | ||
| Bacteria | |||
| Actinomyces turicensis | 11 | (24) | 0 |
| Aeromonas hydrophila | 1 | (25) | 0 |
| Bacteroides fragilis | 31 | (24;26;27) | 0 |
| Borrelia burgdorferi | 1 | (28) | 0 |
| Brucella melitensis | 4 | (29–32) | 0 |
| Campylobacter jejuni | 3 | (33–35) | 0 |
| Capnocytophaga canimorsus | 5 | (36–39) | 0 |
| Chlamydia pneumoniae | 1 | (40) | 0 |
| Clostridium difficile | 2 | (41;42) | 0 |
| Ehrlichia chaffeensis | 1 | (43) | 0 |
| Ehrlichia equi | 1 | (44) | 0 |
| Enterobacter aerogenes | 0 | 1 | |
| Enterobacter cloacae | 0 | 1 | |
| Enterococcus faecalis | 0 | 3 | |
| Fusobacterium necrophorum | 1 | (45) | 0 |
| Legionella pneumophila | 2 | (46;47) | 0 |
| Leptospira 2 | 1 | (48) | 0 |
| Leptospira bataviae | 1 | (49) | 0 |
| Mycobacterium tuberculosis | 2 | (50;51) | 0 |
| Mycoplasma pneumoniae | 2 | (52;53) | 0 |
| Pseudomonas aeruginosa | 0 | 1 | |
| Rickettsiales bartonellaceae3 | 1 | (54) | 0 |
| Rickettsia rickettsii | 1 | (55) | 3 |
| Salmonella typhi | 6 | (56;57) | 0 |
| Staphylococcus aureus | 3 | (58–60) | 1 |
| Staphylococcus epidermidis | 1 | (61) | 1 |
| Streptococcus pneumoniae | 2 | (62;63) | 2 |
| Streptococcus pyogenes | 1 | (64) | 2 |
| Streptococcus viridans | 4 | (58;65–67) | 2 |
| Streptococcus Group C | 1 | (68) | 0 |
| Yersinia pseudotuberculosis | 2 | (69) | 0 |
| Viruses | |||
| Cytomegalovirus | 4 | (22;70–72) | 3 |
| Hepatitis4 | 1 | (73) | 1 |
| HIV | 0 | ||
| Influenza A | 2 | (23;74) | |
| Parvovirus B19 | 2 | (75;76) | |
| Varicella zoster | 1 | (77) | |
| Fungi | |||
| Aspergillus fumigatus5 | 3 | (78–80) | 1 |
| Blastomyces dermatitidis | 0 | 1 | |
| Candida albicans | 0 | 1 | |
| Cryptococcus neoformans | 0 | 1 | |
One patient had infection with both Actinomyces turicensis and Bacteroides fragilis.
Organism documented only by order and family.
Organism not identified by species.
Type of hepatitis not described.
Organism not identified by species in one report.
DISCUSSION
The lack of specific diagnostic criteria often creates uncertainty about the diagnosis of TTP, yet the potential for TTP to be rapidly fatal creates urgency to begin effective treatment with plasma exchange. This management dilemma is made even more difficult because plasma exchange is associated with a high risk for major complications(8) and because patients with TTP are uncommonly encountered.(12)
The perspective of the Oklahoma TTP-HUS Registry is unique because it describes the total community experience with the diagnosis and management of TTP. All patients for whom plasma exchange is requested for a diagnosis of TTP are enrolled and all patients remain in the Registry with complete follow-up, even if the diagnosis of TTP is subsequently excluded. The rationale for follow-up of all patients is that TTP can never be absolutely excluded. Among patients initially diagnosed and treated for TTP, some may have both an alternative disorder and TTP.
In this report, we describe the 22 year experience of the Oklahoma TTP-HUS Registry with 31 patients who were initially diagnosed with TTP and treated with plasma exchange but whose presenting clinical features were subsequently attributed to a systemic infection. All 31 patients had diagnostic criteria for TTP; 16 (52%) had the complete “pentad” of clinical features; most were critically ill; 68% died. Sixteen (52%) of these 31 patients were comatose, perhaps increasing the urgency for initiating plasma exchange treatment. Coma was uncommon among patients with severe ADAMTS13 deficiency and no alternative disorders, however focal neurologic abnormalities, typically transient, were more common in patients with severe ADAMTS13 deficiency than in patients with systemic infections. Although the appropriateness of the decision to diagnose TTP in some of these patients and to treat them with plasma exchange may be questioned, the management decisions for these patients represent community practice, in which concern for withholding a potentially effective treatment often overrides diagnostic uncertainty. These patients were diagnosed with TTP and plasma exchange was requested by 23 different physicians across most of the 22 years of the Registry, documenting that these decisions occurred among many physicians and across many years. Withholding plasma exchange treatment may not be possible in a critically ill patient in whom the diagnosis of TTP has been proposed, even if a diagnosis of systemic infection seems more likely. However once plasma exchange treatment has begun, the appropriateness of continued plasma exchange should be continually reassessed.
