Abstract
The evolutionary origins of Ig-producing B cells appear to be linked to the emergence of fish in this planet. There are three major classes of living fish species, which from most primitive to modern they are referred to as agnathan (e.g., lampreys), Chondrichthyes (e.g., sharks), and teleost fish (e.g., rainbow trout). Agnathans do not have immunoglobulin-producing B cells, however these fish contain a subset of lymphocytes-like cells producing type B variable lymphocyte receptors (VLRBs) that appear to act as functional analogs of immunoglobulins. Chondrichthyes fish represent the most primitive living species containing bona-fide immunoglobulin-producing B cells. Their B cells are known to secrete three types of antibodies, IgM, IgW and IgNAR. Teleost fish are also called bony fish since they represent the most ancient living species containing true bones. Teleost B cells produce three different immunoglobulin isotypes, IgM, IgD and the recently described IgT. While teleost IgM is the principal player in systemic immunity, IgT appears to be a teleost immunoglobulin class specialized in mucosal immune responses. Thus far, three major B cell lineages have been described in teleost, those expressing either IgT or IgD, and the most common lineage which co-expresses IgD and IgM. A few years ago, the study of teleost fish B cells revealed for the first time in vertebrates the existence of B cell subsets with phagocytic and intracellular bactericidal capacities. This finding represented a paradigm shift as professional phagocytosis was believed to be exclusively performed by some cells of the myeloid lineage (i.e., macrophages, monocytes, neutrophils). This phagocytic capacity was also found in amphibians and reptiles, suggesting that this innate capacity was evolutionarily conserved in certain B cell subsets of vertebrates. Recently, the existence of subsets of B cells with phagocytic and bactericidal abilities have also been confirmed in mammals. Moreover, it has been shown that phagocytic B-1 B cells have a potent ability to present particulate antigen to CD4+ T cells. Thus, studies carried out originally on fish B cells have lead to the discovery of new innate and adaptive roles of B cells in mammals. This review will concentrate on the evolutionary and functional relationships of fish and mammalian B cells, focusing mainly on the newly discovered roles of these cells in phagocytosis, intracellular killing and presentation of particulate antigen.
Keywords: Evolution, fish, mammals, phagocytosis, B cells, antigen presentation, intracellular killing
1. GENERAL FEATURES OF IMMUNE MOLECULES AND LYMPHOID ORGANS INVOLVED IN TELEOST FISH ADAPTIVE IMMUNE RESPONSES
Teleost fish represent the oldest living organisms with true bones containing an adaptive immune system similar in many respects to that of mammals [1-3]. At the molecular level, they are known to have bona fide MHC class I and II, TCR, CD4, CD8 and Ig molecules [4-8]. Moreover, teleost fish contain most of the cytokines described thus far in mammals, including the signature cytokines for Th1-, Th2-, and Th17-type responses [9, 10]. Teleost fish leukocytes are known to express critical co-stimulatory molecules, including CD28, CD80/86 and CD40 [11-13]. Important for the recognition of PAMPs and for the modulation of immune responses, teleost fish appear to contain most of the TLR molecules identified thus far in mammals [14]. Due to the tetraploid ancestry of many teleost fish, most of the aforementioned molecules in these species are found in different isoforms, a fact that makes their study more complex. With regards to lymphoid tissues, teleost fish are known to have a true spleen and thymus [15]. However, they lack germinal centers, lymph nodes and bone marrow [16-18]. The anterior part of the fish kidney (head kidney) is an important lymphoid organ, and it has been compared to the bone marrow of mammals since it is known to be a hematopoietic organ. Moreover, the head kidney, similar to the fish spleen, is an important blood filtering and immune responsive organ [16, 18]. Teleost fish contain gut-associated lymphoid tissue (GALT) with a population of intraepithelial lymphocytes (IELs). The teleost GALT is made up of an epithelium with its corresponding basal membrane and the underlying lamina propria [16]. Significantly, teleost fish GALT lacks the organized Peyer Patches and mesenteric lymph nodes present in the GALT of mammals [16, 19, 20].
2. EVOLUTIONARY ORIGINS OF B CELLS
The evolutionary origins of Ig-producing B cells appear to be linked to the emergence of fish in this planet. In that regard, there are three major classes of living fish species, the agnathan or called also mouthless fish (e.g., lampreys, hagfish), the cartilaginous fish (e.g., sharks, rays) and the teleost fish (e.g., tuna, salmon). Lampreys do not have immunoglobulin-producing B cells, however these fish contain leukocytes that bear a striking resemblance to lymphocytes at the morphological level [21]. Instead of producing immunoglobulins, lamprey and hagfish lymphocytes make variable lymphocyte receptors (VLRs), which have been shown to act as functional analogs of immunoglobulins [22, 23]. It is interesting that two lineages of VLR-producing lymphocytes exist in lampreys, those that express VLRA and those expressing VLRB [24, 25]. Based on the gene expression profiles of VLRA(+) and VLRB(+) lymphocytes, it would appear that VLRB(+) lymphocyte gene expression is in many aspects similar to that of mammalian B cells, while that of VLRA(+) lymphocytes would resemble that of T cells [24, 26]. In addition, only VLRB molecules appear to bind native antigens [22]. Thus, it would seem that VLRB(+) lymphocytes are functional analogs of B cells from jawed vertebrates.
The oldest living species containing bona-fide immunoglobulin-producing B cells are the cartilaginous fish (e.g., sharks, rays) [17]. Cartilaginous fish B cells are known to produce three types of immunoglobulins IgM, IgW and Ig-NAR (immunoglobulin new antigen receptor) [17]. Phylogenetic analyses are suggestive of IgW being closely related to IgD [17, 27]. In contrast, IgNAR appears to be a specialized shark-specific immunoglobulin that is composed of disulphide-linked heavy chains which are not associated to light chains [1]. The structure of the IgNAR immunoglobulin reminds that of camelid heavy-chain IgG antibodies, which also lack light chains. It is thought however that the structural similarities of IgNAR with that of camelid heavy-chain IgG antibodies are due to a process of convergent evolution rather than a true phylogenetic relationship between these two immunoglobulin types [28, 29]. Shark B cells are morphologically similar to that of teleost fish and higher vertebrates. In sharks it has been shown that IgNAR and IgM are expressed by different B cell subsets and that these species are devoid of B cells concomitantly expressing both B cell receptors [1]. However, nothing is known at this point regarding the B cells expressing IgW. In summary, shark B cells represent the oldest living ancestors of more modern B cells, including those of teleost fish and mammals.
3. TELEOST FISH B CELLS AND IMMUNOGLOBULINS
Teleost fish are also called bony fish since they represent the oldest living species containing true bones. Teleost fish comprise the most modern fish species as they appeared on earth 400 million years ago, after the emergence of cartilaginous fish. This review will focus primarily on B cells of teleost fish as those are by far the best studied immunoglobulin-producing fish B cells. Before stepping into the world of teleost B cells, it is worth mentioning that teleost fish have leukocytes that are morphologically and functionally similar to mammalian macrophages, dendritic cells, neutrophils, monocytes, thrombocytes, T cells and plasma cells [16, 30-32]. The occurrence of eosinophils, mast cells and basophils is not certain in teleosts although there are several studies reporting cells with similar morphologies and functions [33]. On the other hand, there is good evidence for the existence of natural killer-like cells [34]. Another type of cytotoxic cell that seems to be different from teleost fish NK cells is the nonspecific-cytotoxic cell (NCC), which can recognize and destroy a variety of targets, including transformed human cell lines and protozoan parasites [35, 36]. It should be clarified that while the aforementioned cells seem to be the counterparts of those in mammals, a limited number of functional studies have been performed with most of these cell types, and thus, more work is required to clearly define their functions and relationships with their mammalian counterparts.
Teleost fish species contain three different immunoglobulin isotypes, IgM, IgD and the recently described IgT/Z [6-8] (Table 1). Teleost IgM is a tetrameric molecule, as opposed to the pentameric mammalian IgM [7, 37]. Tetramers in teleost IgM are formed apparently in the absence of inter-molecular interactions mediated by immunoglobulin joining (J) chains [17]. Interestingly, the IgM tetramers are found in different oxidation states believed to be important for function [38]. In all species analyzed thus far, the tetrameric IgM is the most abundant Ig present in serum, and until recently it was thought to be the only teleost fish Ig responding to antigenic stimulation [7, 37]; in 2010 however, IgT was also shown to respond to antigenic stimulation [39]. Memory IgM responses have been demonstrated in teleost fish although it is clear that they are significantly less enduring than those demonstrated in mammals [40-43]. In teleost fish, IgM is secreted by plasmablasts and plasma-like cells [42, 43]. It has been shown that the majority of IgM-secreting cells are localized in the head kidney [42, 43]. Immunoglobulin class-switch does not occur in teleosts. In addition these species are known for having poor affinity maturation of their IgM responses [7, 37, 44]. Interestingly, the time required to generate a significant antigen-specific response is generally much longer than that in mammals and it appears to be temperature-dependent [7, 37]. As an example, to generate significant antigen-specific IgM responses upon intraperitoneal injection, rainbow trout and salmon need at least 3-4 weeks, in contrast to the few days required by mammals [42, 43].
Table 1.
