Abstract
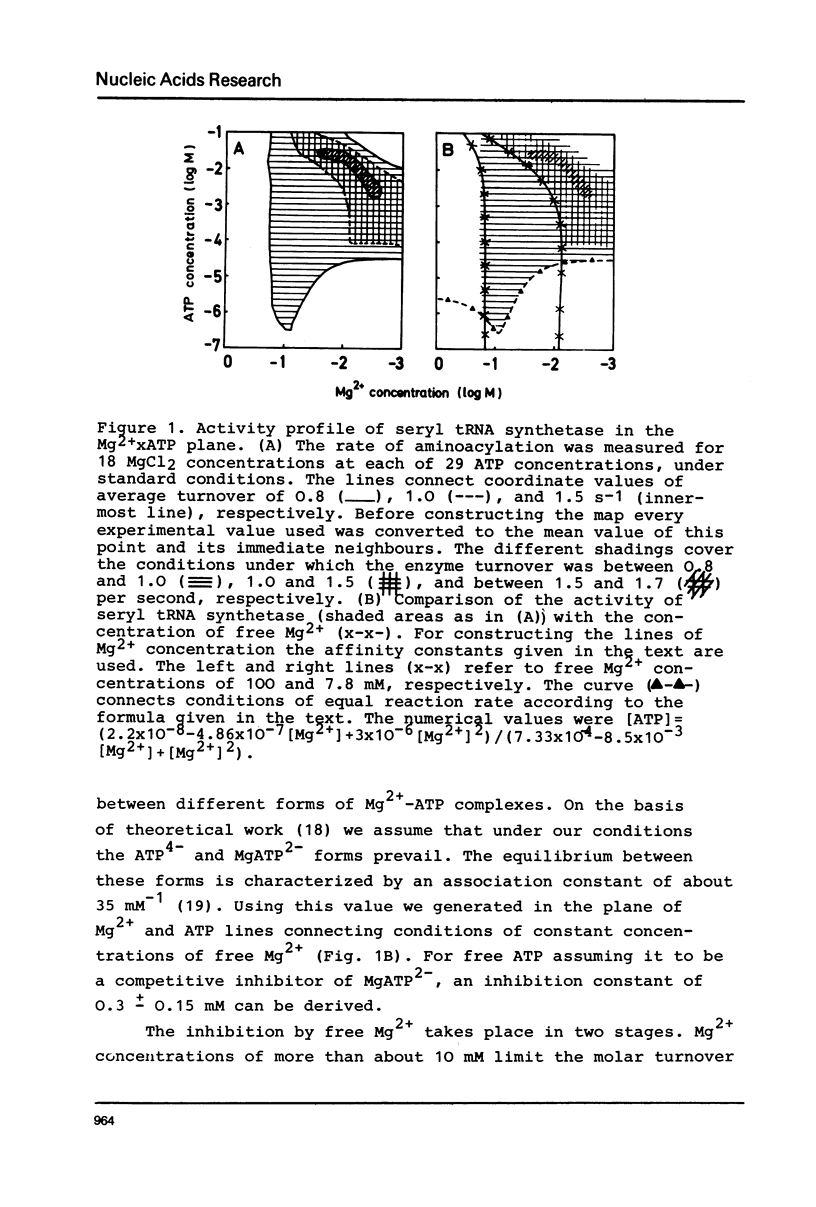
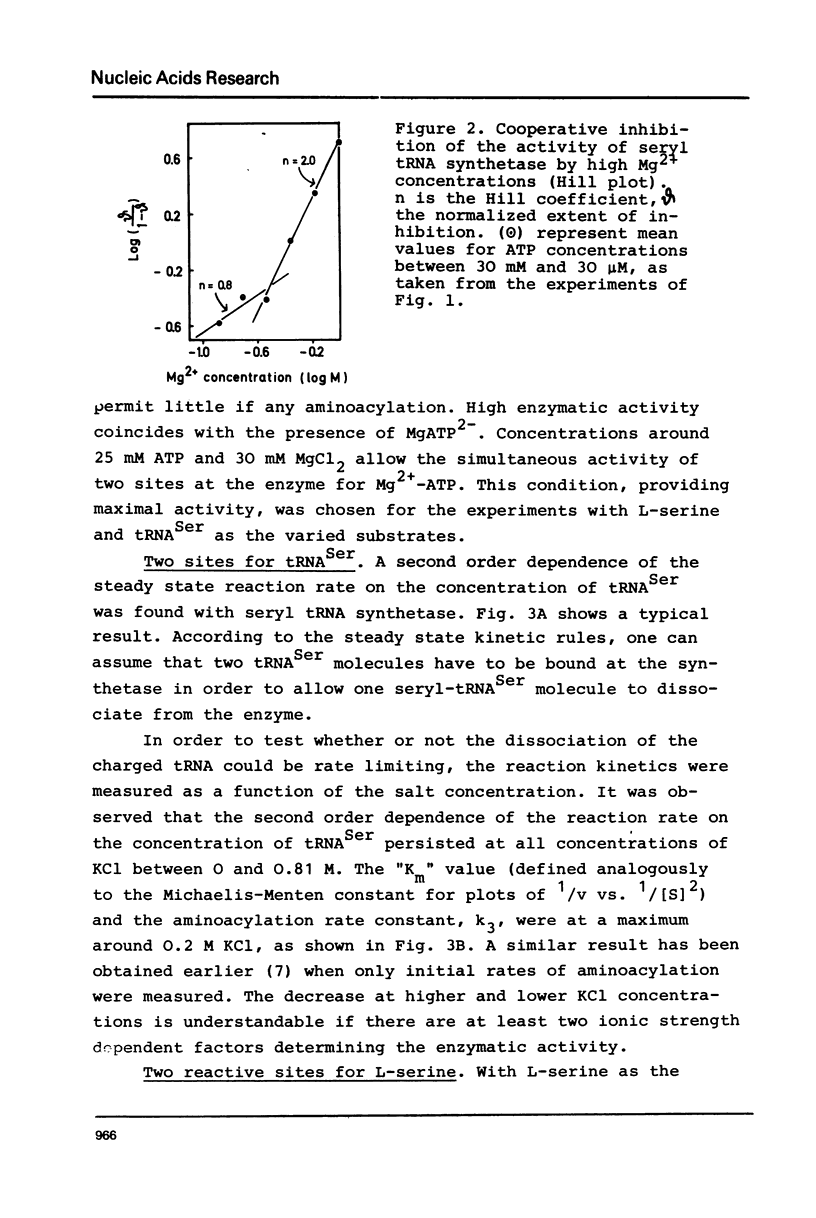
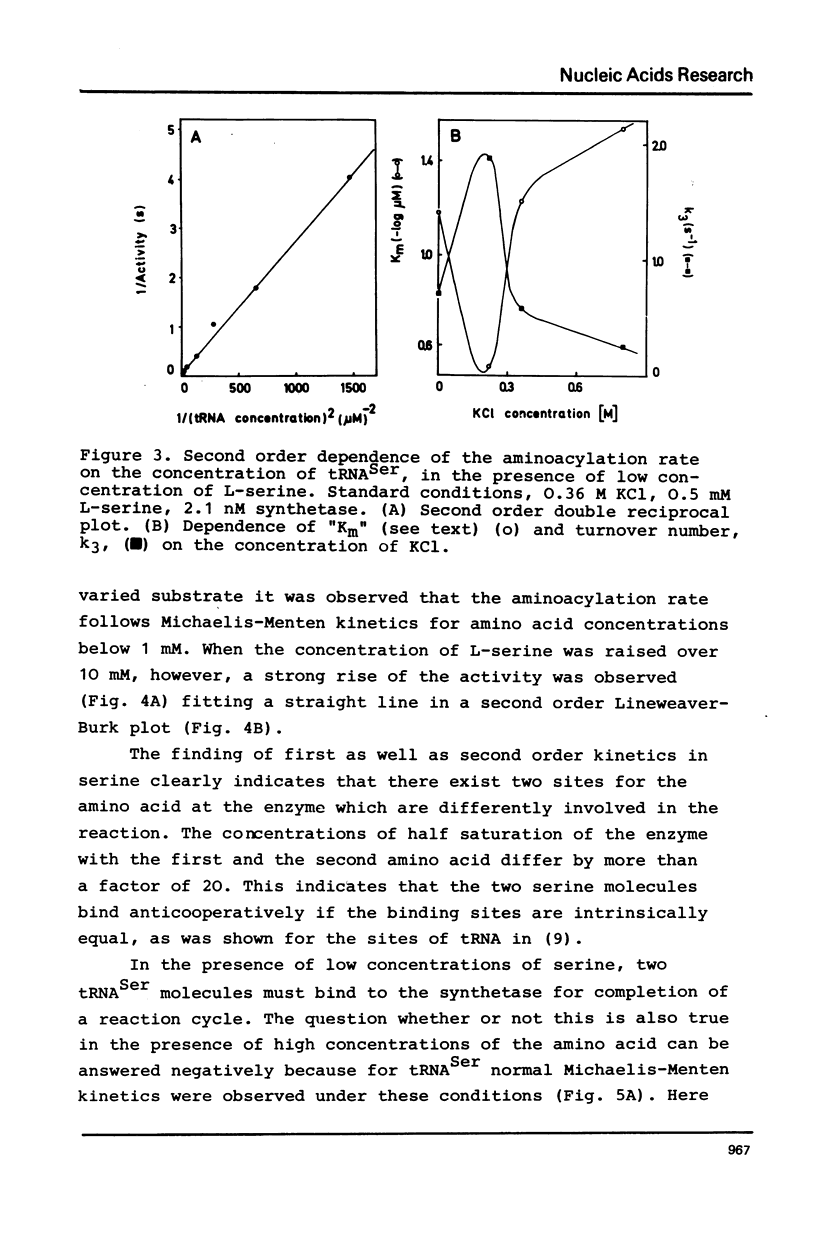
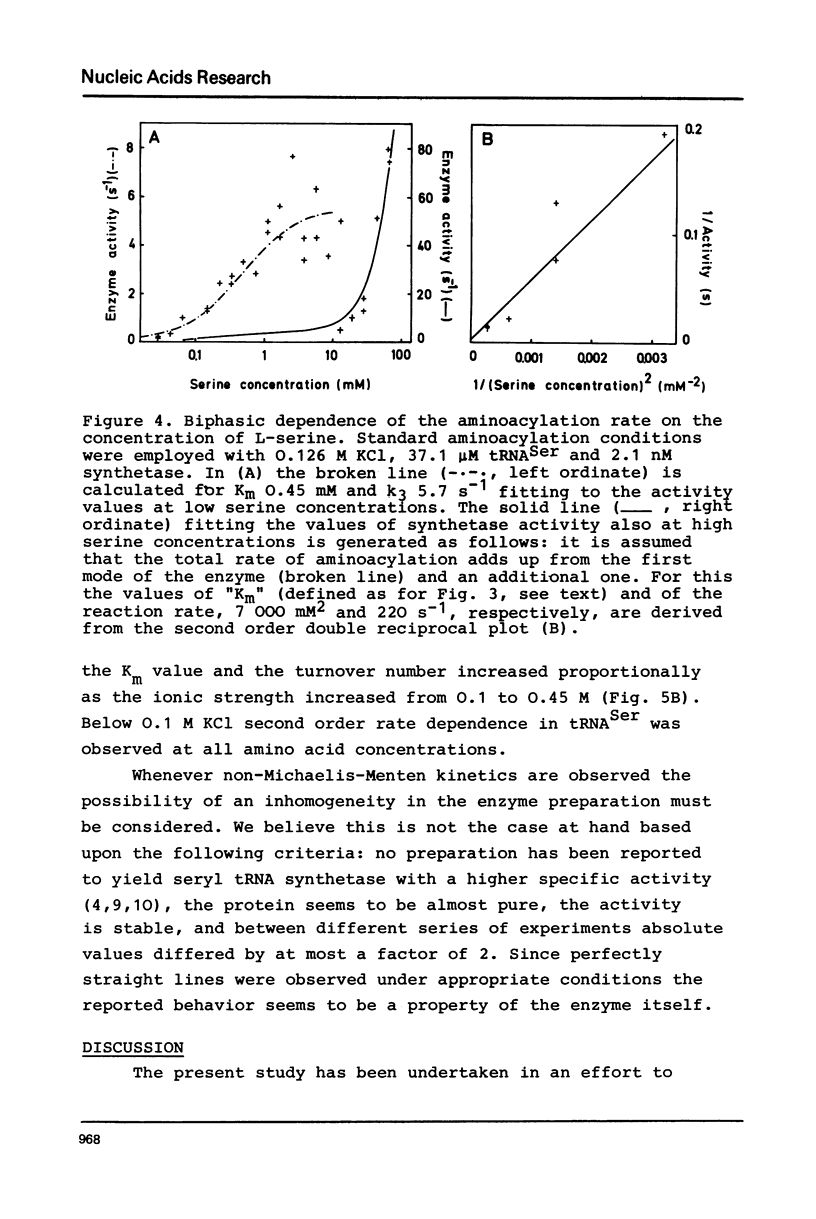
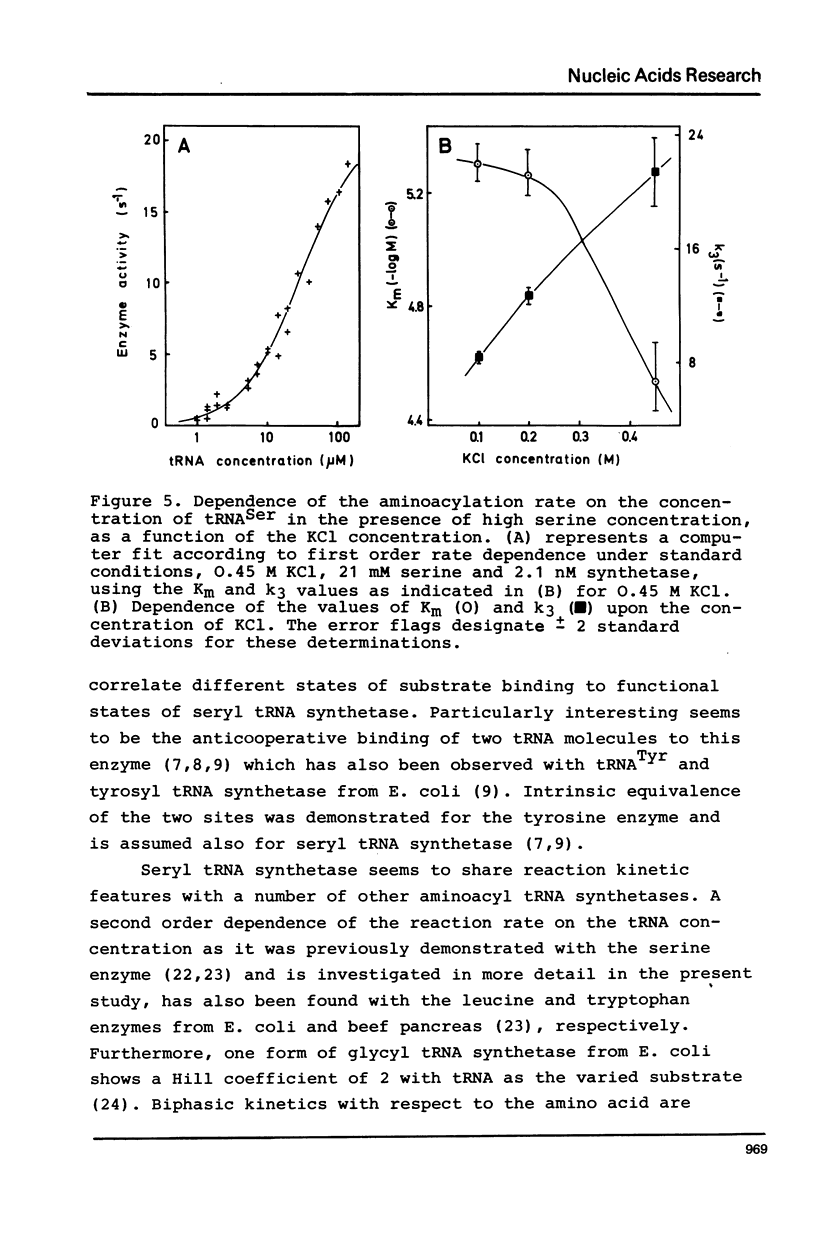
Seryl tRNA synthetase from Saccharomyces Carlsbergensis C836 contains two sets of sites for tRNASer, L-serine, and Mg2+-ATP, both of which are involved in aminoacylation. This is based on the following experimental results: (a) at low serine concentrations, second order kinetics in tRNASer are observed; (b) biphasic kinetics result when the amino acid is the varied substrate indicating anticooperative binding of two serine molecules to the synthetase; (c) when two molecules of serine are bound the rate of aminoacylation increases strongly and becomes first order in tRNASer; (d) the involvement of more than one site for Mg2+ and ATP is deduced from systematic variations of the concentrations of Mg2+ and ATP. Implications of the anticooperative binding of the substrates for possible reaction mechanisms are discussed. The results indicate that under normal conditions, the activity of seryl tRNA synthetase is regulated mainly by tRNASer while at high serine concentrations regulation by the amino acid itself prevails.