Abstract
Streptococcus mutans has been implicated as the major acid-producing (cariogenic) bacterium. Dietary sugars and other factors may cause an imbalance of oral microflora that enables S. mutans to become dominant in the multi-species biofilms on the tooth surface, which could lead to dental caries. The application of broad-spectrum antimicrobials often results in re-colonization and re-dominance of S. mutans within oral flora, while in contrast, therapies capable of selective elimination of S. mutans from oral microbial communities may help to re-establish the normal flora and provide long-term protection. C16G2, a novel synthetic antimicrobial peptide with specificity for S. mutans, was found to have robust killing efficacy and selectivity for S. mutans in vitro. A subsequent pilot human study found that a single application of C16G2 in the oral cavity (formulated in a mouthrinse vehicle) was associated with a reduction in plaque and salivary S. mutans, lactic acid production, and enamel demineralization during the entire 4-day testing period. C16G2 is now being developed as a new anticaries drug.
Keywords: microbial ecology, microbiology, microbial genetics, caries, dental biofilm, microbiota
Introduction
This is a short review with a specific focus on C16G2, a targeted antimicrobial peptide, which was recently accepted by the US FDA as an Investigational New Drug (IND) to be tested for its safety and clinical efficacy against dental caries. The article will briefly review the rationales behind this new investigative drug, as well as the in vitro and in vivo data that support its possible efficacy.
Current Approaches for Treating Dental Caries and Their Possible Limitations
Dental caries is a chronic disease of microbiological origin, and S. mutans has been implicated as one of the major etiological pathogens responsible for the majority of caries (Loesche, 1986; Tanzer et al., 2001). Caries arises from dietary sugars (primarily sucrose) that cause acidogenic microbes, such as S. mutans, to produce acids that damage tooth structure. This also leads to an imbalance in the oral microflora that enables these cariogenic bacteria to become dominant in the multi-species biofilms found on the tooth surface.
Currently, the surgical approach (drilling and filling) is still the main tool to treat dental caries, while the only chemical intervention approved by the FDA for fighting caries involve the use of fluoride-containing varnishes and toothpastes, which are capable of preventing enamel demineralization and reversing the process through remineralization (Stookey et al., 1993; Donly, 2003). Fluoride has been successful in reducing the incidence and prevalence of dental caries and should remain an important part of any anti-caries regimen, but it has limited efficacy in killing cariogenic bacteria residing in dental plaque, even though there have been reports of its inhibitory effects on bacterial metabolism (Hamilton, 1990). This could be a factor contributing to the persistence of dental caries within populations, despite fluoride’s well-documented clinical efficacy (Beighton, 2005; Anderson and Shi, 2006; Milgrom et al., 2009).
Controlling caries by reducing the total bacterial load in saliva and plaque through the use of broad-spectrum antibacterial agents can, in theory, reduce caries incidence; however, broad killing of the bacteria alone allows for equal competition between cariogenic bacteria and non-pathogenic organisms to re-establish the biofilms. Consequently, there is no strong clinical evidence supporting the long-term prevention of re-infection of cariogenic bacteria and very few studies examining the impact on caries reduction (Milgrom et al., 2009; Vollmer et al., 2010; Young et al., 2010; Papas et al., 2012). The reason for the lack of long-term protection could be tied to the persistence of cariogenic bacteria within the dental plaque and the dynamic balance of the biofilm community between a healthy state and cariogenic state. Individual behaviors with respect to oral hygiene and diet play a major role in determining whether dental plaque remains healthy or cariogenic. If an individual has poor oral hygiene and a diet high in refined sugars, the re-established biofilm will retain a community where cariogenic conditions persist and may dominate.
The Rationale Behind the Specifically Targeted Antimicrobial Peptides
It is known that patients with “healthy” dental plaque displaying low levels of S. mutans are resistant to exogenous colonization from cariogenic pathogens and have shown long-term protection from dental caries (Keene and Shklair, 1974; Anderson and Shi, 2006; He et al., 2009; Marsh, 2010). Lack of S. mutans has been associated with sites of healthy dentition, while progressively increasing levels of S. mutans are associated with sites of caries development and cavitation (Becker et al., 2002).