Summary of Features from B Cells and Immunoglobulins of Vertebrates
| Vertebrate Spleen Class Thymus | VLR-Producing Lymphocytes | Ig-Producing Lymphocytes | Ig Classes | Affinity Maturation | Class Switch | L. Nodes/G. Centers | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Agnathan fish | + | - | - | ? | - | - | No spleen, but thymuslike structure |
|
| |||||||
| Cartilaginous fish | - | + | IgM, IgW | Poor | - | - | + |
| IgNAR | |||||||
|
| |||||||
| Teleost fish | - | + | gM, IgD | Poor | - | - | + |
| IgT | |||||||
|
| |||||||
| Amphibians | - | + | IgM, IgY | Poor | + | + | + |
| IgX, IgD | |||||||
|
| |||||||
| Reptiles | - | + | IgM, IgY | ? | + | + | + |
| IgA (?) | |||||||
|
| |||||||
| Birds | - | + | IgM, IgY | Medium | + | + | + |
| IgA | |||||||
|
| |||||||
| Mammals | - | + | IgM, IgG | High | + | + | + |
| IgA, IgD | |||||||
| IgE | |||||||
Abbreviations: Ig=Immunoglobulin; L. nodes= Lymph nodes; G. centers= Germinal Centers.
With regards to IgD, this immunoglobulin has been cloned in several teleost species [7, 45]. At the protein level, IgD has only been characterized in catfish where it appears to be a monomeric immunoglobulin [46]. Interestingly, it has been shown that secretory IgD in catfish lacks the variable region. In that regard, the role of fish IgD in immunity is unclear, although the IgD Fc-region of catfish has been suggested to function as a pattern recognition molecule [46]. Moreover catfish have also been described to contain IgD-armed granulocytes [47]. In catfish, two IgD+ B cell populations have been identified, an IgM+/IgD+ and a IgM-/IgD+ subset [48], although more studies are required to elucidate the distinct roles of these IgD+ B cell subsets in immunity. In all other teleost fish analyzed thus far IgD is always found co-expressed with IgM on B cells.
In addition to IgM and IgD, teleost fish contain IgT, a new immunoglobulin class first identified in 2005 (6). In rainbow trout it has been named IgT [6], whereas in zebrafish this isotype is called IgZ [8]. This isotype has been cloned in all analyzed teleost fish, except in the catfish [49]. Based on the genomic arquitecture of the teleost fish ighτ-ighμ locus, teleost B cells were predicted to express either IgT or IgM. Consistent with this prediction, genetic evidence for two distinc lineages expressing IgM or IgZ were reported in zebrafish [50]. In addition, the identification of a novel subset of B cells uniquely expressing surface IgT was reported in rainbow trout [39]. The same report confirmed that rainbow trout B cells expressed either surface IgM or IgT, but not both immunoglobulins at the same time. Importantly, trout IgT+ B cells were shown to represent the prevalent B cell subset in the gut. While IgT was found as a monomer in serum, in the gut mucus was present for the most part in multimeric form, a feature resembling that of human IgA. In that respect, we have recently demonstrated that IgT is an immunoglobulin class specialized in mucosal immunity, thus representing a functional analog of IgA in teleost fish [39].
In conclusion, teleost fish contain a variety of B cell subsets. In catfish three B cell subsets have been identified: IgM+/IgD-, IgM+/IgD+ and IgM-/IgD+ [48] while in rainbow trout, only two subsets have been described thus far: IgM+/IgD+/IgT- and IgM-/IgD-/IgT+ [39]. However, less is known in terms of the distribution of these different subsets in systemic and mucosal lymphoid tissues. We have shown that IgM-/IgD-/IgT+ B cells (IgT+ B cells) represent a minor B cell subset in the main systemic lymphoid organs (~16-27% of all B cells), whereas in the gut, IgT+ B cells comprise the prevalent (~54% of all B cells) B cell population [39]. In addition, IgT+ B cells appear to be also the prominent B cell subset in trout skin (unpublished results), thus supporting further the concept that IgT is an immunoglobulin specialized in mucosal immunity. One remarkable difference between B cells of teleost fish and mammals is their relative profusion in blood. Thus, in most analyzed teleosts species, B cells represent an average of ~30-60% of all peripheral blood leukocytes (PBLs), while monocytes and neutrophils combined comprise only ~5-20% of PBLs [51-55]. In contrast, the percentage of B cells in human PBLs is only ~2-8%, whereas neutrophils/monocytes comprise ~50-70 % of all blood leukocytes [56]. The aforementioned numbers in teleost fish only take into account the IgM+ B cell subset, while the real percentages of total B cells in blood may be even higher once the percentage of IgT+ and IgD+ B cell subsets will be known in several species. Not only the percentage of B cells is very high in PBLs, but also the total blood B cell numbers in fish appear to be much higher than those of humans. Taking rainbow trout as an example, they contain ~5000-10000 IgM+ B cell per μl blood [51], whereas in humans, the B cell count ranges ~150-720 per μl of blood [57]. Due to the fact that a large percentage of B cells in teleosts are phagocytic and capable of intracellular killing (see below in section 6.1), the high abundance of blood B cells in these species may suggests a prominent role of these cells in the clearance of microbes and particulate antigen.
4. RELATIONSHIP BETWEEN B CELLS AND MACROPHAGES
In the past, evolutionary, developmental and functional relationships between B cells and macrophages have long been recognized. A number of studies have shown that mouse and human malignant B cell lines can switch into macrophage-like cells, having the capacity to phagocytose large particles [58-60]. This switch from a lymphoid to a myeloid lineage gave rise to the term of “lineage switching” or “lineage infidelity” [61]. The biphenotypic features of these malignant cells pointed to a close relationship between CD5+ B-1 cells and macrophages [61]. Moreover, subsets of primary CD5+ B cells have been shown to differentiate in vitro into cells with classical macrophage morphology. These cells were called B/macrophage cells as they were able to express B220, surface IgM, surface IgD, CD5, F4/80 and Mac-1 markers, in addition to containing rearranged Ig genes [62, 63]. Based on the latter findings the authors suggested that subsets of B lymphocytes and macrophages shared a closer lineage relationship than what the models of hematopoietic differentiation at the time predicted [61]. Alternatively, they also suggested that theses B/macrophage biphenotypic cells could represent a primitive B lymphocyte lineage with a great flexibility to adapt to infectious agents. Similar to the studies by Borrello et al., peritoneal cavity murine B-1 cells were reported to differentiate into a mononuclear phagocyte in vitro. These B-1-derived mononuclear phagocytes had a macrophage-like morphology and a potent capacity to phagocytose large particles [64]. Additional evidence for a relationship between B cells and macrophages came from studies showing that differentiated B cells could be reprogrammed in vitro into macrophages [65]. Further support for this lineage relationship between B cells and macrophages came from the identification of B/macrophage progenitors in fetal liver [66] and mammalian adult bone marrow [67]. These progenitor cells are not capable of phagocytosing or acting as B cells; however they are able to differentiating either into macrophage or B cells, depending of the micro-environmental milieu. All of the aforementioned functional and developmental relationships between B cells and macrophages in mammals suggest an evolutionarily close relationship between these cells as already indicated by Katsura [68], implying a common predecessor for both cells in species predating the emergence of jawed vertebrates. Recent findings of subsets of B cells from fish, amphibians, reptiles and mammals with phagocytic activity (see below) give further support to the hypothesis that B cells and macrophages arose from a common phagocytic ancestor [69].
5. PHAGOCYTOSIS IN VERTEBRATES
In vertebrates, phagocytosis appears to have evolved from playing a role in innate immunity to having essential functions in adaptive immunity [70, 71]. Thus in mammals, phagocytosis plays a key role in the uptake and destruction of microbes, as well as in the initiation and development of an adaptive immune response [71]. Whether phagocytosis in lower vertebrates (i.e., fish, amphibians) is involved also in the initiation of adaptive immune processes, remains to be investigated. However, cells specialized in the uptake of foreign material have been described in all classes of vertebrate species, from the most primitive fish (agnathan) to mammals [71, 72].
The process of phagocytosis is known to be triggered by the interaction of surface molecules from the phagocytic target (i.e., PAMPs), with receptors of the phagocyte (i.e., PRRs such as scavenger, Fc, complement receptors, Toll receptors) [70]. Lysosomes fusing to phagosomes form phagolysosomes, the vesicles where internalized microbes will be killed and degraded by a variety of lysosomal hydrolytic enzymes. Formation of phagososmes and phagolysosomes have been described not only in mammals but also in fish [51]. Moreover, phagocytosis is known to elicit several antimicrobial mechanisms that are used by phagocytes to kill microbes [73]. Arguably, the most important and well-known of these mechanisms involve the production of reactive oxygen (i.e., superoxide radicals) and nitrogen (i.e., nitric oxide) intermediates, which are known to kill ingested microbes contained in the phagolysosomes of mammals and fish [73]. In mammals, degraded microbial antigens can be proteolytically processed by intracellular endopeptidases, and the resulting peptides can then be presented to T cells through the MHC-II pathway [71]. In addition, it has also been shown that phagocytosis is involved in MHC-class I-restricted antigen presentation [74, 75], or also called cross-presentation. Whether presentation of particulate antigen in fish occurs through any of the two aforementioned pathways, is thus far not known. In mammals and other vertebrates, including fish, phagocytosis is mainly performed by professional phagocytes, including polymorphonuclear cells (PMNs), monocytes and macrophages [73, 76]. Other cell types in mammals (i.e., epithelial cells, fibroblasts, NKT cells, plasmacytoid dendritic cells) have also been shown to be capable of engulfing particles, albeit with a much restricted capacity [76-78]. Until recently, it was thought that vertebrate primary B cells were unable to perform phagocytosis. Breaking this paradigm, the last few years have seen a number of reports demonstrating the ability of some vertebrate B cell subsets to phagocytose non-opsonized particles and to intracellularly kill microbes (see section 6 below and Table 2). Thus, it would appear that professional phagocytosis is not restricted to cells of myeloid origin, as it can also be carried out by cells of lymphoid origen.