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craine J. E., Peterkofsky A. Studies on arginyl-tRNA synthetase from Escherichia coli B. Dual role of metals in enzyme catalysis. J Biol Chem. 1976 Jan 10;251(1):241–246. [PubMed] [Google Scholar]
- Engel G., Heider H., Maelicke A., von der Haar F., Cramer F. Fluorescence studies on substrate binding to seryl-tRNA synthetase from yeast. Eur J Biochem. 1972 Sep 18;29(2):257–262. doi: 10.1111/j.1432-1033.1972.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Fittler F., Kruppa J., Zachau H. G. Two fractionation methods for transfer RNAs. Biochim Biophys Acta. 1972 Sep 14;277(3):513–522. doi: 10.1016/0005-2787(72)90094-9. [DOI] [PubMed] [Google Scholar]
- Francis T. A., Nagel G. M. Glycyl-tRNA synthetase: evidence for two enzyme forms and sigmoidal saturation kinetics. Biochem Biophys Res Commun. 1976 Jun 7;70(3):862–868. doi: 10.1016/0006-291x(76)90671-9. [DOI] [PubMed] [Google Scholar]
- Freist W., von der Haar F., Sprinzl M., Cramer F. Phenylalanyl-tRNA and seryl-tRNA synthetases from baker's yeast. Substrate specificity with regard to ATP analogs and mechanism of the aminoacylation reaction. Eur J Biochem. 1976 May 1;64(2):389–393. doi: 10.1111/j.1432-1033.1976.tb10313.x. [DOI] [PubMed] [Google Scholar]
