Abstract
Xylitol is a safe dental caries preventive when incorporated into chewing gum or confections used habitually. The goal of this paper is to identify and assess the work on xylitol and other polyols and dental caries since 2008. Xylitol is effective when used by the mother prenatally or after delivery to prevent mutans transmission and subsequent dental caries in the offspring. One new completed trial confirmed that children of mothers who used xylitol lozenges after delivery had less dental caries than a comparison group. A similar study confirmed that the use of xylitol gum by the mother either prevented or postponed MS transmission to the offspring. Xylitol use among schoolchildren delivered via a gummy bear confection reduced S. mutans levels, but a once per day use of xylitol-containing toothpaste did not. Randomized trials, with caries outcomes, assessing xylitol-containing lozenges in adults and xylitol-containing gummy bears in children will release results in the coming year. Other studies are ongoing but are not systematic and will fail to answer important questions about how xylitol, or other polyols, can address the global dental caries problem.
Keywords: xylitol, maltitol, erythritol, dental caries, prevention, chewing gum
Xylitol is a sugar substitute “generally regarded as safe” by the U.S. FDA and has been approved by regulators in Europe and elsewhere. In teens and young adults, it is non-cariogenic and anti-cariogenic (Mäkinen et al., 1995). Xylitol is effective in chewing gum or confections used habitually. It inhibits metabolism of S. mutans (Trahan, 1995). Chewing alone does not cause the effect (Hildebrandt and Sparks, 2000; Ly et al., 2006; Milgrom et al., 2006, 2009). It has also been shown to prevent caries in offspring of mothers who use xylitol gum by reducing maternal transmission of S. mutans (Isokangas et al., 2000). Limited evidence is available on the efficacy of maltitol and erythritol.
The goal of this paper is to assess work on polyols and dental caries since 2008. The aims are to: (1) address results of evidence-based reviews or policy statements, (2) describe findings on the caries-protective mechanisms, (3) summarize clinical trials, and (4) describe ongoing research that may contribute further to an understanding of the usefulness of polyols.
Methods
Although this is not a formal systematic review, multiple broad-based searches were conducted to assemble information: (1) Papers published in 2008 or later were identified from Medline using the search terms “xylitol and dental caries”. Including the terms “polyol” or “maltitol” or “erythritol” did not identify additional papers of clinical relevance. (2) The Web sites clinicaltrials.gov and controlled-trials.com were searched using the same terms. (3) The iadr.org Web site was used to identify abstracts. Research reports were followed up with authors to identify additional research.
Results
Evidence-based Reviews
The American Dental Association published results of an exhaustive review and meta-analysis of non-fluoride caries-preventive agents (Rethman et al., 2011). None reviewed for effectiveness—including polyols—was seen as being supported by unequivocal evidence. Nevertheless, among remedies assessed, only sucrose-free gum and xylitol-containing lozenges used after meals were recommended as effective. Newer studies, such as the xylitol syrup trial in toddlers (Milgrom et al., 2009), were included in the review, but were not recommended because a confirmatory study had not been published.
A meta-analytic study evaluated the pooled effects of 14 polyol chewing gum randomized trials or observational studies (Deshpande and Jadad, 2008). The authors found a large preventive fraction for xylitol-sorbitol blended gums as well as for sorbitol-mannitol gums. A systematic review was published evaluating xylitol candies and lozenges (Antonio et al., 2011). After exclusion of studies not interpretable, only 3 were included in the meta-analysis. The results favored a caries-preventive effect.
Such systematic reviews are important, because dissemination of available knowledge about xylitol is limited among practitioners. As long as clinicians are uncertain about the evidence for maternal transmission or the effectiveness of xylitol (e.g., Huebner et al., 2009), little progress will be made. Greater research work on dissemination of this information is needed.
The American Academy of Pediatric Dentistry (AAPD) strengthened its recommendation regarding xylitol (American Academy of Pediatric Dentistry, 2011). The policy says that AAPD “supports the use of xylitol as part of a preventive strategy aimed specifically at long term caries pathogen suppression and caries (dmf) reduction in higher risk populations.” It recommends better labeling.
