Abstract
Recent rapid advances in “-omics” technologies have yielded new insights into the interaction of the oral microbiome with its host. Associations of species that are usually considered to be acid-tolerant with caries have been confirmed, while some recognized as health-associated are often present in greater proportions in the absence of caries. In addition, some newly identified bacteria have been suggested as potential contributors to the caries process. In spite of this progress, two major challenges remain. The first is that there is a great deal of heterogeneity in the phenotypic capabilities of individual species of oral bacteria. The second is that the most abundant taxa in oral biofilms display remarkable phenotypic plasticity, i.e., the bacteria associated most strongly with health or with caries can morph rapidly in response to alterations in environmental pH, carbohydrate availability and source, and oxygen tension and redox environment. However, new technologic advances coupled with “old-fashioned microbiology” are starting to erode the barriers to a more complete understanding of oral biofilm physiology and ecology, and in doing so are beginning to provide insights for the creation of novel cost-effective caries control therapies.
Keywords: biofilm, 16S rRNA, acid tolerance, arginine metabolism, ecology, pH homeostasis
The understanding of the etiology of dental caries in humans has evolved and changed considerably over the last two decades. It is now widely accepted that dental caries occurs when environmental pressures, primarily in the form of frequent intake of dietary carbohydrate and repeated acidification of oral biofilms, lead to changes in the proportions of particular bacterial species as a caries lesion is initiated and progresses. Arguably, a key conceptual advance that led to the current ecological view of dental caries development was the recognition of the importance of the property of aciduricity (acid tolerance) in S. mutans and its relationship to caries. Specifically, work by Marquis and others focused on the idea that S. mutans thrives in cariogenic plaque because it is better able to tolerate, to grow, and to metabolize carbohydrate in a low pH environment (Bender et al., 1986; Bender and Marquis, 1987; Marquis, 1990; Belli and Marquis, 1991).
Over the past decade or so, the application of DNA-based technologies to the analysis of the composition of the oral microbiome in healthy individuals and in those with caries has allowed for a more comprehensive cataloging of the species that appear to be beneficial to dental health or to contribute to the caries process, respectively.
Oral health researchers have been world leaders in the application of these types of technologies to the illumination of the composition of the oral microbiome in health and disease—in some cases, as a function of the hosts’ overall health. For a comprehensive treatise on progress in the understanding of the oral microbiome, readers are referred to a recent paper from the group at The Forsyth Institute (Dewhirst et al., 2010). In terms of dental caries specifically, hybridization-based and sequence-based technologies have proven effective in developing a better picture of caries etiology, both from the standpoint of providing a more comprehensive understanding of the composition of the microbiome in caries and health, and by identifying species, some of them previously uncharacterized or uncultivated, as possible contributors to the caries process. Some of these data and more detail on the current view of caries and the oral microbiome have been well-summarized in a recent review by Howard Jenkinson (Jenkinson, 2011). In particular, the findings that S. mutans is not always isolable from active lesions and that the proportions of certain species tend to be enhanced in health (e.g., S. sanguinis, S. gordonii), whereas caries lesions contain higher proportions of aciduric S. mutans, non-mutans streptococci, aciduric lactobacilli, bifidobacteria, Actinomyces spp., and Scardovia spp., led Jenkinson to propose that “pathogenic communities” rather than “traditional microbial etiologies” are the drivers of dental caries. A general consensus seems to be developing that this is a reasonable way to view the microbiology of dental caries.
The Known Unknowns
The application of 16S rRNA gene sequencing to analysis of the oral microbiome will continue to contribute to our knowledge of the oral microbiome, and with new technologies, the depth and breadth of the information collected should be remarkable. But this technology is not a panacea, and there is a very good chance that such studies will prove confirmatory at best. In fact, it may be that such studies serve to further complicate the interpretation of the relationships of different bacteria with caries and health. For example, in a study by Griffen and co-workers, species that were previously thought to be associated primarily with health were also elevated in caries-active individuals, leading the authors to conclude that “The relationship of acid-base metabolism to 16S rRNA gene-based species assignments appears to be ‘complex’…” (Gross et al., 2010). This conclusion confirms what many microbiologists who study oral bacteria have known for a long time: Individual species of oral commensals and pathogens display a remarkable degree of genetic heterogeneity. The ramifications of this genetic heterogeneity in the context of caries etiology are that one cannot rely solely on 16S sequence information to understand the cariogenic potential of the oral microbiome. This is because individual isolates of S. mutans, oral commensal streptococci, and other common dental plaque bacteria differ widely in those characteristics—adherence, biofilm formation, acidogenesis, and acid tolerance—that have a direct impact on their ability to contribute to the caries process.