Table 2.
Summary of Features from Phagocytic B Cells of Vertebreates
| Phagocytic B Cell Features | ||||
|---|---|---|---|---|
| Vertebrate Cytokine Classes Production | B Cell Subsets | Intracel Killing | Antigen Presentation | |
| Agnathan fish | ? | ? | ? | ? |
| Cartilaginous fish | ? | ? | ? | ? |
| Teleost fish | IgM+ and IgT+ | + | ? | ? |
| Amphibians | IgM+ (The only studied subset) | ? | ? | ? |
| Reptiles | IgM+ (The only studied subset) | ? | ? | ? |
| Birds | ? | ? | ? | ? |
| Mammals | B-1a, B-1b and to a much lesser degree, B-2 cells. | +, but only in PerC and liver | + | IL-12 |
Abbreviations: Intracel. Killing= Intracellular killing; PerC= Peritoneal Cavity.
6. PHAGOCYTIC B CELLS DISCOVERED IN VERTEBRATES
6.1. Phagocycitic B Cells in Fish
Up until recently, all studies done with fish phagocytes had shown that, like in mammals, monocytes, neutrophils and macrophages were the main phagocytic cells [30, 73]. Changing this view, in 2006 we showed for the first time in vertebrates that fish primary B cells were capable of phagocytosis and intracellular killing [51]. In the past, phagocytosis in fish had typically been studied utilizing the population of glass/plastic adherent leukocytes derived mainly from head kidney, and to a lesser degree, from other lymphoid sites. Moreover, the use of cell markers specific for B cells or other leukocyte types was seldom employed in such studies. Because of the reasons stated above, the ability of B cells to phagocytose particles in fish had probably remained unnoticed.
The original identification of fish phagocytic B cells came while studying the phagocytic activity of rainbow trout blood leukocytes using flow cytometry as means to detect phagocytic cells. The initial results indicated the presence of large numbers of small non-adherent cells avidly engulfing a variety of particles. Characterization of this non-adherent population with a well-known monoclonal antibody against trout IgM [79] provided the first indication that these small phagocytic cells had B-lymphocyte features. Giemsa staining and transmission electronic analysis showed that sorted phagocytic IgM+ cells possessed lymphocyte-like morphology. In addition, from all sorted phagocytic cell populations, only the IgM+ cells expressed B-cell specific markers including sIgM, mIgM and IgD. Moreover, the expression of T cell markers (TCR-α, TCR-β and CD8) was restricted to the non-phagocytic IgM− population, indicating that trout T cells were not phagocytic [51].
The ultrastructure of phagocytic B cells was studied by transmission (TEM) and scanning (SEM) electron microscopy. Phagocytic B cells were characterized by having a large nucleus, a thin rim of agranular cytoplasm and a varying number of small dendrites surrounding the cell. Interestingly, most of the analyzed cells contained significant numbers of mitochondria, a trait that has been identified also in mouse B-1 lymphocytes [80]. In most instances, engulfment of 1 μm beads was carried out by small dendrites, although membrane extensions or pseudopod-like structures were occasionally observed. Moreover, SEM analysis clearly showed the formation of phagocytic cups containing beads in the process of being internalized Fig. (1). Strikingly, over 55% of all blood IgM+ B cells were phagocytic, a number that in blood represented 62% of all phagocytic cells, while in the head-kidney, phagocytic B cells represented only 20% of all phagocytes. While those experiments were done using non-opsonized particles, we demonstrated that phagocytosis by IgM+ B cells was significantly enhanced if bacteria was coated with IgM or complement, thus suggesting that phagocytic B cells contained both Fc and complement receptors that enhanced their ability to internalize opsonized microbes. The capacity to augment particle uptake by IgM and complement opsonization has also been reported in fish granulocytes and monocytes [81-84]. To demonstrate the physiological relevance of phagocytic B cells, in vivo studies showed that a significant percentage of peritoneal cavity IgM+ B cells (29-44%) could also phagocytose inert particles and bacteria [51].
Fig. (1). Transmission and scanning electron microscopy analysis of fish phagocytic B cells.
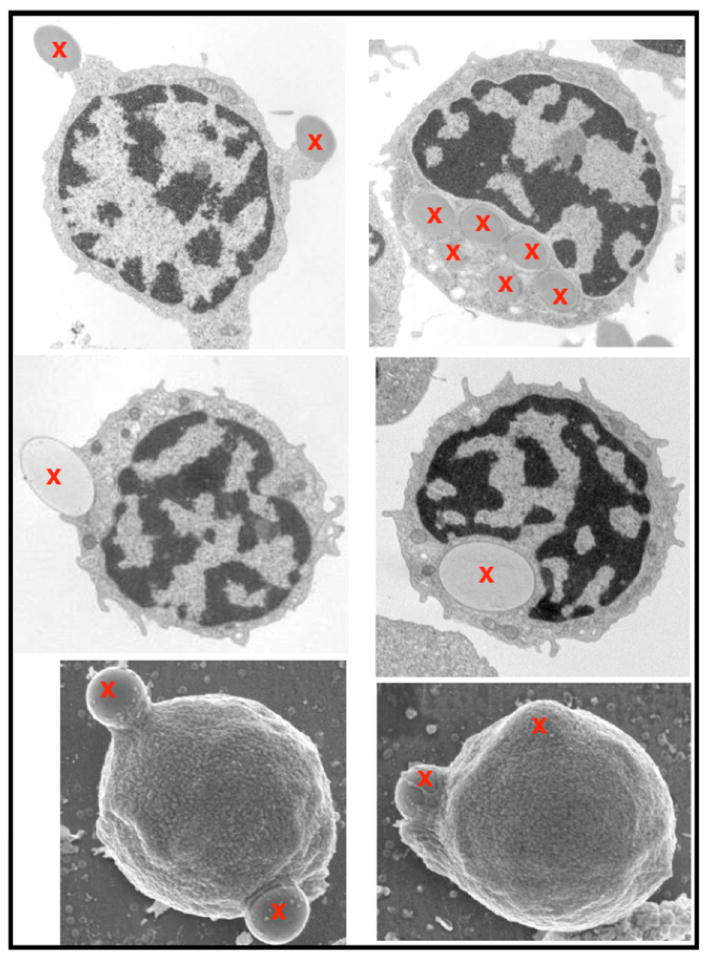
Rainbow trout IgM+ B cells can be seen in the process of internalizing 1 μm or 2 μm latex beads (left images) or with internalized beads (right images). Upper and middle panels are TEM images, whereas the bottom panels are SEM images. Beads are marked with a red X. Images were taken from (51) and reproduced with permission of Nature Immunology.
Professional phagocyte internalization of particles is known to trigger signaling cascades that result in the formation of phagolysosome vesicles in which microbes are killed and degraded. TEM analysis showed that bacteria ingested by phagocytic B cells appeared to be contained in a phagosome-like compartment, suggesting that processes of phagolysosome formation might also occur upon internalization of particles by trout B cells (51). In addition, we confirmed that particle internalization by phagocytic B cells resulted in the fusion of lysosome vesicles to phagosomal compartments [51]. The formation of phagolysosomes in trout phagocytic B cells presented exciting possibilities for a possible contribution of these cells to bacterial killing. Experiments to address the aforementioned hypothesis confirmed that these cells were capable of intracellular killing. All combined, these findings showed for the first time the presence of primary B cells in a vertebrate species with the capacity of phagocytose and kill internalized microbes. The findings described above were generated using the IgM+ B cell subset of rainbow trout. Recently these findings were extended to a newly found rainbow trout B cell lineage uniquely expressing IgT [39]. This report revealed that a large percentage of blood IgT+ B cells were also phagocytic and capable of killing internalized bacteria. In blood, IgT+ B cells represented ~20% of all B cells, and the percentage of blood IgT+ B cells with phagocytic capacity was similar (~57%) to that of IgM+ B cells. Whether this capacity is kept by IgT+ B cells from other lymphoid tissues, including those of mucosal surfaces, is a question that requires further investigation.
In addition to rainbow trout, other teleost fish species have also been shown to contain phagocytic IgM+ B cells, including catfish, cod and atlantic salmon. Catfish blood was shown to have large amounts of phagocytic IgM+ B cells and by TEM, their ultrastructure was very similar to that of rainbow trout [51]. In a different study, both salmon and atlantic cod contained significant numbers of phagocytic IgM+ B cells in head kidney and blood [85]. Interestingly, phagocytic IgM+ B cells in salmon represented the vast majority of blood phagocytes whereas in cod they represented only 8% of these cells. Moreover, the phagocytic capacity of cod IgM+ B cells was higher than that of salmon since over 70-80% of the cod cells ingested three or more beads in contrast to salmon in which that number was below 50% [85]. Thus it seems apparent that this phagocytic capacity is a general feature of all B cell lineages in teleost fish, although these results need to be confirmed in other fish species.