New Work on Mechanisms of Action
Caries reduction is usually attributed to growth inhibition of MS (Marsh et al., 2009). However, consumption of xylitol can lead to less plaque and less MS bound to plaque (Söderling, 2009). Söderling and Hietala-Lenkkeri (2010) have shown that polysaccharide-mediated cell adherence of S. mutans and sanguinis is not related to growth inhibition (Fig. 1). These findings further help explain why xylitol lowers the amount of MS found in plaque.
Figure 1.
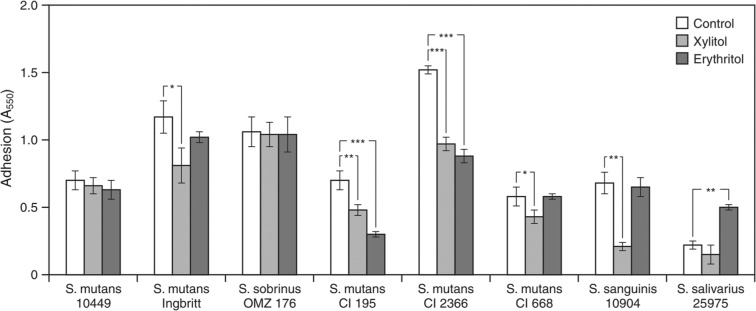
The adhesion (A550) of 8 oral streptococci grown in the presence of 4% (0.26 mol/l) xylitol or 4% (0.33 mol/l) erythritol to a smooth glass surface. Significant differences shown between control vs. xylitol and vs. erythritol are indicated as follows: *P < -.05; **P < 0.01; ***P < 0.001. (Reprinted from Söderling and Hietala-Lenkkeri, 2010, with permission.)
Campus and colleagues (2009) showed that children who chewed xylitol gum for 3 and 6 mos had a reduction in plaque acidogenicity. Xylitol-induced decreases in salivary MS were smaller. This finding is in line with the ecological plaque hypothesis (Marsh et al., 2009) and may partially explain the clinical effectiveness of xylitol. Similar findings were reported for maltitol gum (Macioce et al., 2010).
Recently, a randomized, controlled, double-blind, cross-over pilot study with 12 participants suggested that although xylitol consumption for 4 wks decreased plaque MS, no changes were detected in 14 other plaque species (Söderling et al., 2011).
New Clinical Studies
Estonia Chewing Gum Study
Mothers at a maternity clinic followed a 6 g/day regimen of xylitol lozenges of 4 doses/day until their children were 36 mos old (Olak et al., 2012). This was compared with those receiving no treatment. Offspring were examined at 2 and 3 yrs of age. Children in the control group were more likely to have caries at 2 yrs (OR = 6.6, 95% CI 1.8, 25.0) or 3 yrs (OR = 3.9, 95% CI 1.5, 10.0). A limitation is absence of randomization.