The original goal of this report was to describe the variety of systemic infections that can mimic TTP. Among the 31 patients from the Oklahoma Registry, 17 different infectious etiologies were identified. The patient selection criteria for our systematic review were more stringent than the characteristics of the patients from the Oklahoma Registry, to allow consistent interpretation of the selected case reports. In spite of these more stringent selection criteria, we identified 67 additional patients in whom the diagnosis of TTP or HUS was preceded by a systemic infection; 33 different infectious etiologies were identified. Among the combined group of 98 patients with 41 different infectious etiologies, no single etiology was identified in more than six patients; 18 (44%) infectious etiologies were reported only once. The types of infections were diverse; 31 different bacteria, six different viruses, and four different fungi were identified. These observations suggest that no single infectious etiology is a major mimic of TTP and that perhaps any severe infection may mimic TTP. For some infections, the pathogenesis of microangiopathic hemolytic anemia has been described. Examples are bacterial endocarditis,(60) bacterial infections associated with microvascular injury such as brucellosis,(30;31) streptococcal infections resulting in acute glomerulonephritis,(64) angioinvasive fungi such as aspergillosis,(81), and viruses and rickettsiae that cause endothelial injury such as CMV,(69–71) HIV,(16) erlichiosis,(42) and Rocky Mountain spotted fever.(54) However for other infections the mechanisms of microangiopathic hemolytic anemia are unclear.
In each of our 31 patients, the final clinical impression was that a systemic infection, rather than TTP, was responsible for the presenting clinical features, including the four patients with severe ADAMTS13 deficiency. This clinical impression was supported by the absence of pathologic evidence for TTP in all six patients who had autopsies. However we learned from our review of published reports that most authors concluded that their patients had both a systemic infection and TTP or HUS; in some reports it was suggested that the infection may have “triggered” or caused the TTP or HUS. This different interpretation may be related to our inclusion only of patients with an acute, systemic infection at the time of diagnosis of TTP and exclusion of patients who were clearly recovering from a preceding infection at the time that symptoms attributable to TTP began. These different interpretations, that patients either had both an infection and TTP or that the infection was merely mimicking TTP, cannot be completely resolved because the diagnosis of TTP can never be conclusively excluded.
Although severe deficiency of ADAMTS13 is consistent with the diagnosis of TTP, it is not specific for the diagnosis of TTP.(3;7) Four of our 31 patients had ADAMTS13 activity <10%; two had demonstrable inhibitors including one who had no autopsy evidence for TTP; one patient with ADAMTS13 activity of 12% also had a demonstrable inhibitor. ADAMTS13 activity was reported for only two of the 67 patients in previous publications; one was normal(70) and one was <5%.(75) The patient identified in our literature review who had severe ADAMTS13 deficiency was the clearest example of an infection that appeared to trigger an acute episode of TTP: she had documented influenza A that was followed in two days by TTP.(75) Other reports have suggested that infections(9;10) as well as conditions such as pregnancy,(83;84) surgery,(85) and pancreatitis,(86) may trigger acute episodes of TTP.
The observations from our review of published reports created a new perspective for our Registry. Because of the difficult differential diagnosis of systemic infections and TTP, we have revised our Registry protocol to systemically collect of data on coagulation parameters. Furthermore, we recognize that systemic infections can “trigger” an acute episode of TTP and therefore that patients can have both a systemic infection and TTP. Therefore we have also revised our Registry protocol to systematically document all symptoms and medical care during the three weeks preceding the diagnosis of TTP to identify events that may have triggered the acute episode.(87) Whether “trigger events” preceding acute episodes of TTP are uncommon or unrecognized is unknown.