6.2. Phagocytic B Cells in Amphibians and Reptiles
Our investigations on the phagocytic ability of B cells were extended to amphibians. These studies showed that while a significant portion of Xenopus laevis blood IgM+ B cells where capable of phagocytosing latex beads, the percentage of phagocytic IgM+ B cells was significantly lower (14%) than that found for trout and other teleost fish blood B cells [51]. By TEM, the ultrastructure of Xenopus laevis phagocytic IgM+ B was almost indistinguishable from that described for rainbow trout phagocytic B cells. Whether these amphibian phagocytic B cells are capable of intracellularly killing internalized bacteria, is not known at this point. Similar to what has been described in Xenopus laevis, a recent study has demonstrated the presence of small numbers of blood IgM+ B cells with phagocytic capacity in the turtle red-eared slider, a reptile [86]. The aforementioned studies on amphibians and reptile phagocytic B cells were quite limited in scope, and thus, other lymphoid organs as well as B cell subsets (i.e., IgX+ and IgY+ B cells) of these species need to be investigated.
6.3. Phagocytic B Cells in Mammals
Until recently, it was thought that mammalian primary B cells were unable to perform phagocytosis. One study showed a BCR-mediated uptake of live Salmonella typhimurium by human primary B cells. However, dead Salmonella could not be taken up by these cells, and it was shown that Salmonella internalization required bacterial-mediated processes [87]. Thus, it was not clear from these studies that Salmonella internalization was due to an active process of BCR-mediated phagocytosis or to bacterial-mediated mechanisms.
Earlier findings on phagocytic B cells in fish and amphibians, coupled with the more recent description of phagocytic B cells in reptiles, suggested that this phagocytic capacity in certain B cell subsets of vertebrates was evolutionarily preserved. To investigate whether this capacity was conserved in subsets of mammalian B cells, we investigated the phagocytic ability of murine B cells from bone marrow, spleen, blood and peritoneal cavity. Preliminary results showed that the peritoneal cavity (PerC) contained the largest percentage of phagocytic B cells (~10-17%), whereas in the other lymphoid sites, the amount of B cells with phagocytic capacity was less than 1.6%. Thus, the efforts to characterize further the functions and phenotypes of phagocytic B cells focused on the study of murine PerC B cell subsets. It was found that most of the PerC phagocytic B cells were B-1 B cells [88]. Hence, around 9-17% of the B-1 B cells were phagocytic, while less than 1.5% of B-2 B cells contained such ability. Interestingly, the PerC B-1a subset possessed a significantly higher percentage of phagocytic cells (14-17%) than the PerC B-1b subset (8.6-11.4%). The phagocytic capacity of both B-1 B cell subsets was demonstrated both in vitro and in vivo using latex beads as well as E. coli as phagocytic targets. Importantly, it was shown that phagocytosis by PerC B-1a and PerC B-1b B cells occurred in a B cell receptor (BCR)-independent manner, although the receptors involved in the recognition and uptake of the used phagocytic targets is at this point unknown. The ultrastructure of phagocytic B-1a and B-1b B cells was very similar to that previously described for normal cells of these two B cell subsets [80]. In that regard, phagocytic B-1a and B-1b B cells displayed a large nucleus with a cytoplasm containing a considerable number of mitochondria. Moreover, phagocytic B-1 B cells were surrounded by small dendrites that were often seen in the process of particle internalization Fig. (2).
Fig. (2). Transmission electron microscopy analysis of murine phagocytic B-1b B cells.
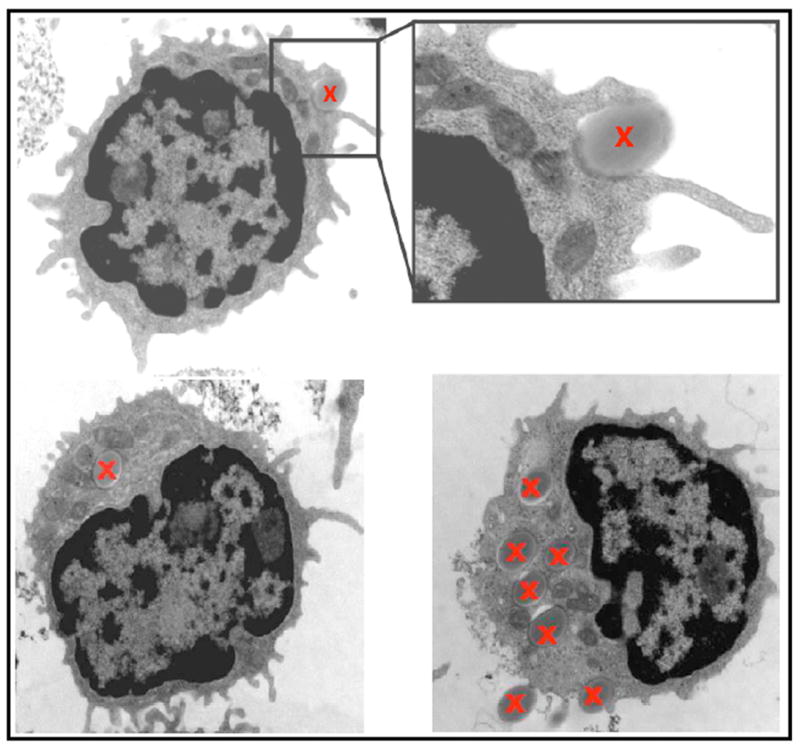
Murine B-1b B cells can be seen in the process of internalizing 1μm latex beads (top images) or with internalized beads (bottom images). Beads are marked with a red X. Images were taken from (88) and reproduced with permission of Journal of Leukocyte Biology.
Similar to fish phagocytic B cells, murine phagocytic PerC B-1a and PerC B-1b B cells were able to mature their phagosomes into phagolysosomes and exhibited the ability to kill internalized bacteria. However their phagocytic and bactericidal capacities were significantly lower than those found for PerC macrophages. In that sense, PerC macrophages were able to ingest a considerably higher number of phagocytic targets, and they were able to kill most internalized bacteria in a much faster manner than phagocytic PerC B-1 B cells. It is possible that the high efficiency of PerC macrophages to kill bacteria is due to the larger amount of lysosomes of these cells when compared to that of PerC B-1 B cells (88).
It is well-known that phagocytosis by macrophages and dendritic cells leads to the presentation of internalized antigen to T cells [89]. Using the OVA antigen model [90], it was demonstrated that PerC B-1a and PerC B-1b B cells were able to efficiently present antigen from internalized particles to CD4+ T cells. In the absence of particle internalization, antigen presentation was hampered. Critically, the capacity of both PerC B-1 subsets to present particulate antigen was far superior than that seen for the same amount of soluble antigen. With regards to particulate antigen, it was apparent however that PerC B-1a were better APCs than PerC B-1b cells, probably due to the smaller number of cells of the later subset with a phagocytic ability. Similar to PerC B-1 cells, macrophages and dendritic cells were also more efficient at presenting particulate antigen, although macrophages were significantly less potent APCs than both PerC B-1 subsets. However, the capacity to present both particulate and soluble antigen by BMDCs cells was superior than that of PerC B-1 cells [88]. On the other end, PerC B-2 cells displayed the lowest capacity to present particulate antigen, probably because very few B-2 cells had the capacity to internalize particles, as stated above. In addition, the capacity of B-2 cells to present soluble antigen was also lower than that of PerC B-1 cells.
At the same time we reported the identification of murine phagocytic PerC B-1 cells, another study by Nakashima et al., described the existence of phagocytic B cells in the liver and spleen of mice [91]. In agreement with our findings [88], their in vitro studies showed that the percentage of liver and spleen B-1 B cells with phagocytic capacity was greater than that of B-2 cells. However, the percentage of liver and spleen phagocytic B-2 B cells shown by the later study [91], is greater than what we reported for PerC and spleen B-2 B cells [88]. This disparity in the results seen for phagocytic B-2 B cells in the two studies could be due to at least two possible reasons: 1-) The bed:cell ratio used for the in vitro phagocytic assays by Nakashima et al. [91] was 200:1 or 400:1, far superior than the ratio (10:1) used in most of our studies [88]. Hence, it is likely that at such high bed:cell ratios, the percentage of cells with non-specifically attached beads is larger than that seen when using a much lower ratio. In fact, our preliminary studies had shown that when using bed:cell ratios higher than 40:1, the number of B cells with non-internalized beads was far more plentiful than when using ratios of 20:1 or less (unpublished results). Moreover in the study by Nakashima et al. [91] when 10:1 or 20:1 bed:cell ratios where used, very low percentages of B cells with phagocytic capacity (1-3%) were obtained, similar to those observed by us in the spleen, bone marrow and blood when using the same bed:cell ratios [88]. However, at these low bed:cell ratios (10:1-20:1), we found that as many as 11-14% of PerC B-1 were phagocytic, while only 1-2% of B-2 B cells had this capacity. All combined, the aforementioned studies suggest that the most potent phagocytic B cells are the PerC B-1 B cells since that subset presented the highest percentage of cells with phagocytic ability when using more physiologically relevant (lower) bed:cell ratios. 2-) Another reason that may explain the differences in the numbers of phagocytic B-2 B cells between the [88] and [91] reports, may reside in the activation state and/or the differential expression of PRRs of B cells, depending on the lymphoid tissue where these cells are localized. This in turn would influence the recognition and uptake of particles. Thus, the possibility exists that B-2 B cells from the liver, spleen and peritoneal cavity differ in the aforementioned parameters; however this would not explain the higher percentage of phagocytic B-2 B cells observed in the spleen by Nakashima et al. [91] when compared to that reported in [88]. In this case, the disparity of results could be attributed to methodological differences. In that regard, it is somewhat puzzling that in contrast to what was found when employing latex beads, the use of non-opsonized pHrodo-E. coli in the same study resulted in very low percentages of spleen phagocytic B cells, similar to those observed by us in [88].