Another reason why the 16S sequence alone appears inadequate to predict associations of particular bacteria with oral diseases is related to the concept of phenotypic plasticity. That is, depending on the environment in which the organisms are growing, the phenotypes of the bacteria can be quite different (Lemos and Burne, 2008). Environmental factors, including pH, oxygen and redox, carbohydrate source and availability, population density, and the growth phase of the bacteria, have been shown to have a profound impact on the gene expression profiles of many oral streptococci and to influence phenotypes that directly influence cariogenicity (Burne et al., 2009). Therefore, the cariogenic potential of particular strains of commensals or pathogens could be greatly enhanced in vivo given the right host environment, making it impossible to predict solely from 16S sequence or using simple in vitro assays which bacteria have the greatest potential to damage dental tissue. In light of these findings, one can posit that the most crucial goal for caries microbiology today is to correlate genotypic data effectively with the phenotypic capacities of organisms and clinical status of the individuals. Said another way, microbiologists need to develop the knowledge base to make reliable correlations of the gene content of individual species with the ability of oral commensals to enhance the stability and beneficial nature of oral biofilms or the capacity of cariogenic organisms to damage host tissues.
Exploring the Relationship of Genotype and Cariogenic Potential in Streptococcus Mutans
Numerous studies have demonstrated substantial genetic heterogeneity in clinical isolates of S. mutans. [For recent examples, see Zhang et al. (2009), Arthur et al. (2011), Cheon et al. (2011), and Phattarataratip et al. (2011).] Although these studies have enhanced our basic understanding of the biology and epidemiology of S. mutans, our present knowledge of gene content across the species and its relationship to phenotypes is minimal. As part of a collaborative project between the University of Florida and Cornell University, we have completed a high-coverage DNA sequence of 57 genomes of S. mutans, as well as genomes of the closely related mutans streptococci S. ratti and S. cricetus. A manuscript detailing the gene content and evolutionary biology has been submitted, and all sequences will be posted to a convenient Web browser. One of the goals of this undertaking was to begin to correlate gene content with phenotype, which we have undertaken by initiating phenotypic characterization of 16 genetically diverse isolates of S. mutans for which we have the high-coverage sequence. The Table shows the results of analysis of a selected group of phenotypic properties that highlights the diversity of this species. Collectively, the genetic diversity observed from analysis of a whole-genome sequence of these strains of S. mutans is mirrored by a high degree of phenotypic diversity. Importantly, there is diversity in traits that directly affect the establishment, persistence, and cariogenic potential of S. mutans in humans. Studies are ongoing to explore correlations of gene content with critical phenotypic traits.
Table.
Overview of Phenotypic Properties of 16 S. mutans Isolates for Which a High-coverage DNA Sequence Is Now Available
| Condition | Doubling Time (Range in min) |
|---|---|
| Normal1 | 65-125 |
| pH 5.52 | 164-328 |
| Aerated3 | 87-218 |
| Paraquat4 | 105-387 (1 NG) 5 |
Growth in static culture in Brain Heart Infusion broth in a 5% CO2 atmosphere.
Growth in static culture in a 5% CO2 atmosphere in Brain Heart Infusion broth that was acidified to pH 5.5.
Growth in Brain Heart Infusion broth with shaking at 150 RPM in an aerobic environment.
Growth in Brain Heart Infusion broth in static culture with 25 mM paraquat.
NG = No growth. One isolate was unable to initiate any growth in the presence of paraquat.
Understanding pH Homeostasis in Health-associated Dental Biofilms
Embodied in the ecological plaque hypothesis (Marsh, 2009) for dental caries is the concept that certain functions are well-represented in health-associated biofilms, whereas certain other functions, such as acidogenesis and acid tolerance, are better represented in biofilms on or near active caries lesions. Since caries is a disease that does not occur without the production of a low pH environment by oral biofilms, one might predict that activities that help to promote a more neutral pH could have a strong inhibitory effect on caries development. It is known that there are two substrates provided in saliva and the diet that are particularly effective at eliciting an increase in the pH when metabolized by populations of oral bacteria: urea and arginine (Fig.). A more comprehensive review of oral arginine and urea metabolism is provided elsewhere (Burne and Marquis, 2000). Here we focus on new information related to arginine metabolism and caries resistance in humans.