- Heider H., Gottschalk E., Cramer F. Isolation and characterization of seryl-tRNA synthetase from yeast. Eur J Biochem. 1971 May 11;20(1):144–152. doi: 10.1111/j.1432-1033.1971.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Hertz H. S., Zachau H. G. Kinetic properties of phenylalanyl-tRNA and seryl-tRNA synthetases for normal substrates and fluorescent analogs. Eur J Biochem. 1973 Aug 17;37(2):203–213. doi: 10.1111/j.1432-1033.1973.tb02977.x. [DOI] [PubMed] [Google Scholar]
- Hirsch R., Zachau H. G. Isolierung und Charakterisierung der Seryl- und Phenylalanyl-tRNA-Synthetase aus Hefe. Hoppe Seylers Z Physiol Chem. 1976 Apr;357(4):509–526. [PubMed] [Google Scholar]
- Holler E. Rearrangement of an abortive aminoacyl-tRNA synthetase complex with aminoacyl-tRNA could be rate-determining for catalytic charging. J Biol Chem. 1976 Dec 10;251(23):7717–7719. [PubMed] [Google Scholar]
- Jacques Y., Blanquet S. Interrelation between transfer RNA and amino-acid-activating sites of methionyl transfer RNA synthetase from Escherichia coli. Eur J Biochem. 1977 Oct 3;79(2):433–441. doi: 10.1111/j.1432-1033.1977.tb11825.x. [DOI] [PubMed] [Google Scholar]