While liver and spleen B cells appeared to internalize non-opsonized beads and bacteria, the same report showed that complement opsonization of bacteria promoted their uptake by a significantly larger number of B cells [91]. This finding is similar to what had previously been described for phagocytic B cells of fish [51]. Whether opsonization of particles with antibody will also enhance their uptake by phagocytic B cells, is a question that remains to be investigated.
In addition to showing the presence of liver and spleen phagocytic B cells using in vitro strategies, Nakashima et al., identified the same cells also when performing in vivo studies
[91. Similar to what was found for PerC B-1 cells {Parra, #2438], it was revealed that liver phagocytic B cells had the capacity to form phagolysosomes and to kill internalized bacteria, although this capacity was not observed in spleen phagocytic B cells [91]. The reasons for the differences seen in bactericidal activity between liver and spleen phagocytic B cells were not clear. While our studies showed that PerC B-1 cells efficiently presented particulate antigen to T cells [88], this capacity was not investigated for liver and spleen phagocytic B cells [91]. On the other hand, a very interesting finding by Nakashima et al., was the ability of liver (but not spleen) phagocytic B cells to secrete Il-12 [91]. The authors argued that this could enhance the killing of bacteria since Il-12-mediated induction of IFN-γ by NK and T cells would augment the bactericidal activity of macrophages. It will be interesting in the future to analyze which other cytokines are produced by phagocytic B cells. In that respect, phagocytosis by professional phagocytes induces the release of proinflammatory cytokines; whether this is the case also for phagocytic B cells, remains to be investigated.
In conclusion, while some disparities exist on which murine B cell subsets display phagocytic and bactericidal abilities, it seems clear that B-1 B cells are the most abundant at exhibiting such capacities. It could be argued that these subsets of phagocytic B cells may contribute significantly to the clearing of microbes through phagocytosis. However, it is clear from the recently reported data that macrophages and neutrophils were able to uptake many more particles than phagocytic B cells [88, 91]. Moreover, macrophages killed internalized bacteria with a significantly faster killing kinetics than phagocytic B cells [88]. Therefore, it would be conceivable to state that the main role of phagocytic B cells is probably not the clearing of microbes (although that may happen in some local environments), but the efficient presentation of low amounts of particulate antigen to T cells. In that regard, when using equal amounts of particulate antigen, phagocytic PerC B-1 B cells were considerably superior than macrophages in presenting antigen to CD4+ T cells [88]. While the aforementioned studies represent a significant advance on the roles of B-1 B cells in immunity, much work remains to be carried out to further characterize the phenotypes of all mammalian phagocytic B subsets and to further dissect their roles in innate and adaptive immunity (discussed below).
7. DISCUSSION AND FUTURE PERSPECTIVES
The evolutionary history of Ig-producing B cells remains thus far unresolved, although several hypotheses have been proposed for their emergence in the missing ancestors that gave rise to jawed vertebrate species. VDJ recombination, a hallmark of B and T cell receptors, has been hypothesized to have arisen as result of an ancient lateral gene-transfer event setting of a transposon-mediated insertion of an ancestral RAG gene in the predecessor gene from which BCR/TCRs emerged later in jawed vertebrates [18, 92]. Whether the insertion of this ancient RAG gene occurred in a TCR- or BCR-like gene, is a matter of debate. It has been argued that this gene was probably more similar to TCR than BCR, since it has been shown that while lampreys lack VDJ recombination, they possess a single TCR-like gene with features of V and J regions [93]; thus this TCR-like gene could have been the target of the aforementioned transposon event [18]. Thereafter, the BCR would have evolved later by gene duplication from this ancestral RAG-containing TCR-like gene cluster, and both genes would have gradually diversified to give raise to modern BCRs and TCRs. Alternatively, it has been suggested that this transposon insertion event took place in species predating the emergence of jawed vertebrates which had distinct populations of cytotoxic killer cells and phagocytes. Both cell populations would have contained a BCR/TCR prototype, and after the transposon insertion event, the cytotoxic killer and the phagocytes would have evolved to T- and B-cells respectively [69]. It was also argued that the discovery of B cells with phagocytic capacity in fish supported the aforementioned hypothesis [51].
While the evolutionary intermediate cell that gave rise to Ig-producing B cells probably remains hidden in the fossil records, it is quite apparent that fish B cells represent the predecessors from which amphibian, reptilian, avian and mammalian B cells evolved. In that respect, there are clear morphological and functional parallels between mammalian B-1 B cells and fish IgM-bearing B cells. For example, both of them can innately secrete IgM when directly activated by a number of PAMPs, they are both capable of phagocytosis and intracellular killing, and they both can be induced to produce antigen-specific IgM. Therefore, one could speculate that the B-1 B cell lineage evolved from IgM-bearing B cells of ectotherms and, later in evolution, the B-2 B cell lineage emerged as a more evolved B cell subset that gradually specialized to play a prevailing role in adaptive immunity. This hypothesis would fit well with the concept of an evolutionarily layered immune system proposed by L.A. Herzenberg [94]. Accordingly, B-1 B cells would constitute the evolutionary oldest B cell layer, and as such, they embody more innate features than B-2 B cells, the most evolved layer of all B cell subsets with a more prominent role in adaptive immunity. This evolutionary pathway might explain also the much lower phagocytic capacity observed in B-2 B cells when compared to that of B-1 B lymphocytes [88], as phagocytosis is considered an innate immune process. Nevertheless, it is also possible that the predecessor of B-1 and B-2 lineages might have evolved concurrently from B cell lineages or subsets that remain to be characterized in fish or other cold-blooded vertebrates. Therefore, the evolutionary origins of B-1 and B-2 B cell lineages cannot be accurately traced until we identify further the existing B cell lineages and subsets of lower vertebrates and we investigate their specific roles in innate and adaptive immunity.
Here we illustrate how studies carried out originally on fish B cells have lead to the discovery of new innate and adaptive roles of mammalian B cells. Accordingly, recent studies have shown that several subsets of mammalian B cells, prevalently B-1 B cells, possess potent phagocytic and bactericidal capacities [88, 91]. Critical for the initiation of adaptive immune responses, it was also shown that PerC B-1B cells, but not B-2 B cells, were able to efficiently present non-opsonized phagocytosed particulate antigen to CD4+ T cells [88]. Remarkably, phagocytic PerC B cells had a much higher capacity to present particulate antigen than PerC macrophages, despite of the fact that the later could ingest much larger numbers of particles than PerC B cells. It was argued that this greater antigen-presenting capacity of phagocytic PerC B cells could be due to the observed lower potential for phagolysosome acidification of these cells when compared to that of macrophages [88]. In that regard, it has been demonstrated in DCs that a delayed acidification of their phagolysosome leads to an increased efficiency of these cells to present antigen [95, 96]. In fact, a lower degree of phagolysosome acidification was shown to translate into reduced degradation and greater retention of antigen, thus leading to a more efficient antigen-presenting capacity of DCs [95, 97] and perhaps by extension, of phagocytic PerC B cells [88]. In line with the aforementioned arguments, it has been reported that murine B cells contain lower levels of lysosomal proteases than macrophages, an attribute that allows them to retain antigen for longer time periods [98]. Because of the much higher phagocytic capacity of macrophages when compared to that of phagocytic PerC B, but the superior antigen presenting abilities of the later cells, it was hypothesized that rather than clearing microbes, a main task of phagocytic PerC B would be to present particulate antigen to T cells [88]. To confirm this hypothesis, future in vivo studies will have to address the specific contributions and prevalent roles of phagocytic PerC B-1 B cells and macrophages in clearing microbes as well as in presenting particulate microbial antigens to T cells. Moreover, these studies will have to be extended to evaluating the capacity and specific contributions of phagocytic PerC B-1 B cells to cross-present particulate antigen to CD8+ T cells, an ability already demonstrated in macrophages and dendritic cells [75, 99].
While PerC B-1 B cells were shown to possess a high capacity to present non-opsonized particulate antigen to CD4+ T cells [88], this only occurred when the particle was internalized. Interestingly, earlier studies had shown that murine B cells could extract and present antigen from non-internalized particles, although that process was BCR-dependent [100, 101]. Therefore it would appear that B-1 and B-2 B cells have different but complementary abilities in the presentation of particulate antigen. Accordingly, B-1 B cells would seem to be more specialized in presenting “innately” recognized and internalized particulate antigen, whereas B-2 B cells would be more proficient at presenting “adaptively” recognized particulate antigen. To confirm this hypothesis, further studies will have to investigate the capability of B-1 B cells to present non-internalized antigen in a BCR-dependent manner, and to compare their efficiency to that B-2 B cells. In addition, to further dissect the specific antigen presenting capabilities of these B cell subsets, it will also be necessary to compare the efficiencies of B-1 and B-2 B cells at phagocytosing and presenting antibody- or complement-opsonized particulate antigen.