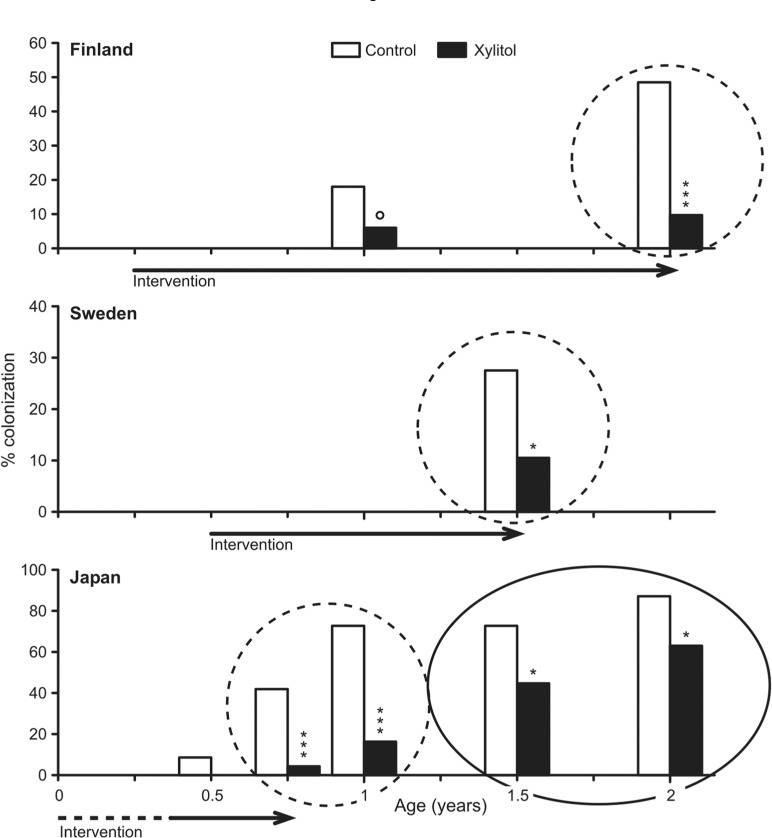
Similarly, a Japanese study examined xylitol gum use (1 piece 100% xylitol 1.32 g/pellet, 5 times/day) among 56 women during pregnancy and after delivery compared with a no-gum control group (Nakai et al., 2009). Actual xylitol use averaged 3.8 g/day, 2.9 times/day. Lower SM were shown for the xylitol group up to 7 mos after initiation, but subsequent results were mixed. Another publication from the same group (Nakai et al., 2010) reported that, compared with offspring of the controls, offspring of the xylitol group were less likely to acquire MS at 9-24 mos of age, and that, overall, the children in the xylitol group acquired MS 8.8 mos later than did children of controls. Fig. 2 illustrates the results compared with results from Finland and Sweden.
Figure 2.
The results of three mother-child intervention studies showing that maternal interventions result in lowered colonization rates in the offspring. The studies were conducted in Finland, Sweden, and Japan and vary in the timing of the intervention and dose of xylitol; the circled areas indicate where the results are similar (Fig. courtesy of Dr. Eva Söderling, University of Turku). Significance tests are indicated as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
In a third study, S. mutans and Lactobacillus spp. were studied in a 6-week evaluation of gummy bear snacks in 154 children of various levels of caries risk, providing daily consumption of 11.7 g or 15.6 g xylitol vs. 44.7 g maltitol divided into 3 doses (Ly et al., 2008). The maltitol condition was intended as an inactive control. The results (Fig. 3) showed reductions of plaque S. mutans with exposure to both doses of xylitol and to maltitol, when the data were evaluated both with and without children with no measurable S. mutans at baseline. Lactobacillus spp. were unchanged. There were no differences in the effects of xylitol and maltitol, even when the xylitol groups were combined.
Figure 3.
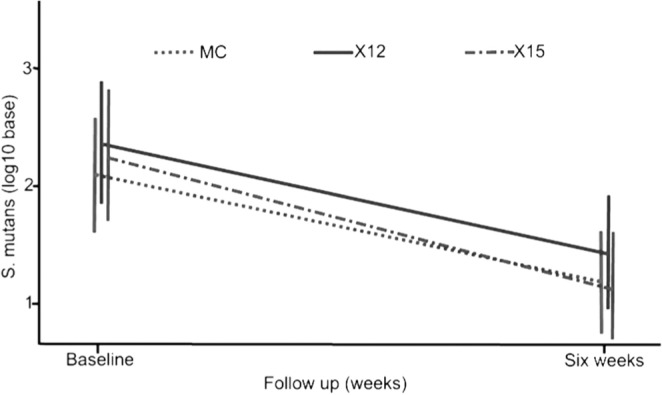
Mean S. mutans/sobrinus levels in plaque at baseline and after 6 wks of gummy bear exposure for schoolchildren exposed to either 11.7 g/d (X12) or 15.6 g/d (X15) xylitol or 44.7 g/d maltitol (MC) total dose, divided into 3 doses per school day. At 6 wks, log10 S. mutans/sobrinus levels showed significant reductions for all groups (p = 0.0001): X16 = 1.13 (SD = 1.65); X12 = 0.89 (SD = 1.11); M45 = 0.91 (SD = 1.46). Reprinted with permission from Ly et al., 2008.