- Jakes R., Fersht A. R. Tyrosyl-tRNA synthetase from Escherichia coli. Stoichiometry of ligand binding and half-of-the-sites reactivity in aminoacylation. Biochemistry. 1975 Jul 29;14(15):3344–3350. doi: 10.1021/bi00686a009. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O. Aminoacyl-tRNA synthetases: sone recent results and achievements. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):141–238. doi: 10.1002/9780470122853.ch5. [DOI] [PubMed] [Google Scholar]
- Krauss G., Pingoud A., Boehme D., Riesner D., Peters F., Maas G. Equivalent and non-equivalent binding sites for tRNA on aminoacyl-tRNA synthetases. Eur J Biochem. 1975 Jul 15;55(3):517–529. doi: 10.1111/j.1432-1033.1975.tb02189.x. [DOI] [PubMed] [Google Scholar]
- Malygin E. G., Zinoviev V. V., Fasiolo F., Kisselev L. L., Kochkina L. L., Achverdyan V. Z. Interaction of aminoacyl-tRNA synthetases and tRNA: positive and negative cooperativity of their active centres. Mol Biol Rep. 1976 Jul;2(6):445–454. doi: 10.1007/BF00356933. [DOI] [PubMed] [Google Scholar]
- Noat G., Ricard J., Borel M., Got C. Kinetic study of yeast hexokinase. Inhibition of the reaction by magnesium and ATP. Eur J Biochem. 1970 Apr;13(2):347–363. doi: 10.1111/j.1432-1033.1970.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Pachmann U., Cronvall E., Rigler R., Hirsch R., Wintermeyer W., Zachau H. G. On the specificity of interactions between transfer ribonucleic acids and aminoacyl-tRNA synthetases. Eur J Biochem. 1973 Nov 1;39(1):265–273. doi: 10.1111/j.1432-1033.1973.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Pachmann U., Zachau H. G. Yeast seryl tRNA synthetase: interactions between the ATP binding site and the sites for tRNASer and L-serine. Nucleic Acids Res. 1978 Mar;5(3):975–985. doi: 10.1093/nar/5.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- Riesner D., Pingoud A., Boehme D., Peters F., Maass G. Distinct steps in the specific binding of tRNA to aminoacyl-tRNA synthetase. Temperature-jump studies on the serine-specific system from yeast and the tyrosine-specific system from Escherichia coli. Eur J Biochem. 1976 Sep;68(1):71–80. doi: 10.1111/j.1432-1033.1976.tb10765.x. [DOI] [PubMed] [Google Scholar]