The capacity described in several vertebrate B cell subsets to phagocytose particles occurred in a non-BCR-mediated manner. Whether this process triggers the production of “antigen-nonspecific immunoglobulin” is a crucial point that needs to be addressed. Hence, upon internalization of the particle in a BCR-independent manner, two scenarios could be pictured. In the first scenario Fig. (3), the phagocytic B cells would not be activated to produce immunoglobulins or alternatively, their ability to generate them would be inhibited. Under such circumstances, the roles of phagocytic B cells might be limited to those played by other phagocytes and APCs, including microbial killing, antigen presentation, and production of IL-12. In addition, these cells could also play other immune roles that remain to be investigated, including their ability to produce pro-inflammatory or anti-inflammatory cytokines. In that respect, phagocytosis of microbes is an inflammatory process that leads to the production of pro-inflammatory cytokines [71], and B cells are known to produce under certain conditions a long list of these cytokines (eg., effector or regulatory B cells [102]). Thus, it is conceivable that phagocytic B cells could upregulate the expression of some of these cytokines upon uptake of foreign material. Alternatively, phagocytosis of apoptotic bodies (AB) could lead to the generation of antiinflammatory cytokines, as it has been shown for other phagocytes [103, 104]. Hence, it will be interesting to see if AB uptake is one of the roles played by IL-10 producingregulatory B cells [105, 106] or other B cell subsets. In connection to the later, it has been shown that apoptotic bodies can attach to B-1 B cells and that TIM-4 mediates part of this binding [107]. In the same report, immunofluorescence microscopy studies appeared to indicate that perC CD11b+ cells internalized AB, thus suggesting that B-1 B cells were implicated in AB uptake. However, further studies are required to clarify the direct involvement of B-1 B cells in AB phagocytosis as well as the functional consequences of this process, including the potential induction of anti-inflammatory cytokines by these cells.
Fig. (3). Schematic representation of potential roles of phagocytic B cells in mammalian immunity.
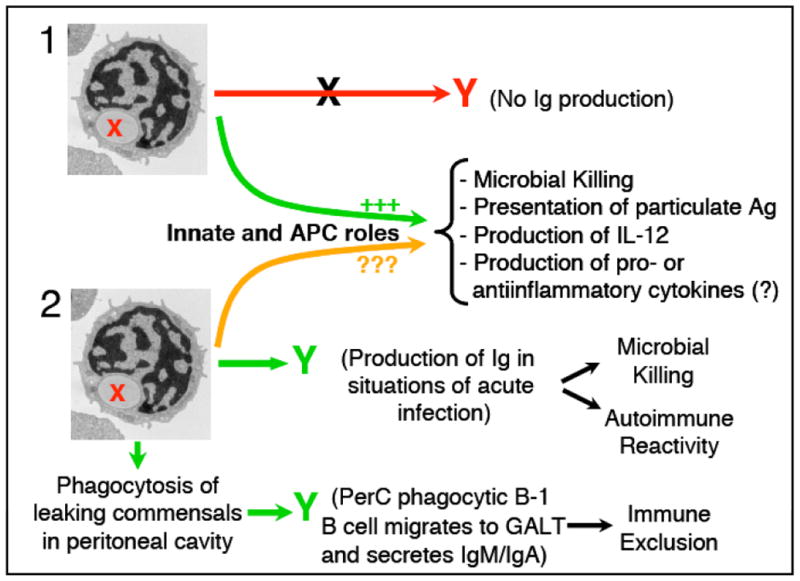
Upon phagocytosis of the particle, scenario 1 (top) hypothesizes a lack or inhibition of antibody production. In this scenario, phagocytic B cells could embrace functions characteristic of professional phagocytes and APCs. Some of these functions, including microbial killing, presentation of particulate antigent to CD4 T cells and secretion of Il-12, have recently been reported (88, 91). In addition, these phagocytic B cells could also produce pro- or antiinflammatory cytokines depending on whether they have ingested microbes or apoptotic bodies respectively. Scenario 2 (bottom) hypothesizes that upon phagocytosis of the particle, phagocytic B cells are activated and produce immunoglobulins. These natural Igs could be produced in situations of acute infection with the goal of recognizing microbial PAMPs and clearing microbes. Alternatively PerC B-1 B cells could ingest commensal bacteria leaking from the gut and going into the PerC. Upon phagocytosis of the commensal, the phagocytic PerC B-1 B cells would migrate into into GALT where this cell would either produce IgM or would class-switch to produce low affinity IgA. The secreted antibody would be transported into the gut lumen where it would contribute to the process of immune exclusion. Red or green Y represents non-produced or produced immunoglobulin respectively. Green, orange and red lines represent processes that will, may or won’t happen respectively.
In the second scenario Fig. (3), the uptake of particles in a non-BCR manner would trigger the production of “antigen-nonspecific” immunoglobulins. One could envision this process as an evolutionarily conserved strategy to produce “innate” antibodies that act as pattern recognition molecules (i.e., natural antibodies [108]), similar to the process of polyclonal activation induced by LPS in B-1 B cells. Such a strategy could be very useful in situations of acute infection in which these immunoglobulins would function in a first line of defense recognizing common microbial PAMPs. This scenario however, could potentially have harmful effects as the phagocytic B cells would be producing immunoglobulins with specificities unrelated to the antigen they have internalized. In turn, this might enhance autoimmune reactivity Fig. (3). This could explain at least in some cases, the involvement attributed to B-1 B cells in autoimmune diseases [109]. In addition to producing antibodies in situations of acute infection, in the case of PerC B-1 B cells one could envision that these antibodies would be generated upon migration of the phagocytic B cell into the GALT. In this alternative scenario Fig. (3), the PerC B-1 B cells could phagocytose and kill commensals leaking from the gut. Thereafter, the phagocytic PerC B-1 B cell would probably migrate into the GALT (i.e., gut lamina propria) to class-switch and produce low affinity IgA antibodies. These IgA antibodies would be transported into the gut lumen where they would play a role in immune exclusion. In that regard, production of extrafollicular IgA is known to occur in the GALT [110], although the mechanisms and pathways involved are thus far unresolved [111]. Whether or not the production of these “innate” IgM or IgA antibodies would occur concurrently with the activation of other immune processes stated in the first scenario (e.g., production of pro- or anti-inflammatory cytokines, antigen presentation to T cells), is a key point to investigate if we are to resolve further the functional implications of particle uptake by B cells.
In conclusion, much remains to be investigated concerning the evolutionary origins and functional relationships of B cell subsets from fish and mammals. We should be reminded that several critical discoveries on B cells have been made with the use of non-mammalian species. Most notably, the origen of the name B cells comes from the first description of these cells in the Bursa of birds [112, 113]. With regards to fish models, studies on catfish IgD were recently critical to uncover functions on mammalian IgD that had remain elusive for decades [47, 114]. Another example is the one provided in this review in which the initial discovery of phagocytic B cells in fish lead to the recent identification of mammalian B cell subsets with similar capacities [51]. Moreover, the studies on mammals also revealed that these phagocytic B cells efficiently presented particulate antigen to CD4+ T cells. Thus, these recent findings on mammalian phagocytic B cells will be now be instrumental in illuminating similar antigen-presenting capacities of these cells in fish.
Overall, the new findings described herein on phagocytic B cells, indicate that these cells are positioned at the crossroads that link innate with adaptive immunity. Thus, it has been proposed that phagocytosis-mediated uptake and presentation of particulate antigen, would provide these cells with the potential to initiate and modulate immune responses against microbes and particulate self-antigens. We are convinced that further comparative studies using B cells of nonmammalian and mammalian species will help unveiling clues on the aforementioned proposed roles of phagocytic B cells in these species. Moreover such studies will be instrumental in elucidating the evolutionary origins of B cells as well as potential new functions of phagocytic B cells and other B cell subsets in innate and adaptive immune responses.
Acknowledgments
This work was supported by the National Science Foundation (NSF-MCB-0719599 to J.O.S.), the National Institutes of Health (R01GM085207-01 to J.O.S.), the United States Department of Agriculture (USDA-NRI 2006-01619 and USDA-NRI 2007-01719 to J.O.S.), a 2008 University of Pennsylvania-School of Veterinary Medicine VCID award to J.O.S., and a 2010 University of Pennsylvania Research Foundation (URF) award to J.O.S.