One conclusion from these observations is that the “pentad” of clinical features described 45 years ago(1) continues to be influential for many physicians. A common clinical belief is that if more features of the “pentad” are present and if the abnormalities are more severe, then the diagnosis of TTP is more likely. However the “pentad” is rare among patients with TTP with no alternative disorders and in whom the diagnosis is supported by the presence of severe ADAMTS13 deficiency. Therefore physicians should have a lower index of suspicion for TTP when the complete “pentad” of clinical features is present, especially in the presence of fever and chills. Another conclusion is that documentation of severe deficiency of ADAMTS13 activity or the presence of an ADAMTS13 inhibitor does not exclude the presence of a systemic infection that is mimicking TTP. Patients with a systemic infection and no evidence of TTP at autopsy may have severe ADAMTS13 deficiency with an inhibitor. However patients with TTP may also have a concurrent preceding systemic infection that triggered the acute episode.(10;75)
A strength of this study is the observation of actual clinical practice of many different physicians in our community across many years, including all patients identified prospectively at the time of the initial diagnosis of TTP and request for plasma exchange treatment. In contrast, most published case series of patients with TTP are retrospectively assembled, excluding patients with probable alternative etiologies. A weakness of this study is that the clinical impressions of systemic infections were often imprecise because of the severe and sometimes brief clinical course.
Our experience and the experience from published case reports emphasize the broad spectrum of infectious diseases that can cause microangiopathic hemolytic anemia, thrombocytopenia and often the complete “pentad” of clinical features of TTP. Because plasma exchange treatment has a high frequency of major complications, including a 2.8% risk for death,(8) a continuing search for a systemic infection in patients with suspected TTP is essential to avoid unnecessary plasma exchange treatment.
Acknowledgments
Grant support: Hematology Research Fund of the University of Oklahoma. Dr. Terrell is partially supported by NIH 1U01HL72283-09S1.
Footnotes
Conflict of interest: The authors have no conflict with this topic or these data. Drs. Terrell and George serve as consultants for Baxter, Inc. for the development of rADAMTS13 as a potential treatment for TTP.
Reference List
- (1).Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45:139–59. [Google Scholar]
- (2).Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. New Eng J Med. 1991;325:393–97. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- (3).George JN. How I treat patients with thrombotic thrombocytopenic purpura - 2010. Blood. 2010;116:4060–4069. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- (4).Clark WF, Garg AX, Blake PG, Rock GA, Heidenhwim AP, Sackett DL. Effect of awareness of a randomized controlled trial on use of experimental therapy. JAMA. 2003;290:1351–55. doi: 10.1001/jama.290.10.1351. [DOI] [PubMed] [Google Scholar]
- (5).Brain MC, Dacie JV, Hourihane OB. Microangiopathic hemolytic anemia: the possible role of vascular lesions in pathogenesis. Br J Haematol. 1962;8:358–74. doi: 10.1111/j.1365-2141.1962.tb06541.x. [DOI] [PubMed] [Google Scholar]
- (6).Francis KK, Kalyanam N, Terrell DR, Vesely SK, George JN. Disseminated malignancy misdiagnosed as thrombotic thrombocytopenic purpura: a report of 10 cases and a systematic review of the literature. The Oncologist. 2007;12:11–19. doi: 10.1634/theoncologist.12-1-11. [DOI] [PubMed] [Google Scholar]