- Rigler R., Cronvall E., Ehrenberg M., Pachmann U., Hirsch R., Zachau H. G. Interactions between aminoacyl tRNA synthetases, tRNAs, and fluorescent dyes. FEBS Lett. 1971 Nov 1;18(2):193–198. doi: 10.1016/0014-5793(71)80443-x. [DOI] [PubMed] [Google Scholar]
- Rigler R., Cronvall E., Hirsch R., Pachmann U., Zachau H. G. Interactions of seryl-tRNA synthetase with serine and phenylalanine specific tRNA. FEBS Lett. 1970 Dec 18;11(5):320–323. doi: 10.1016/0014-5793(70)80558-0. [DOI] [PubMed] [Google Scholar]
- Rigler R., Pachmann U., Hirsch R., Zachau H. G. On the interaction of seryl-tRNA synthetase with tRNA Ser. A contribution to the problem of synthetase-tRNA recognition. Eur J Biochem. 1976 May 17;65(1):307–315. doi: 10.1111/j.1432-1033.1976.tb10418.x. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebe R. Magnesium ions still necessary in isoleucyl-tRNA formation. FEBS Lett. 1977 Jul 1;79(1):212–214. doi: 10.1016/0014-5793(77)80386-4. [DOI] [PubMed] [Google Scholar]
- Zachau H. G., Hertz H. S. Lack of incorporation of 1:N6-ethenoadenosine triphosphate into yeast tRNAPhe and tRNASer under standard conditions. Eur J Biochem. 1974 May 2;44(1):289–291. doi: 10.1111/j.1432-1033.1974.tb03484.x. [DOI] [PubMed] [Google Scholar]