Based on these findings, it was suggested that targeted antimicrobial therapies against S. mutans may be a good alternative approach to treat dental caries (Eckert et al., 2006). As illustrated in Fig. 1, with the exception of a limited number of oral pathogens (S. mutans being a major example), most of the micro-organisms within the indigenous oral microflora are benign or beneficial. The application of broad-spectrum antimicrobials often results in the re-colonization and re-dominance of cariogenic bacteria within the oral flora (Fig. 1A). In contrast, therapies capable of selective elimination of cariogenic bacteria from the oral flora may help to re-establish normal flora to provide long-term protection (Fig. 1B).
Figure 1.
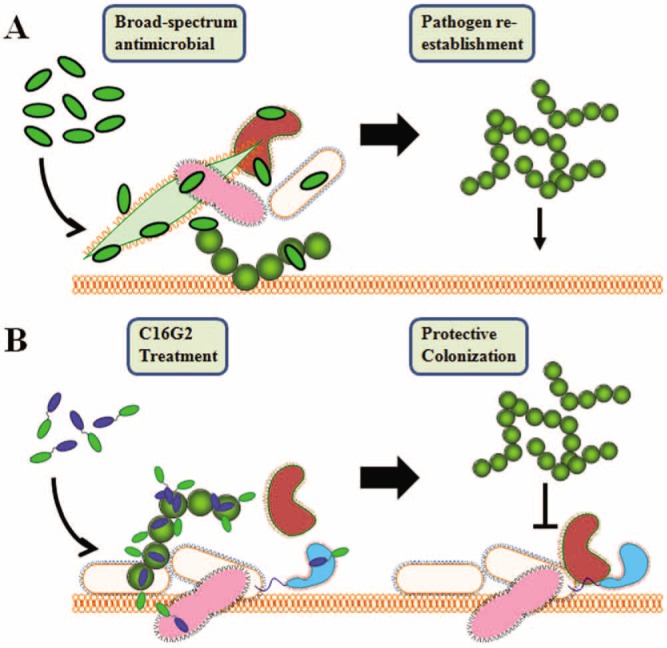
Illustration of targeted antimicrobial treatment for long-term protection. (A) Broad-spectrum antimicrobial therapy results in killing of pathogenic and normal flora bacteria. The mucosal surface can be re-colonized by pathogens. (B) Targeted STAMP (C16G2) treatment results in selective pathogen removal and protective colonization associated with the remaining and established normal flora.
To achieve the selective elimination of a particular bacterial species from a multi-species microbial community, a novel technology called Specifically Targeted Antimicrobial Peptides (STAMPs) was developed. As illustrated in Fig. 2, a “STAMP” is a fusion peptide with 2 main domains: a killing domain, made of a non-specific antimicrobial peptide; and a targeting domain, containing a species-specific targeting peptide. The targeting domain provides specific binding to a selected pathogen and facilitates the targeted delivery of an attached antimicrobial peptide.
Figure 2.
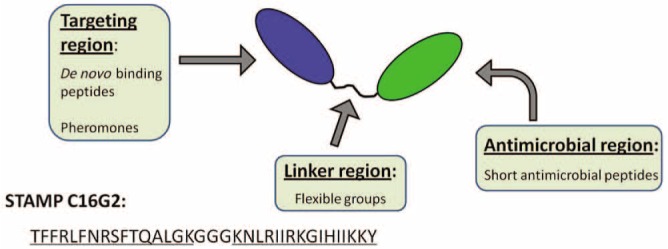
Illustration of the basic structure for the specifically targeted antimicrobial peptides (STAMPs). Adapted from Eckert (2011).
C16G2 is a STAMP designed with antimicrobial specificity for S. mutans with 2 peptide sequences for specific functional domains (Fig. 2). The first is a S. mutans-selective ‘targeting domain’ derived from a fragment of the S. mutans competence stimulating peptide (CSP), designated as C16 (or CSPC16), and comprised of amino acids 1 through 16. The second is a “killing domain” derived from a broad-spectrum antimicrobial peptide, designated as G2, and comprised of amino acids 20 through 35. These 2 peptide sequences are conjoined by a sequence of 3 glycine residues comprised of amino acids 17 through 19, which allows both domains to function properly.