- (7).Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115:1500–1511. doi: 10.1182/blood-2009-09-243790. [DOI] [PubMed] [Google Scholar]
- (8).Nguyen L, Terrell DR, Duvall D, Vesely SK, George JN. Complications of plasma exchange in patients treated for thrombotic thrombocytopenic purpura. IV. An additional study of 43 consecutive patients, 2005–2008. Transfusion. 2009;49:392–94. doi: 10.1111/j.1537-2995.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- (9).Cserti CM, Landaw SA, Uhl L. Do infections provoke exacerbations and relapses of thrombotic thrombocytopenic purpura? J Clin Apheresis. 2007;22:21–25. doi: 10.1002/jca.20114. [DOI] [PubMed] [Google Scholar]
- (10).Douglas KW, Pollock KGJ, Young D, Catlow J, Green R. Infection frequently triggers thrombotic microangiopathy in patients with pre-existing factors: a single-institution experience. J Clin Apheresis. 2010;25:47–53. doi: 10.1002/jca.20226. [DOI] [PubMed] [Google Scholar]
- (11).Vesely SK, George JN, Lämmle B, Studt J-D, Alberio L, El-Harake MA, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;101:60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- (12).Terrell DR, Williams LA, Vesely SK, Lammle B, Kremer Hovinga JA, George JN. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13 deficiency. J Thromb Haemost. 2005;3:1432–36. doi: 10.1111/j.1538-7836.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- (13).Roy V, Rizvi MA, Vesely SK, George JN. Thrombotic thrombocytopenic purpura-like syndromes following bone marrow transplantation: an analysis of associated conditions and clinical outcomes. Bone Marrow Transplantation. 2001;27:641–46. doi: 10.1038/sj.bmt.1702849. [DOI] [PubMed] [Google Scholar]
- (14).Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–86. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- (15).Karpac CA, Li X, Terrell DR, Kremer Hovinga JA, Lämmle B, Vesely SK, et al. Sporadic bloody diarrhoea-associated thrombotic thrombocytopenic purpura-haemolytic uraemic syndrome: an adult and paediatric comparison. Br J Haematol. 2008;141:696–707. doi: 10.1111/j.1365-2141.2008.07116.x. [DOI] [PubMed] [Google Scholar]
- (16).Benjamin M, Terrell DR, Vesely SK, Voskuhl GW, Dezube BJ, Kremer Hovinga JA, et al. Frequency and significance of HIV infection among patients diagnosed with thrombotic thrombocytopenic purpura. Clin Infect Dis. 2009;48:1129–37. doi: 10.1086/597471. [DOI] [PubMed] [Google Scholar]
- (17).Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, et al. Von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. New Engl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- (18).Bianchi V, Robles R, Alberio L, Furlan M, Lämmle B. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–713. doi: 10.1182/blood-2002-02-0344. [DOI] [PubMed] [Google Scholar]
- (19).Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- (20).Kremer Hovinga JA, Mottini M, Lämmle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost. 2006;4:1146–48. doi: 10.1111/j.1538-7836.2006.01904.x. [DOI] [PubMed] [Google Scholar]
- (21).George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic hematopoietic stem cell transplantation: a diagnostic dilemma. Transfusion. 2004;44:294–304. doi: 10.1111/j.1537-2995.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- (22).Terrell DR, Vesely SK, Kremer Hovinga JA, Lämmle B, George JN. Different disparities of gender and race among the thrombotic thrombocytopenic purpura and hemolytic-uremic syndromes. Amer J Hematol. 2010;85:844–47. doi: 10.1002/ajh.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Riegert-Johnson DL, Sandhu N, Rajkumar SV, Patel R. Thrombotic thrombocytopenic purpura associated with a hepatic abscess due to Actinomyces turicensis. Clin Infect Dis. 2002;35:636–37. doi: 10.1086/342327. [DOI] [PubMed] [Google Scholar]