Figure.
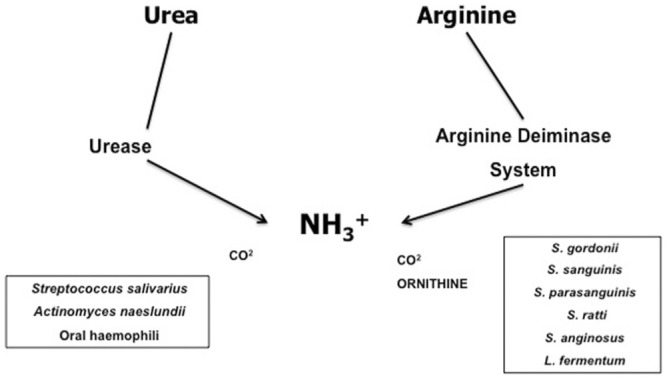
The principle sources of, and pathways for, pH homeostasis in oral biofilms. Urea is present in all salivary gland secretions and in gingival crevicular fluid at concentrations approximating those in serum (1-10 mM). Arginine is present in low µM quantities in salivary secretions, but is abundant in peptide form. Urease enzymes are encoded by a relatively small number of oral bacteria, mainly Streptococcus salivarius, some Actinomyces spp., and oral Haemophilus spp., and cleave urea into 2 molecules of ammonia and 1 of CO2. The arginine deiminase pathway (ADS) is a 3-enzyme system that cleaves arginine to ornithine, 2 molecules of ammonia, and 1 of CO2. The most abundant ADS-positive bacteria are oral Streptococcus spp. and some lactobacilli, including Lactobacillus fermentum. The oral streptococci tend to express the ADS at much higher levels than other oral bacteria.
Arginine is metabolized by oral biofilms primarily by a 3-enzyme pathway called the arginine deiminase system (ADS). The net reaction is that arginine is converted to ornithine and 2 molecules of ammonia, which causes the pH to rise. The bacteria also gain an ATP in the process (Burne and Marquis, 2000). Thus, metabolism of arginine via the ADS is beneficial to the host because it raises biofilm pH, which discourages the outgrowth of cariogenic species and reduces damage to enamel. The ADS is also beneficial to the bacteria that have it because the ammonia it generates neutralizes the cytoplasm of the bacteria, which protects them from acid damage, and the ATP produced can be used for growth or homeostasis.
One major impetus for generating a better understanding of the ADS in oral bacteria was the idea that enhancing ADS activity in dental plaque could have a beneficial effect on biofilm ecology and provide protection against caries. It is only recently, though, that the arginolytic capacity of the bacteria in dental plaque and saliva has been compared in caries-active and caries-free individuals. Consistent with its predicted role, individuals who had no clinical evidence of past or present caries activity had significantly higher levels of the arginine deiminase enzyme measurable in their plaque and salivary bacterial populations compared with those who had active caries lesions (Nascimento et al., 2009). This seminal study was conducted with plaque that had been pooled from different sites in adult humans, but more recently, Nascimento and co-workers have shown that ADS activity in plaques isolated from carious surfaces in children is markedly lower than that from sound surfaces. Notably, the range of ADS activity among these children was greater than 10,000-fold, so there is huge variation in the arginolytic potential of plaque in children. Given that we have shown in vitro that the ability of ammonia production by bacteria to temper acidification during a glucose challenge is directly correlated with the quantity of ADS enzymes present in the bacteria, these findings have tremendous implications for caries control. However, more information on the organisms responsible for arginolysis in dental plaque is needed.
It is here that the heterogeneity of human oral bacteria becomes particularly germane and interesting. Specifically, Nascimento and co-workers, using PCR-based methods, explored whether dental plaque that had low arginolytic capacity also had decreased proportions of the most common ADS-positive bacteria (Nascimento et al., 2009). Surprisingly, high ADS activity could not be correlated with high numbers of the more prominent known ADS-positive bacteria. After additional experimentation, it was determined that there is remarkable heterogeneity within the species of bacteria that possess the ADS, vis-à-vis their ability to produce high levels of the enzymes. Interestingly, within a given species, a wide range of abilities to break down arginine was noted (manuscript in preparation). For example, individual isolates of S. sanguinis differed by as much as 100-fold in the levels of AD enzyme activity. Therefore, intraspecies variability in the capacity to break down arginine may account, in large part, for the variability in ADS activity in caries-active and -free individuals, reinforcing the idea that species- or taxa-level identification of organisms in the microbiome is not adequate to come to terms with the pathogenic potential of oral biofilms.