ABBREVIATIONS
- TLR
Toll-Like Receptor
- IEL
Intraepithelial Lymphocytes
- APC
Antigen Presenting Cell
- GALT
Gut-associated Lymphoid Tissue
- AB
Apoptotic Body
- PerC
Peritoneal Cavity
- PRR
Pattern Recognition Receptor
- PAMPs
Pathogen-associated Molecular Patterns
- BCR
B cell Receptor
- TCR
T cell Receptor
- Dc
Dendrictic cell
- BMDC
Bone Marrow-derived Dendritic cell
- TEM
Transmission Electron Microscopy
- SEM
Scanning Electron Microscopy
- VLR
Variable Lymphocyte Receptors
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2(9):688–698. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 2.Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25(12):640–644. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr Top Microbiol Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 4.Wilson MR, Zhou H, Bengten E, Clem LW, Stuge TB, Warr GW, et al. T-cell receptors in channel catfish: structure and expression of TCR alpha and beta genes. Mol Immunol. 1998;35(9):545–557. doi: 10.1016/s0161-5890(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 5.Bengten E, Wilson M, Miller N, Clem LW, Pilstrom L, Warr GW. Immunoglobulin isotypes: structure, function, and genetics. Curr Top Microbiol Immunol. 2000;248:189–219. doi: 10.1007/978-3-642-59674-2_9. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci USA. 2005;102(19):6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solem ST, Stenvik J. Antibody repertoire development in teleosts-a review with emphasis on salmonids and Gadus morhua L. Dev Comp Immunol. 2006;30(1-2):57–76. doi: 10.1016/j.dci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6(3):295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 9.Secombes CJ, Wang T, Bird S. The interleukins of fish. Dev Comp Immunol. 2011;35:1336–1345. doi: 10.1016/j.dci.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Bird S, Zou J, Secombes CJ. Advances in fish cytokine biology give clues to the evolution of a complex network. Curr Pharm Des. 2006;12(24):3051–3069. doi: 10.2174/138161206777947434. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YA, Hikima J, Li J, LaPatra SE, Luo YP, Sunyer JO. Conservation of structural and functional features in a primordial CD80/86 molecule from rainbow trout (Oncorhynchus mykiss), a primitive teleost fish. J Immunol. 2009;183(1):83–96. doi: 10.4049/jimmunol.0900605. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JD, Du Pasquier L, Lefranc MP, Lopez V, Benmansour A, Boudinot P. The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol Immunol. 2009;46(3):457–472. doi: 10.1016/j.molimm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Gong YF, Xiang LX, Shao JZ. CD154-CD40 interactions are essential for thymus-dependent antibody production in zebrafish: insights into the origin of costimulatory pathway in helper T cell-regulated adaptive immunity in early vertebrates. J Immunol. 2009;182(12):7749–7762. doi: 10.4049/jimmunol.0804370. [DOI] [PubMed] [Google Scholar]
- 14.Palti Y. Toll-like receptors in bony fish: From genomics to function. Dev Comp Immunol. 2011;35:1263–1272. doi: 10.1016/j.dci.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Zapata A, Diez B, Cejalvo T, Gutierrez-de Frias C, Cortes A. Ontogeny of the immune system of fish. Fish & Shellfish Immunology. 2006;20(2):126–136. doi: 10.1016/j.fsi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Current Topics in Microbiology & Immunology. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 17.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007;8(2):131–135. doi: 10.1038/ni1435. [DOI] [PubMed] [Google Scholar]
- 19.Bernard D, Six A, Rigottier-Gois L, Messiaen S, Chilmonczyk S, Quillet E, et al. Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. J Immunol. 2006;176(7):3942–3949. doi: 10.4049/jimmunol.176.7.3942. [DOI] [PubMed] [Google Scholar]
- 20.Rombout JH, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol. 2010 doi: 10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Mayer WE, Uinuk-Ool T, Tichy H, Gartland LA, Klein J, Cooper MD. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc Natl Acad Sci USA. 2002;99(22):14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9(3):319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 23.Pancer Z, Saha NR, Kasamatsu J, Suzuki T, Amemiya CT, Kasahara M, et al. Variable lymphocyte receptors in hagfish. Proc Natl Acad Sci USA. 2005;102(26):9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, et al. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459(7248):796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, Kerzic MC, et al. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc Natl Acad Sci USA. 2010;107(30):13408–13413. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, McCurley N, et al. A thymus candidate in lampreys. Nature. 2011;470(7332):90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- 27.Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proc Natl Acad Sci USA. 2006;103(28):10723–10728. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374(6518):168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 29.Roux KH, Greenberg AS, Greene L, Strelets L, Avila D, McKinney EC, et al. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proc Natl Acad Sci USA. 1998;95(20):11804–11809. doi: 10.1073/pnas.95.20.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Companjen AR, Florack DEA, Slootweg T, Borst JW, Rombout J. Improved uptake of plant-derived LTB-linked proteins in carp gut and induction of specific humoral immune responses upon infeed delivery. Fish & Shellfish Immunology. 2006;21(3):251–260. doi: 10.1016/j.fsi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi T, Toda H, Shibasaki Y, Somamoto T. Cytotoxic T cells in teleost fish. Dev Comp Immunol. 2011;35:1317–1323. doi: 10.1016/j.dci.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Levraud JP, Boudinot P. The immune system of teleost fish. Med Sci (Paris) 2009;25(4):405–411. doi: 10.1051/medsci/2009254405. [DOI] [PubMed] [Google Scholar]
- 33.Reite OB, Evensen O. Inflammatory cells of teleostean fish: A review focusing on mast cells/eosinophilic granule cells and rodlet cells. Fish & Shellfish Immunology. 2006;20(2):192–208. doi: 10.1016/j.fsi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Shen L, Stuge TB, Bengten E, Wilson M, Chinchar VG, Naftel JP, et al. Identification and characterization of clonal NK-like cells from channel catfish (Ictalurus punctatus) Dev Comp Immunol. 2004;28(2):139–152. doi: 10.1016/s0145-305x(03)00119-8. [DOI] [PubMed] [Google Scholar]
- 35.Yoder JA. Investigating the morphology, function and genetics of cytotoxic cells in bony fish. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138(3):271–280. doi: 10.1016/j.cca.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Jaso-Friedmann L, Leary JH, 3rd, Evans DL. The non-specific cytotoxic cell receptor (NCCRP-1): molecular organization and signaling properties. Dev Comp Immunol. 2001;25(8-9):701–711. doi: 10.1016/s0145-305x(01)00031-3. [DOI] [PubMed] [Google Scholar]
- 37.Warr GW. The adaptive immune system of fish. Dev Biol Stand. 1997;90:15–21. [PubMed] [Google Scholar]
- 38.Kaattari S, Evans D, Klemer J. Varied redox forms of teleost IgM: an alternative to isotypic diversity? Immunol Rev. 1998;166:133–142. doi: 10.1111/j.1600-065x.1998.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Muiswinkel WB, Wiegertjes GF. Immune responses after injection vaccination of fish. Dev Biol Stand. 1997;90:55–57. [PubMed] [Google Scholar]
- 41.Arkoosh MR, Kaattari SL. Development of immunological memory in rainbow trout (Oncorhynchus mykiss). I. An immunochemical and cellular analysis of the B cell response. Dev Comp Immunol. 1991;15(4):279–293. doi: 10.1016/0145-305x(91)90021-p. [DOI] [PubMed] [Google Scholar]
- 42.Bromage ES, Kaattari IM, Zwollo P, Kaattari SL. Plasmablast and plasma cell production and distribution in trout immune tissues. J Immunol. 2004;173(12):7317–7323. doi: 10.4049/jimmunol.173.12.7317. [DOI] [PubMed] [Google Scholar]
- 43.Zwollo P, Cole S, Bromage E, Kaattari S. B cell heterogeneity in the teleost kidney: evidence for a maturation gradient from anterior to posterior kidney. J Immunol. 2005;174(11):6608–6616. doi: 10.4049/jimmunol.174.11.6608. [DOI] [PubMed] [Google Scholar]
- 44.Kaattari SL, Zhang HL, Khor IW, Kaattari IM, Shapiro DA. Affinity maturation in trout: clonal dominance of high affinity antibodies late in the immune response. Dev Comp Immunol. 2002;26(2):191–200. doi: 10.1016/s0145-305x(01)00064-7. [DOI] [PubMed] [Google Scholar]
- 45.Wilson M, Bengten E, Miller NW, Clem LW, Du Pasquier L, Warr GW. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc Natl Acad Sci USA. 1997;94(9):4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edholm ES, Bengten E, Wilson M. Insights into the function of IgD. Dev Comp Immunol. 2011;35:1309–1316. doi: 10.1016/j.dci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10(8):889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edholm ES, Bengten E, Stafford JL, Sahoo M, Taylor EB, Miller NW, et al. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J Immunol. 2010;185(7):4082–4094. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- 49.Zhang YA, Salinas I, Sunyer JO. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011;31(5):627–634. doi: 10.1016/j.fsi.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, Maischein HM, et al. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J Immunol. 2006;177(4):2463–2476. doi: 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7(10):1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 52.Jansson E, Gronvik KO, Johannisson A, Naslund K, Westergren E, Pilstrom L. Monoclonal antibodies to lymphocytes of rainbow trout (Oncorhynchus mykiss) Fish & Shellfish Immunology. 2003;14(3):239–257. doi: 10.1006/fsim.2002.0434. [DOI] [PubMed] [Google Scholar]
- 53.Miyadai T, Ootani M, Tahara D, Aoki M, Saitoh K. Monoclonal antibodies recognising serum immunoglobulins and surface immunoglobulin-positive cells of puffer fish, torafugu (Takifugu rubripes) Fish Shellfish Immunol. 2004;17(3):211–222. doi: 10.1016/j.fsi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki Y, Maita M, Okamoto N. Rainbow trout neutrophils are responsible for non-specific cytotoxicity. Fish Shellfish Immunol. 2002;12(3):243–252. doi: 10.1006/fsim.2001.0368. [DOI] [PubMed] [Google Scholar]