Xylitol/Fluoride Toothpaste
An evaluation assessed the effectiveness of supervised brushing 1/day with fluoride-sorbitol vs. fluoride-xylitol toothpaste to control MS and prevent dental caries among high-caries-risk children ages 3-5 yrs (N = 196) in the Marshall Islands (Chi et al., 2012). Four preschool classrooms were randomly assigned to 1 of 2 treatments: 1400-ppm fluoride-sorbitol toothpaste or 1400-ppm fluoride–31% xylitol toothpaste (Epic Dental, Provo, UT, USA). This toothpaste contains sodium lauroyl sarcosinate, similar to sodium lauryl sulfate, a common ingredient known to interfere with the intracellular uptake of xylitol (Assev et al., 1997). Children had examinations at the start and end of the school year. Plaque and saliva MS were assessed. There was no significant difference in baseline caries scores between the 2 groups. At the end of the year, there were no differences by treatment in the proportions of children with high levels of MS. While the number of primary tooth surfaces affected by caries increased in both groups, the median caries increment in the fluoride-xylitol group was greater than that in the fluoride-sorbitol group (0.0375 vs. 0.0114 surfaces, p = 0.009).
Xylitol/Maltitol and Erythritol/Maltitol Lozenges
A 4-year cluster trial investigated the preventive effects of xylitol/maltitol (49% xylitol, 47.46% maltitol) and erythritol/maltitol lozenges (49.43% xylitol, 47.04% erythritol) delivered at school (Hietala-Lenkkeri et al., 2011). From 21 schools, 579 low-caries-risk 10-year-olds were randomly assigned to 1 of 5 groups. Four groups used the lozenges on school days in 3 teacher-supervised sessions daily, over 1 or 2 yrs. The daily amount was 4.7g/4.6g for xylitol/maltitol and 4.5g/4.2g for erythritol/maltitol. The control group received no treatment. Use of xylitol/maltitol or erythritol/maltitol lozenges did not result in caries reduction.
Ongoing Work
Bader and colleagues (2010) have conducted a 3-year multicenter randomized trial to test 5 g/day xylitol in 1-g lozenges among 691 adults ages 21-80 yrs with at least 1 coronal or root-surface cavitated caries lesion either at entry or within 12 mos. Lozenges were chosen to overcome the adults’ resistance to using gum. Nelson and Milgrom (2011) are assessing the effectiveness of xylitol-sweetened (7.8 g xylitol) gummy bears (2/dose, 3 times/day) during school among 562 high-caries-risk kindergarten children followed until 2nd grade in a cluster (classroom) randomized design. The gummy bears were chosen as the xylitol vehicle to enhance cooperation of the children and overcome resistance to chewing gum in the schools.
A trial is ongoing in which 450 Estonian children, 7-9 yrs old at enrollment, are randomized to 1 of 3 conditions: erythritol lozenges 2.5 g 3 times/day; xylitol lozenges 2.5 g 3 times/day; or sorbitol lozenges 2.5 g 3 times/day (University of Tartu, 2010). The outcome is caries reduction at 3 yrs post-baseline. A trial is also taking place in Scotland among 180 mothers with high MS to determine the effect of 6 g/day xylitol (12 lozenges or 9 pieces of gum) in 4 exposures/day when their children are 3-24 mos old (NHS Fife, 2011). A final trial involves 60 2- to 6-year-olds chewing xylitol tablets up to 7 times/day with an intended total dose of 5 g/day (Y. Nakai, personal communication). The comparison group (N = 60) chews a sorbitol tablet. MS levels are to be measured at baseline and at 1, 3, and 6 mos.