- (24).Fang JS, Chen JB, Chen WJ, Hsu KT. Haemolytic-uraemic syndrome in an adult male with Aeromonas hydrophila enterocolitis. Nephrol Dial Transplant. 1999;14:439–40. doi: 10.1093/ndt/14.2.439. [DOI] [PubMed] [Google Scholar]
- (25).Shalev O, Karni A, Kornberg A, Brezis M. Thrombotic thrombocytopenic purpura associated with Bacteroides bacteremia. Arch Intern Med. 1981;141:682–92. [PubMed] [Google Scholar]
- (26).Fiala M, Bauer H, Khaleeli M, Giorgio A. Dog bite, bacteroides infection, coagulopathy, renal microangiopathy. Ann Int Med. 1977;87:248–49. doi: 10.7326/0003-4819-87-2-248_2. [DOI] [PubMed] [Google Scholar]
- (27).Schröder S, Spyridopoulos I, König J, Jaschonek KG, Luft D, Seif FJ. Thrombotic Thrombocytopenic Purpura (TTP) associated with a Borrelia burgdorferi infection. Am J Hematol. 1995;50:72–73. doi: 10.1002/ajh.2830500128. [DOI] [PubMed] [Google Scholar]
- (28).Erdem F, Kiki I, Gundogdu M, Kaya H. Thrombotic thrombocytopenic purpura in a patient with brucella infection is highly responsive to combined plasma infusion and antimicrobial therapy. Medical Principles and Practice. 2007;16:324–26. doi: 10.1159/000102159. [DOI] [PubMed] [Google Scholar]
- (29).Kiki I, Gundogdu M, Albayrak B, Bilgic Y. Thrombotic thrombocytopenic purpura associated with brucella infection. American Journal of the Medical Sciences. 2008;335:230–232. doi: 10.1097/MAJ.0b013e3180d09f19. [DOI] [PubMed] [Google Scholar]
- (30).Di Mario A, Sica S, Zini G, Salutari P, Leone G. Microangiopathic hemolytic anemia and severe thrombocytopenia in Brucella infection. Ann Hematol. 1995;70:59–60. doi: 10.1007/BF01715385. [DOI] [PubMed] [Google Scholar]
- (31).Altuntas F, Eser B, Sari I, Yildiz O, Cetin M, Unal A. Severe thrombotic microangiopathy associated with brucellosis: successful treatment with plasmapheresis. Clin Appl Thrombosis/Haemostasis. 2005;11:105–8. doi: 10.1177/107602960501100114. [DOI] [PubMed] [Google Scholar]
- (32).Delans RJ, Biuso JD, Saba SR, Ramirez G. Hemolytic uremic syndrome after Campylobacter-induced diarrhea in an adult. Arch Intern Med. 1984;144:1074–76. [PubMed] [Google Scholar]
- (33).Chamovitz BN, Hartstein AI, Alexander SR, Terry AB, Short P, Katon R. Campylobacter jejuni-associated hemolytic-uremic syndrome in a mother and daughter. Pediatrics. 1983;71:253–56. [PubMed] [Google Scholar]
- (34).Sillero M, Almirall J. Campylobacter jejuni and hemolytic-uremic syndrome. Nephron. 1999;82:363–64. doi: 10.1159/000045457. [DOI] [PubMed] [Google Scholar]
- (35).Mulder AH, Gerlag PGG, Verhoef LHM, van den Wall Bake AWL. Hemolytic uremic syndrome after Capnocytophaga canimorsus (DF-2) septicemia. Clin Nephrol. 2001;55:167–70. [PubMed] [Google Scholar]
- (36).Kennedy JS, Gerety BM, Silverman R, Pattison ME, Siskind MS, Pond GD. A syndrome resembling thrombotic thrombocytopenic purpura associated with Capnocytophaga canimorsus septicemia. The American Journal of Medicine. 1991;90:127–28. doi: 10.1016/0002-9343(91)90517-2. [DOI] [PubMed] [Google Scholar]
- (37).Finn M, Dale B, Isles C. Beware of the dog! A syndrome resembling thrombotic thrombocytopenic purpura associated with Capnocytophaga canimorsus septicaemia. Nephrol Dial Transplant. 1996;11:1839–40. [PubMed] [Google Scholar]
- (38).Tobe TJM, Franssen CFM, Zijlstra JG, de Jong PE, Stegeman CA. Hemolytic Uremic Syndrome due to Capnocytophaga canimorsus bacteremia after a dog bite. Am J Kidney Dis. 1999;33:1–3. doi: 10.1016/s0272-6386(99)70172-1. [DOI] [PubMed] [Google Scholar]
- (39).Knox-Macaulay HHM, Adil SN, Ahmed EME. Acute thrombotic thrombocytopenic purpura following doxycycline treatment of Chlamydia pneumoniae infection in a patient with dermatomyositis. Clin Lab Haem. 2004;26:147–51. doi: 10.1111/j.1365-2257.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- (40).Mbonu CC, Davison DL, El-Jazzar KM, Simon GL. Clostridium difficile colitis associated with hemolytic uremic syndrome. Am J Kidney Dis. 2003;41:E14. doi: 10.1016/s0272-6386(03)00210-5. [DOI] [PubMed] [Google Scholar]