- 55.Kollner B, Blohm U, Kotterba G, Fischer U. A monoclonal antibody recognising a surface marker on rainbow trout (Oncorhynchus mykiss) monocytes. Fish Shellfish Immunol. 2001;11(2):127–142. doi: 10.1006/fsim.2000.0300. [DOI] [PubMed] [Google Scholar]
- 56.de Jong JL, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39:481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- 57.Berger S, Ballo H, Stutte HJ. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur J Immunol. 1996;26(6):1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 58.Bauer SR, Holmes KL, Morse HC, 3rd, Potter M. Clonal relationship of the lymphoblastic cell line P388 to the macrophage cell line P388D1 as evidenced by immunoglobulin gene rearrangements and expression of cell surface antigens. J Immunol. 1986;136(12):4695–4699. [PubMed] [Google Scholar]
- 59.Hanecak R, Zovich DC, Pattengale PK, Fan H. Differentiation in vitro of a leukemia virus-induced B-cell lymphoma into macrophages. Mol Cell Biol. 1989;9(5):2264–2268. doi: 10.1128/mcb.9.5.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka T, Wu GE, Paige CJ. Characterization of the B cellmacrophage lineage transition in 70Z/3 cells. Eur J Immunol. 1994;24(7):1544–1548. doi: 10.1002/eji.1830240713. [DOI] [PubMed] [Google Scholar]
- 61.Borrello MA, Phipps RP. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol Today. 1996;17(10):471–475. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- 62.Borrello MA, Phipps RP. Fibroblasts support outgrowth of splenocytes simultaneously expressing B lymphocyte and macrophage characteristics. J Immunol. 1995;155(9):4155–4161. [PubMed] [Google Scholar]
- 63.Borrello MA, Phipps RP. Fibroblast-secreted macrophage colony-stimulating factor is responsible for generation of biphenotypic B/macrophage cells from a subset of mouse B lymphocytes. J Immunol. 1999;163(7):3605–3611. [PubMed] [Google Scholar]
- 64.Almeida SR, Aroeira LS, Frymuller E, Dias MA, Bogsan CS, Lopes JD, et al. Mouse B-1 cell-derived mononuclear phagocyte, a novel cellular component of acute non-specific inflammatory exudate. Int Immunol. 2001;13(9):1193–1201. doi: 10.1093/intimm/13.9.1193. [DOI] [PubMed] [Google Scholar]
- 65.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117(5):663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 66.Cumano A, Paige CJ, Iscove NN, Brady G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature. 1992;356(6370):612–615. doi: 10.1038/356612a0. [DOI] [PubMed] [Google Scholar]
- 67.Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;2(1):83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- 68.Katsura Y. Redefinition of lymphoid progenitors. Nat Rev Immunol. 2002;2(2):127–132. doi: 10.1038/nri721. [DOI] [PubMed] [Google Scholar]
- 69.Kawamoto H, Katsura Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 2009;30(5):193–200. doi: 10.1016/j.it.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Desjardins M, Houde M, Gagnon E. Phagocytosis: the convoluted way from nutrition to adaptive immunity. Immunol Rev. 2005;207:158–165. doi: 10.1111/j.0105-2896.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 71.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22(5):539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284(5418):1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 73.Neumann NF, Stafford JL, Barreda D, Ainsworth AJ, Belosevic M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Developmental & Comparative Immunology. 2001;25(8-9):807–825. doi: 10.1016/s0145-305x(01)00037-4. [DOI] [PubMed] [Google Scholar]
- 74.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 75.Brode S, Macary PA. Cross-presentation: dendritic cells and macrophages bite off more than they can chew! Immunology. 2004;112(3):345–351. doi: 10.1111/j.1365-2567.2004.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5(3):85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 77.Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, et al. Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183(9):5622–5629. doi: 10.4049/jimmunol.0901772. [DOI] [PubMed] [Google Scholar]
- 78.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 79.DeLuca D, Wilson M, Warr GW. Lymphocyte heterogeneity in the trout, Salmo gairdneri, defined with monoclonal antibodies to IgM. European Journal of Immunology. 1983;13(7):546–551. doi: 10.1002/eji.1830130706. [DOI] [PubMed] [Google Scholar]
- 80.Abrahao TB, Freymuller E, Mortara RA, Lopes JD, Mariano M. Morphological characterization of mouse B-1 cells. Immunobiology. 2003;208(4):401–411. doi: 10.1078/0171-2985-00287. [DOI] [PubMed] [Google Scholar]
- 81.Leiro J, Ortega M, Estevez J, Ubeira FM, Sanmartin ML. The role of opsonization by antibody and complement in in vitro phagocytosis of microsporidian parasites by turbot spleen cells. Vet Immunol Immunopathol. 1996;51(1-2):201–210. doi: 10.1016/0165-2427(95)05509-6. [DOI] [PubMed] [Google Scholar]
- 82.Nonaka M, Iwaki M, Nakai C, Nozaki M, Kaidoh T, Natsuume-Sakai S, et al. Purification of a major serum protein of rainbow trout (Salmo gairdneri) homologous to the third component of mammalian complement. J Biol Chem. 1984;259(10):6327–6333. [PubMed] [Google Scholar]
- 83.Honda A, Kodama H, Moustafa M, Yamada F, Mikami T, Izawa H. Phagocytic activity of macrophages of rainbow trout against Vibrio anguillarum and the opsonising effect of antibody and complement. Res Vet Sci. 1986;40(3):328–332. [PubMed] [Google Scholar]
- 84.Jenkins JA, Ourth DD. Opsonic effect of the alternative complement pathway on channel catfish peripheral blood phagocytes. Vet Immunol Immunopathol. 1993;39(4):447–459. doi: 10.1016/0165-2427(93)90074-e. [DOI] [PubMed] [Google Scholar]
- 85.Overland HS, Pettersen EF, Ronneseth A, Wergeland HI. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2009 doi: 10.1016/j.fsi.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 86.Zimmerman LM, Vogel LA, Edwards KA, Bowden RM. Phagocytic B cells in a reptile. Biol Lett. 6(2):270–273. doi: 10.1098/rsbl.2009.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Souwer Y, Griekspoor A, Jorritsma T, de Wit J, Janssen H, Neefjes J, et al. B cell receptor-mediated internalization of salmonella: a novel pathway for autonomous B cell activation and antibody production. J Immunol. 2009;182(12):7473–7481. doi: 10.4049/jimmunol.0802831. [DOI] [PubMed] [Google Scholar]
- 88.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, et al. Pivotal Advance: Peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2011 Nov 4; doi: 10.1189/jlb.0711372. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watts C, Amigorena S. Phagocytosis and antigen presentation. Semin Immunol. 2001;13(6):373–379. doi: 10.1006/smim.2001.0334. [DOI] [PubMed] [Google Scholar]
- 90.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 91.Nakashima M, Kinoshita M, Nakashima H, Habu Y, Miyazaki H, Shono S, et al. Pivotal Advance: Characterization of mouse liver phagocytic B cells in innate immunity. J Leukoc Biol. 2011 Nov 4; doi: 10.1189/jlb.0411214. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 92.Schatz DG. Antigen receptor genes and the evolution of a recombinase. Semin Immunol. 2004;16(4):245–256. doi: 10.1016/j.smim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Pancer Z, Mayer WE, Klein J, Cooper MD. Prototypic T cell receptor and CD4-like coreceptor are expressed by lymphocytes in the agnathan sea lamprey. Proc Natl Acad Sci USA. 2004;101(36):13273–13278. doi: 10.1073/pnas.0405529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann NY Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 95.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 96.Ramachandra L, Noss E, Boom WH, Harding CV. Phagocytic processing of antigens for presentation by class II major histocompatibility complex molecules. Cell Microbiol. 1999;1(3):205–214. doi: 10.1046/j.1462-5822.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 97.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126(1):205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 98.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 99.Ramachandra L, Simmons D, Harding CV. MHC molecules and microbial antigen processing in phagosomes. Curr Opin Immunol. 2009;21(1):98–104. doi: 10.1016/j.coi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batista FD, Neuberger MS. B cells extract and present immobilized antigen: implications for affinity discrimination. Embo J. 2000;19(4):513–520. doi: 10.1093/emboj/19.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312(5774):738–741. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 102.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10(4):236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu G, Wu C, Wu Y, Zhao Y. Phagocytosis of apoptotic cells and immune regulation. Scand J Immunol. 2006;64(1):1–9. doi: 10.1111/j.1365-3083.2006.01771.x. [DOI] [PubMed] [Google Scholar]
- 104.de Almeida CJ, Linden R. Phagocytosis of apoptotic cells: a matter of balance. Cell Mol Life Sci. 2005;62(14):1532–1546. doi: 10.1007/s00018-005-4511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176(2):705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 106.Hoehlig K, Lampropoulou V, Roch T, Neves P, Calderon-Gomez E, Anderton SM, et al. Immune regulation by B cells and antibodies a view towards the clinic. Adv Immunol. 2008;98:1–38. doi: 10.1016/S0065-2776(08)00401-X. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA. 2010;107(19):8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26(4):347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 109.Viau M, Zouali M. B-lymphocytes, innate immunity, and autoimmunity. Clin Immunol. 2005;114(1):17–26. doi: 10.1016/j.clim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 110.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsuji M, Suzuki K, Kinoshita K, Fagarasan S. Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin Immunol. 2008;20(1):59–66. doi: 10.1016/j.smim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Coope MD, Raymond DA, Peterson RD, South MA, Good RA. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966;123(1):75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glick B, Chang TS, Jaap RG. The bursa of Fabricius and antibody production. Poult Sci. 1956;35:224. [Google Scholar]
- 114.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010;237(1):160–179. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