Recently, we have begun to investigate the molecular basis for variability in the expression of the ADS in these isolates. What is emerging is that the differences are, in part, associated with the absolute capacity of the bacteria to produce the enzymes, but are also strongly related to the way the ADS genes are regulated in response to environmental factors. Extrapolating these findings to the in vivo setting, one may conclude that there are certain isolates of common commensal strains of oral streptococci that have a high constitutional capacity to provide protection against caries. Rapid methods to discriminate highly arginolytic isolates from weakly ADS-positive strains could be a very powerful addition to caries risk assessment protocols.
Summary
Technological advances in the use of high-throughput sequencing platforms for analysis of the composition of the oral microbiome, coupled with traditional microbiological studies of the organisms isolated from human dental plaque, have provided a new framework for interpreting caries etiology. However, genetic diversity and complexities in the adaptive strategies of oral bacteria to fluctuating biofilm conditions diminish the utility of taxa-level identification of the microbiome for developing correlations of populations of specific organisms with health, especially, but also with caries. The marriage of functional studies of the microbiome with whole-genome and phenotypic analyses of multiple species and multiple isolates from individuals of known caries status is needed to yield the level of knowledge that will facilitate the rational design of preventive and treatment strategies to eradicate dental caries on a global scale.
Footnotes
This work was supported by the NIH-NIDCR (DE10362), the NIH-NIAID (AI073368), and the Colgate-Palmolive Co.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arthur RA, Cury AA, Graner RO, Rosalen PL, Vale GC, Paes Leme AF, et al. (2011). Genotypic and phenotypic analysis of S. mutans isolated from dental biofilms formed in vivo under high cariogenic conditions. Braz Dent J 22:267-274 [DOI] [PubMed] [Google Scholar]
- Belli WA, Marquis RE. (1991). Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol 57:1134-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender GR, Marquis RE. (1987). Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl Environ Microbiol 53:2124-2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender GR, Sutton SV, Marquis RE. (1986). Acid tolerance, proton permeabilities, membrane ATPases of oral streptococci. Infect Immun 53:331-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Marquis RE. (2000). Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193:1-6 [DOI] [PubMed] [Google Scholar]
- Burne RA, Ahn SJ, Wen ZT, Zeng L, Lemos JA, Abranches J, et al. (2009). Opportunities for disrupting cariogenic biofilms. Adv Dent Res 21:17-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon K, Moser SA, Whiddon J, Osgood RC, Momeni S, Ruby JD, et al. (2011). Genetic diversity of plaque mutans streptococci with rep-PCR. J Dent Res 90:331-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. (2010). The human oral microbiome. J Bacteriol 192:5002-5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, et al. (2010). Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol 48:4121-4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson HF. (2011). Beyond the oral microbiome. Environ Microbiol 13:3077-3087 [DOI] [PubMed] [Google Scholar]
- Lemos JA, Burne RA. (2008). A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154(Pt 11):3247-3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis RE. (1990). Diminished acid tolerance of plaque bacteria caused by fluoride. J Dent Res 69(Spec Iss):672-675 [DOI] [PubMed] [Google Scholar]
- Marsh PD. (2009). Dental plaque as a biofilm: the significance of pH in health and caries. Compend Contin Educ Dent 30:76-87 [PubMed] [Google Scholar]
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. (2009). Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol 24:89-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phattarataratip E, Olson B, Broffitt B, Qian F, Brogden KA, Drake DR, et al. (2011). Streptococcus mutans strains recovered from caries-active or caries-free individuals differ in sensitivity to host antimicrobial peptides. Mol Oral Microbiol 26:187-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Foxman B, Drake DR, Srinivasan U, Henderson J, Olson B, et al. (2009). Comparative whole-genome analysis of Streptococcus mutans isolates within and among individuals of different caries status. Oral Microbiol Immunol 24:197-203 [DOI] [PMC free article] [PubMed] [Google Scholar]