The Robust in vitro Killing Activity and Selectivity of C16G2 for S. mutans
In the original publication describing C16G2 (Eckert et al., 2006), the group discovered that C16G2 was potent against S. mutans grown in a planktonic or biofilm state and did not affect other oral streptococci tested. C16G2 has rapid bactericidal activity against S. mutans, killing bacteria within seconds of contact, but is slower against untargeted bacteria, where several minutes to hours are required to reach similar levels of killing (Kaplan et al., 2011). Multi-species biofilms from which S. mutans has been eliminated by C16G2 resist re-colonization by S. mutans, demonstrating the protective colonization effect in vitro (Li et al., 2010). Mechanistic studies indicate that C16G2 is bactericidal via a cytoplasmic membrane disruption mechanism. Accumulation of C16G2 on the cell surface of S. mutans leads to loss of membrane potential, leakage of intracellular adenosine triphosphate (ATP), and loss of membrane integrity, followed by cell death (Kaplan et al., 2011).
The Clinical Efficacy of C16G2 in a Pilot Study in Humans
A pilot study was conducted in humans to evaluate the efficacy of C16G2 in vivo (Sullivan et al., 2011). The objectives of the study were to assess the effects of a single application of C16G2 on S. mutans in saliva and dental plaque, on plaque pH, on lactic acid production after a sucrose challenge, and mineral loss from tooth enamel.
Study participants arrived at the dental clinic on Day 0 (baseline) without having performed morning oral hygiene, and samples of fasting plaque and whole saliva were collected for analysis of S. mutans and plaque pH. Participants then rinsed with a 10% sucrose solution for 2 min, and 8 min later a second plaque sample was collected for pH analysis. Mouth retainers containing 4 ground and polished blocks of bovine enamel were put into the mouth of each participant. Participants then rinsed for 40 sec with either placebo or 8 mg C16G2. They were allowed to leave the clinic and were instructed to wear the retainer at all times, except to dip the mouth retainer in 10% sucrose at 4 different times throughout the day before placing it back into the mouth. Participants returned to the clinic daily on Days 1-4. Plaque and saliva samples were taken before and after a 10% sucrose rinse, and a block of the bovine enamel was also removed and assessed for demineralization on Day 4.
Twelve healthy individuals were enrolled in the study. As summarized in the Table, the results showed that 8 mg C16G2 was able to selectively eliminate S. mutans from plaque and saliva while leaving the remaining flora, including closely related non-mutans oral streptococci, relatively undisturbed. In the placebo group, S. mutans increased during the course of the study. A reduction of S. mutans in the C16G2 group resulted in a higher resting plaque pH, lower lactic acid production, and a significant reduction in enamel mineralization (Table). Based on these findings, the authors concluded that C16G2 produced reductions in plaque and salivary S. mutans, lactic acid production, and enamel demineralization. The impact on total plaque bacteria was minimal.
Table.
Statistically Significant Improvement vs. Placebo in S. mutans Levels, Plaque pH, Acid Production, and Enamel Demineralization after C16G2 Rinse
| Treatment | Δ S. mutans CFU from Baseline, Plaque (× 106) | Δ S. mutans CFU from Baseline, Saliva (× 106) | Resting pH, Plaque | Lactate Concentration (nM/mg plaque) | % Demineralization |
|---|---|---|---|---|---|
| Placebo | 5.02 | 1.50 | 6.68 | 8.81 | 23.0 |
| C16G2-treated | −0.906 | −0.5 | 7.22 | 6.3 | −3.73 |
Current Development Status of C16G2
C16G2 is currently being developed by a Los Angeles-based biotechnology company, C3 Jian Inc. (www.c3-jian.com). An Investigational New Drug (IND) application has been accepted by the US FDA, and the phase I clinical trial is due to start in the summer of 2012.
Summary and Discussion
It is now clear that dental caries is a microbial-ecology-related disease. Any effective antimicrobial treatment against dental caries should modulate the microbial ecology of dental plaque in a pathogen-targeted manner, since indiscriminant antibacterial killing could lead to the disruption of the ecological balance of normal oral flora and result in persistent pathogenesis and possibly unknown clinical consequences. The STAMP technology offers a novel approach and a promising future for the treatment of microbial infections and dental caries at oral mucosal surfaces, where protective residential flora play a dynamic role in maintaining the health of the host and whose preservation should be maximized.