Discussion
This paper reviews published as well as ongoing studies to provide an update on polyol clinical efficacy. Although it is not a formal systematic review, an attempt was made to be comprehensive and balanced by a broad search, with multiple authors searching, and outside review of the paper. While some possibility of bias remains, nevertheless, if the studies, reviews, and policies are a fair indication, there is increased recognition of the value of xylitol as part of dental caries prevention for individuals at high risk. Newer findings shed light on the mechanisms by which xylitol, and perhaps maltitol and erythritol, function to disrupt biofilm organization and adherence and thus prevent dental caries. As in other areas of medicine, clinical trials have been conducted, while aspects of mechanisms of action remain to be elucidated.
The Estonia mothers study and the study by Nakai et al. (2010) provide a valuable confirmation that xylitol during or after pregnancy prevents colonization in some children and postpones colonization in others. The Estonia study further confirms caries reduction among the offspring. Three studies, although varying in design and dose and frequency of xylitol, now suggest this as a viable clinical intervention to be adopted more widely.
What will we know if ongoing studies are reported? The quality of the designs is mixed, and the studies are obviously not coordinated. The studies by Bader and colleagues and Nelson and colleagues are the only definitive trials. Bader and colleagues studied adults using a xylitol lozenge vehicle but with a weak definition of risk and a wide age range. Nelson and Milgrom (2011), using a xylitol confection vehicle, have studied children who are known as high risk.
The other studies use surrogate outcomes or have other threats to their validity. Using MS as a surrogate is logical for screening products, because the polyols affect MS, and, for the most part, the results of previous surrogate studies agree with the clinical findings. Based on what we now see in the clinical trials literature, and the disincentives for manufacturers to support polyol studies, it will be a long time before enough clinical trial data accumulate to satisfy the criteria of the systematic reviews. It might be better for researchers and policy-makers to agree on a rigorous surrogate design. The European Food Safety Agency has recently accepted claims for the benefits of high-concentration xylitol chewing gum based on the reduction of dental plaque (EFSA, 2011).
Bader and colleagues (2010) used lozenges as their xylitol delivery vehicle. This is logical, because gum chewing is uncommon in adults and previous evidence supports the use of lozenges. Their design, however, raises concerns about a failure to collect information about modifiers such as xerostomic drug use or to plan for subgroup analyses of individuals at higher risk. The attempt to conduct one study that covers an entire population ignores the fact that dental caries is not uniformly distributed in the population and across the life-course.
The Nelson and Milgrom work uses xylitol-sweetened confections as the delivery vehicle. This makes sense in that American schools are unlikely to allow chewing gum at school, and gummy bears deliver equivalent amounts of xylitol to the saliva. Also, a study showed that consumption of the gummy bears lowered S. mutans levels. However, all of the children have received fluoride treatments, so only the additive effect of the xylitol gummy bears can be assessed.
In both of these studies, repeated use is needed. There is a need for a vehicle where xylitol is released more slowly so that it can be used less frequently. In this light, findings from the xylitol toothpaste study by Chi and colleagues are disappointing. There was no benefit from a single use each day. However, this may be because ingredients in the particular toothpaste interfere with the action of xylitol. Studies should be carried out to evaluate other formulations. Work has been proposed (P. Milgrom, personal communication) to create a syrup that might be used once per day based on the syrup used in the trial in the Marshall Islands. Informally, we have examined the U.S. product XyliMelts®. This is a 500-mg disk of xylitol that adheres to the buccal gingiva and dissolves slowly. Daily use of 2 disks at night lowered plaque S. mutans significantly after 1 wk (Milgrom, personal communication), but this product has never been formally evaluated. A slow-release vehicle might also allow for greater use of xylitol to prevent S. mutans transmission from mother to child and early colonization, resulting in Early Childhood Caries.
There are limited data on maltitol and erythritol. Maltitol is cheaper than xylitol and merits further investigation. Nevertheless, the current situation with studies of weak design is unlikely to yield strong evidence.
Conclusion
New studies have emerged since the initial ICNARA meeting, but many questions remain regarding the clinical efficacy of polyols. Higher-quality studies and greater coordination among studies going forward may yield more uniform and practically useful results. In addition, it would be desirable to have all products on the world market formally tested, but regulation is lacking.