- (41).Mogyorosi A, Carley MD. Hemolytic-uremic syndrome associated with pseudomembranous colitis caused by Clostridium difficile. Nephron. 1997;76:491. doi: 10.1159/000190237. [DOI] [PubMed] [Google Scholar]
- (42).Marty AM, Dumler JS, Imes G, Brusman HP, Smrkovski LL, Frisman DM. Ehrlichiosis mimicking thrombotic thrombocytopenic purpura. Case report and pathological correlation. Hum Pathol. 1995;26:920–925. doi: 10.1016/0046-8177(95)90017-9. [DOI] [PubMed] [Google Scholar]
- (43).Modi KS, Dahl DC, Berkseth RO, Schut R, Greeno E. Human granulocytic ehrlichiosis presenting with acute renal failure and mimicking thrombotic thrombocytopenic purpura. A case report and review. Am J Nephrol. 1999;19:677–81. doi: 10.1159/000013541. [DOI] [PubMed] [Google Scholar]
- (44).Chand DH, Brady RC, Bissler JJ. Hemolytic uremic syndrome in an adolescent with Fusobacterium necrophorum bacteremia. Am J Kidney Dis. 2001;37:1–4. doi: 10.1053/ajkd.2001.22099. [DOI] [PubMed] [Google Scholar]
- (45).Riggs SA, Wray NP, Waddell CC, Rossen RD, Gyorkey F. Thrombotic thrombocytopenic purpura complicating Legionnaires' Disease. Arch Intern Med. 1982;142:2275–80. doi: 10.1001/archinte.142.13.2275. [DOI] [PubMed] [Google Scholar]
- (46).Canaud B, Beraud JJ, Mion C, Baldet P, Mimran A. Successful treatment of Legionella-associated haemolytic uraemic syndrome with acute renal failure and malignant hpertension by prostacyclin (epoprostenol) Nephrol Dial Transplant. 1989;4:996–99. doi: 10.1093/ndt/4.11.996. [DOI] [PubMed] [Google Scholar]
- (47).Laing RW, Teh C, Toh CH. Thrombotic thrombocytopenic purpura (TTP) complicating leptospirosis: a previously undescribed association. J Clin Path. 1990;43:961–62. doi: 10.1136/jcp.43.11.961-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hanvanich M, Moollaor P, Suwangool P, Sitprija V. Hemolytic uremic syndrome in Leptospira bataviae. Nephron. 1985;40:230–231. doi: 10.1159/000183465. [DOI] [PubMed] [Google Scholar]
- (49).Cserti CM, Landaw S, Uhl L. Do infections provoke exacerbations and relapses of thrombotic thrombocytopenic purpura? Journal of Clinical Apheresis. 2007;22:21–25. doi: 10.1002/jca.20114. [DOI] [PubMed] [Google Scholar]
- (50).Toscano V, Bontadini A, Falsone G, Conte R. Thrombotic thrombocytopenic purpura associated with primary tuberculosis. Infection. 1995;23:58–59. doi: 10.1007/BF01710061. [DOI] [PubMed] [Google Scholar]
- (51).Meir EB, Amital H, Levy Y, Kneller A, Bar-Dayan Y, Shoenfeld Y. Mycoplasma pneumoniae-induced thrombotic thrombocytopenic purpura. Acta Haematol. 2000;103:112–15. doi: 10.1159/000041030. [DOI] [PubMed] [Google Scholar]
- (52).Cameron D, Turner M. Thrombotic thrombocytopenic due to Mycoplasma pneumoniae. Postgrad Med J. 1992;68:393–94. doi: 10.1136/pgmj.68.799.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Mettler NE. Isolation of a microtatobiote from patients with hemolytic-uremic syndrome and thrombotic thrombocytopenic purpura and from mites in the United States. N Engl J Med. 1969;281:1023–27. doi: 10.1056/NEJM196911062811901. [DOI] [PubMed] [Google Scholar]
- (54).Turner RC, Chaplinski TJ, Adams HG. Rocky mountain spotted fever presenting as thrombotic thrombocytopenic purpura. The American Journal of Medicine. 1986;81:153–57. doi: 10.1016/0002-9343(86)90201-9. [DOI] [PubMed] [Google Scholar]
- (55).Albaqali A, Ghuloom A, Arrayed AA, Ajami AA, et al. Hemolytic uremic syndrome in association with typhoid fever. Am J Kidney Dis. 2003;41:709–13. doi: 10.1053/ajkd.2003.50135. [DOI] [PubMed] [Google Scholar]
- (56).Baker NM, Mills AE, Rachman I, Thomas JEP. Haemolytic-uraemic syndrome in typhoid fever. Brit Med J. 1974;2:84–87. doi: 10.1136/bmj.2.5910.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Bayer AS, Theofilopoulos AN, Eisenberg R, Friedman SG, Guze LB. Thrombotic thrombocytopenic purpura-like syndrome associated with infective endocarditis. A possible immune complex disorder. JAMA. 1977;238:408–10. [PubMed] [Google Scholar]
- (58).Chee CE, Mohr DN. 71 year old woman with fever and altered mental status. Mayo Clin Proc. 2007;82:237–40. doi: 10.4065/82.2.237. [DOI] [PubMed] [Google Scholar]
- (59).Ibrahim TM, Iheonunekwu N. Thrombotic thrombocytopenic purpura associated with Staphylococcus aureus endocarditis in a diabetic with furunculosis. Nigerian Journal of Medicine. 2009;18:422–23. doi: 10.4314/njm.v18i4.51257. [DOI] [PubMed] [Google Scholar]
- (60).Selleng K, Warkentin TE, Greinacher A, Morris AM, Walker IR, Heggtveit HA, et al. Very severe thrombocytopenia and fragmentation hemolysis mimicking thrombotic thrombocytopenic purpura associated with a giant intracardiac vegetation infected with Staphylococcus epidermidis: role of monocyte procoagulant activity induced by bacterial supernatant. Amer J Hematol. 2006;82:766–71. doi: 10.1002/ajh.20821. [DOI] [PubMed] [Google Scholar]
- (61).Myers KA, Marrie TJ. Thrombotic microangiopathy associated with Streptococcus pneumoniae bacteremia: case report and review. Clin Infect Dis. 1993;17:1037–40. doi: 10.1093/clinids/17.6.1037. [DOI] [PubMed] [Google Scholar]
- (62).von Eyben FE, Szpirt W. Pneumococcal sepsis with hemolytic-uremic syndrome in the adult. Nephron. 1985;40:501–2. doi: 10.1159/000183533. [DOI] [PubMed] [Google Scholar]
- (63).Drenger B, Israeli A, Or R, Leitersdorf E. Plasmapheresis for streptococcal sepsis. Lancet. 1985;2:945. doi: 10.1016/s0140-6736(85)90870-0. [DOI] [PubMed] [Google Scholar]
- (64).Izumi T, Hyodo T, Kikuchi Y, Imakiire T, et al. An adult with acute poststreptococcal glomerulonephritis complicated by hemolytic uremic syndrome and nephrotic syndrome. Am J Kidney Dis. 2005;46:E59–E63. doi: 10.1053/j.ajkd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- (65).Castleman B, McNeely BU. Case records of the Massachusetts General Hospital. Weekly Clinicopathological Exercises. NEJM. 1970;283:1042–50. doi: 10.1056/NEJM197110072851510. [DOI] [PubMed] [Google Scholar]
- (66).Lin C-Y, Lin S-H. Fatal thrombotic thrombocytopenic purpura coexisting with bacterial infection: a case report. Acta Neurologica Taiwanica. 2008;17:42–45. [PubMed] [Google Scholar]
- (67).Thomas MP, Wang A. Taken out of context. New Eng J Med. 2008;359:2478–82. doi: 10.1056/NEJMcps0801308. [DOI] [PubMed] [Google Scholar]
- (68).Davenport A, Finn R. Haemolytic uraemic syndrome induced by Yersinia pseudotuberculosis. Lancet. 1988;1:358–59. doi: 10.1016/s0140-6736(88)91149-x. [DOI] [PubMed] [Google Scholar]
- (69).Yamazaki M, Takei T, Otsubo S, Iwasa Y, Yabuki Yea. Wegener's granulomatosis complicated by intestinal ulcer due to cytomegalovirus infection and by thrombotic thrombocytopenia purpura. Internal Medicine. 2007;46:1435–40. doi: 10.2169/internalmedicine.46.0050. [DOI] [PubMed] [Google Scholar]
- (70).Humblot S, Martin T, Pasquali J-L, Korganow A-S. Blood coagulation disorders during primary cytomegalovirus infection. Arch Intern Med. 2001;161:2149–50. doi: 10.1001/archinte.161.17.2149. [DOI] [PubMed] [Google Scholar]
- (71).Neau D, Bonnet F, Viallard JF, Longy-Boursier M, Le Bras M. Thrombotic thrombocytopenic purpura and cytomegalovirus infection in an immunocompetent adult. Clin Infect Dis. 1997;25:1495–96. doi: 10.1086/517014. [DOI] [PubMed] [Google Scholar]