Supplementary Material
Footnotes
Dr. Milgrom’s work was supported by the NIDCR (Grant 1U54DE019346). Dr. Söderling’s work was supported by the Finnish Dental Association. Dr. Nelson’s research was funded by HRSA (Grant R40 MC07838), the NIH (Grants NCRR/NIH 1 UL RR024989), and in-kind gifts from Colgate-Palmolive, 3M ESPE, and Federal Express. Dr. Chi’s work was supported by the NIH (Grants K08DE020856 and L60D003921). Dr. Nakai’s work was supported by a Grant-in-Aid for Scientific Research (C) KAKENHI (Grant 22592284) from the Japan Society for the Promotion of Science.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://adr.sagepub.com/supplemental.
References
- American Academy of Pediatric Dentistry (2011). Policy on the use of xylitol in caries prevention. Pediatr Dent 33(special issue):42-44 [Google Scholar]
- Antonio AG, Pierro VS, Maia LC. (2011). Caries preventive effects of xyltitol-based candies and lozenges: a systematic review. J Public Health Dent 71:117-124 [DOI] [PubMed] [Google Scholar]
- Assev S, Wåler SM, Rølla G. (1997). Are sodium lauryl sulfate-containing toothpastes suitable vehicles for xylitol? Eur J Oral Sci 105:178-182 [DOI] [PubMed] [Google Scholar]
- Bader JD, Shugars DA, Vollmer WM, Gullion CM, Gilbert GH, Amaechi BT, et al. (2010). Design of the Xylitol for Adult Caries Trial (X-ACT). BMC Oral Health 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campus G, Cagetti MG, Sacco G, Solinas G, Mastroberardino S, Lingström P. (2009). Six months of daily high-dose xylitol in high-risk schoolchildren: a randomized clinical trial on plaque pH and salivary mutans streptococci. Caries Res 43:455-461 [DOI] [PubMed] [Google Scholar]
- Chi DL, Tut O, Milgrom P. (2012). Is toothpaste containing fluoride + xylitol more effective than toothpaste containing only fluoride in preventing dental caries among preschoolers? URL accessed on 5/3/2012 at: http://www.controlled-trials.com/ISRCTN51682476/
- Deshpande A, Jadad AR. (2008). The impact of polyol-containing chewing gums on dental caries: a systematic review of original randomized controlled trials and observational studies. J Am Dent Assoc 139:1602-1614 [DOI] [PubMed] [Google Scholar]
- EFSA (2011). Scientific opinion. EFSA J 9(6):2266 URL accessed on 5/3/2012 at: http://www.efsa.europa.eu/en/efsajournal/doc/2266.pdf [Google Scholar]
- Hietala-Lenkkeri AM, Pienihäkkinen K, Hurme S, Alanen P. (2011). The caries-preventive effect of xylitol/maltitol and erythritol/maltitol lozenges: results of a double-blinded, cluster-randomized clinical trial in an area of natural fluoridation. Int J Paediatr Dent 22:180-190 [DOI] [PubMed] [Google Scholar]
- Hildebrandt GH, Sparks BS. (2000). Maintaining mutans streptococci suppression with xylitol chewing gum. J Am Dent Assoc 131:909-916 [DOI] [PubMed] [Google Scholar]
- Huebner CE, Milgrom P, Conrad D, Lee RS. (2009). Providing dental care to pregnant patients: a survey of Oregon general dentists. J Am Dent Assoc 140:211-222 [DOI] [PubMed] [Google Scholar]
- Isokangas P, Söderling E, Pienihäkkinen K, Alanen P. (2000). Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res 79:1885-1889 [DOI] [PubMed] [Google Scholar]
- Ly KA, Milgrom P, Roberts MC, Yamaguchi DK, Rothen M, Mueller G. (2006). Linear response of mutans streptococci to increasing frequency of xylitol chewing gum use. BMC Oral Health 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KA, Riedy CA, Milgrom P, Rothen M, Roberts MC, Zhou L. (2008). Xylitol gummy bear snacks: a school-based randomized clinical trial. BMC Oral Health 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macioce V, Thabuis C, Zhong B, Wang X, Lefranc-Millot C, Berard M, et al. (2010). Maltitol and xylitol chewing gums influence parameters related to caries development. J Dent Res 89(Spec Iss):IADR Abstract 87, Barcelona, Spain: URL accessed on 5/3/2012 at: http://iadr.confex.com/iadr/2010barce/webprogram/Paper134615.html [Google Scholar]
- Mäkinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR, Jr, et al. (1995). Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res 74:1904-1913 [DOI] [PubMed] [Google Scholar]
- Marsh PD, Martin MV, Lewis MA, Williams DW. (2009). Oral microbiology. 5th ed. Edinburgh, UK: Elsevier [Google Scholar]
- Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. (2006). Mutans streptococci dose response to xylitol chewing gum. J Dent Res 85:177-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Ly KA, Tut OK, Mancl L, Roberts MC, Briand K, et al. (2009). Xylitol pediatric topical oral syrup to prevent dental caries: a double-blind randomized clinical trial of efficacy. Arch Pediatr Adolesc Med 163:601-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Shinga C, Kaji M, Moriya K, Takimura M, Murakami K, et al. (2009). Long-term effect of xylitol chewing gum on salivary MS counts. J Dent Res 88(Spec Iss):IADR Abstract 673, Miami, FL: URL accessed on 5/3/2012 at: http://iadr.confex.com/iadr/2009miami/webprogram/Paper115038.html [Google Scholar]
- Nakai Y, Shinga-Ishihara C, Kaji M, Moriya K, Murakamai-Yamanaka K, Takimura M. (2010). Xylitol gum and maternal transmission of mutans streptococci. J Dent Res 89:56-60 [DOI] [PubMed] [Google Scholar]
- Nelson S, Milgrom P. (2011). Minority participation in a school-based randomized clinical trial of tooth decay prevention in the United States. Contemp Clin Trials 33:60-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Fife (2011). Maternal consumption of xylitol to reduce early childhood decay (MaXED Study). NCT01038479. URL accessed on 5/3/2012 at: http://clinicaltrials.gov/ct2/show/NCT01038479?term=Xylitol+and+streptococcus+mutans&rank=2
- Olak J, Saag M, Vahlberg T, Söderling E, Karjalainen S. (2012). Caries prevention with xylitol lozenges in children related to maternal anxiety. A demonstration project. Eur Arch Paediatr Dent 13:64-69 [DOI] [PubMed] [Google Scholar]
- Rethman MP, Beltrán-Aguilar ED, Billings RJ, Hujoel PP, Katz BP, Milgrom P, et al. (2011). Nonfluoride caries-preventive agents: executive summary of evidence-based clinical recommendations. J Am Dent Assoc 142:1065-1071 [DOI] [PubMed] [Google Scholar]
- Söderling EM. (2009). Xylitol, mutans streptococci, and dental plaque. Adv Dent Res 21:74-78 [DOI] [PubMed] [Google Scholar]
- Söderling EM, Hietala-Lenkkeri AM. (2010). Xylitol and erythritol decrease adherence of polysaccharide-producing oral streptococci. Curr Microbiol 60:25-29 [DOI] [PubMed] [Google Scholar]
- Söderling E, Hirvonen A, Karjalainen S, Fontana M, Catt D, Seppä L. (2011). The effect of xylitol on the composition of the oral flora: a pilot study. Eur J Dent 5:24-31 [PMC free article] [PubMed] [Google Scholar]
- Trahan L. (1995). Xylitol: a review of its action on mutans streptococci and dental plaque—its clinical significance. Int Dent J 45(1 Suppl 1):77S-92S [PubMed] [Google Scholar]
- University of Tartu (2010). Effect of erythritol and xylitol on dental caries prevention in children. NCT01062633. URL accessed on 5/3/2012 at: http://clinicaltrials.gov/ct2/show/NCT01062633?term=Saag&rank=2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.