- (72).Caton B, de Otazu RD, Aldamiz-Echebarria M, Viguri A. Haemolytic-uraemic syndrome with thrombotic microangiopathy of the retina following cytomegalovirus infection: postmortem findings. Postgrad Med J. 1993;69:653–55. doi: 10.1136/pgmj.69.814.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Zilliacus H, Pollanen L. Sepsis, thrombotic thrombocytopenic purpura or hepatitis? A problem case. Annales Chirurgiae et Gynaecikiguae Fenniae. 1968;57:398–401. [PubMed] [Google Scholar]
- (74).Wasserstein A, Hill G, Goldfarb S, Goldberg M. Recurrent thrombotic thrombocytopenic purpura after viral infection. Arch Intern Med. 1981;141:685–87. [PubMed] [Google Scholar]
- (75).Kosugi N, Tsurutani Y, Isonishi A, Hori Y, Matsumoto M, Fujimura Y. Influenza A infection triggers thrombotic thrombocytopenic purpura by producing the anti-ADAMTS13 IgG inhibitor. Internal Med. 2010;49:689–93. doi: 10.2169/internalmedicine.49.2957. [DOI] [PubMed] [Google Scholar]
- (76).Kok RHJ, Wolfhagen MJHM, Klosters G. A syndrome resembling thrombotic thrombocytopenic purpura associated with human parvovirus B19 infection. Clin Infect Dis. 2001;32:311–12. doi: 10.1086/318481. [DOI] [PubMed] [Google Scholar]
- (77).Seward EW, Rustom R, Nye FJ, Bone JM. Haemolytic-uraemic syndrome following human parvovirus infection in a previously fit adult. Nephrology Dialysis Transplantation. 1999;14:2472–73. doi: 10.1093/ndt/14.10.2472. [DOI] [PubMed] [Google Scholar]
- (78).Satoh K, Takahashi A, Nagai K, Shibata A. Thrombotic thrombocytopenic purpura and Herpes zoster infection. Ann Int Med. 1988;108:154–55. doi: 10.7326/0003-4819-108-1-154_2. [DOI] [PubMed] [Google Scholar]
- (79).Guidotti TL, Luetzeler J, Di Sant Agnese PA, Escaro DU. Case report of fatal disseminated aspergillosis in a previously well young adult with cystic fibrosis. American Journal of the Medical Sciences. 1982;283:157–60. doi: 10.1097/00000441-198205000-00007. [DOI] [PubMed] [Google Scholar]
- (80).Fantini F, Cimaz R. A fatal case of systemic lupus erythematosus complicated by acute pancreatitis, invasive aspergillosis and features of thrombotic thrombocytopenic purpura. Lupus. 2003;12:418–21. doi: 10.1191/0961203303lu375cr. [DOI] [PubMed] [Google Scholar]
- (81).Robboy SJ, Salisbury K, Ragsdale B, Bobroff LM, Jacobson BM, Colman RW. Mechanism of Aspergillus-induced microangiopathic hemolytic anemia. Arch Intern Med. 1971;128:790–793. [PubMed] [Google Scholar]
- (82).Tsai HM, Lian ECY. Antibodies to von-Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. New Eng J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).George JN. The association of pregnancy with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Current Opinion in Hematology. 2003;10:339–44. doi: 10.1097/00062752-200309000-00003. [DOI] [PubMed] [Google Scholar]
- (84).Fujimura Y, Matsumoto M, Kokame K, Isonishi A, Soejima K, Akiyama N, et al. Pregnancy-induced thrombocytopenia and TTP, and the risk of fetal death, in Upshaw-Schulman syndrome: a series of 15 pregnancies in 9 genotyped patients. Br J Haematol. 2009;144:742–54. doi: 10.1111/j.1365-2141.2008.07515.x. [DOI] [PubMed] [Google Scholar]
- (85).Robertson MD, Zumberg M. Post-appendectomy thrombotic thrombocytopenic purpura: a case report and review of the literature. Amer J Hematol. 2007;82:224–28. doi: 10.1002/ajh.20793. [DOI] [PubMed] [Google Scholar]
- (86).Swisher KK, Doan JT, Vesely SK, Kwaan HC, Kim B, Lammle B, et al. Pancreatitis preceding acute episodes of thrombotic thrombocytopenic purpura: report of five patients with a systematic review of the literature. Haematologica. 2007;92:936–43. doi: 10.3324/haematol.10963. [DOI] [PubMed] [Google Scholar]
- (87).Griffin D, Al-Nouri Z, Muthuraja D, Ross J, Ballard R, Terrell DR, et al. First symptoms in idiopathic thrombotic thrombocytopenic purpura: what are they and when do they occur? Blood. 2010;116:612–13. [Google Scholar]