Given the fact that S. mutans is the major cariogenic bacterium, it is likely that C16G2 will demonstrate some anticaries activities if its bioactivities against S. mutans are retained in vivo. Since S. mutans is not the only cariogenic bacterium, a diagnostic device which could instantly detect the level of S. mutans in saliva and plaque would be very useful to screen for needy individuals and to monitor treatment efficacy. Such a device is currently being developed by C3 Jian Inc. The biggest unknowns at this point are: (1) how long it takes for S. mutans to re-invade and re-establish themselves within the treated oral microbial flora after being initially eliminated by C16G2; and (2) whether elimination of S. mutans would lead to the dominance of new oral pathogens. These two questions will be addressed in the IND clinical trials.
Footnotes
This study was supported by NIH grants MD01831 and DE20102 and by funds from C3 Jian, Inc., Delta Dental, and Colgate-Palmolive. Drs. R. Sullivan and R. Eckert are employees of Colgate-Palmolive and C3 Jian, respectively, both of which have partially funded the in vitro and in vivo pilot human studies reported in this article. Dr. Wenyuan Shi is the co-founder of C3 Jian and is currently serving as a part-time Chief Scientific Officer of the company.
References
- Anderson MH, Shi W. (2006). A probiotic approach to caries management. Pediatr Dent 28:151-153 [PubMed] [Google Scholar]
- Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. (2002). Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D. (2005). The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol 33:248-255 [DOI] [PubMed] [Google Scholar]
- Donly KJ. (2003). Fluoride varnishes. J Calif Dent Assoc 31:217-219 [PubMed] [Google Scholar]
- Eckert R. (2011). Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol 6:635-651 [DOI] [PubMed] [Google Scholar]
- Eckert R, He J, Yarbrough DK, Qi F, Anderson MH, Shi W. (2006). Targeted killing of Streptococcus mutans by a pheromone-guided “Smart” antimicrobial peptide. Antimicrob Agents Chemother 50:3651-3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton IR. (1990). Biochemical effects of fluoride on oral bacteria. J Dent Res 69(Spec Iss):660-667 [DOI] [PubMed] [Google Scholar]
- He X, Lux R, Kuramitsu HK, Anderson MH, Shi W. (2009). Achieving probiotic effects via modulating oral microbial ecology. Adv Dent Res 21:53-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CW, Sim JH, Shah KR, Kolesnikova A, Shi W, Eckert R. (2011). Selective membrane disruption: the mode of action of C16G2, a specifically-targeted antimicrobial peptide. Antimicrob Agents Chemother 55:3446-3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene HJ, Shklair IL. (1974). Relationship of Streptococcus mutans carrier status to the development of carious lesions in initially cariesfree recruits. J Dent Res 53:1295 [DOI] [PubMed] [Google Scholar]
- Li LN, Guo LH, Lux R, Eckert R, Yarbrough D, He J, et al. (2010). Targeted antimicrobial therapy against Streptococcus mutans establishes protective non-cariogenic oral biofilms and reduces subsequent infection. Int J Oral Sci 2:66-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. (1986). Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. (2010). Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am 54:441-454 [DOI] [PubMed] [Google Scholar]
- Milgrom P, Zero DT, Tanzer JM. (2009). An examination of the advances in science and technology of prevention of tooth decay in young children since the Surgeon General’s Report on Oral Health. Acad Pediatr 9:404-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas AS, Vollmer WM, Gullion CM, Bader J, Laws R, Fellows J, et al. (2012). Efficacy of chlorhexidine varnish for the prevention of adult caries: a randomized trial. J Dent Res 91:150-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stookey GK, DePaola PF, Featherstone JD, Fejerskov O, Möller IJ, Rotberg S, et al. (1993). A critical review of the relative anticaries efficacy of sodium fluoride and sodium monofluorophosphate dentifrices. Caries Res 27:337-360 [DOI] [PubMed] [Google Scholar]
- Sullivan R, Santarpia P, Lavender S, Gittins E, Liu Z, Anderson MH, et al. (2011). Clinical efficacy of a specifically-tarted antimicrobial peptide mouth rinse: targeted elimination of Streptococcus mutans and prevention of demineralization. Caries Res 45:415-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Livingston J, Thompson AM. (2001). The microbiology of primary dental caries in humans. J Dent Educ 65:1028-1037 [PubMed] [Google Scholar]
- Vollmer WM, Papas AS, Bader JD, Maupome G, Gullion CM, Hollis JF, et al. (2010). Design of the Prevention of Adult Caries Study (PACS): a randomized clinical trial assessing the effect of a chlorhexidine dental coating for the prevention of adult caries. BMC Oral Health 10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DA, Lyon L, Azevedo S. (2010). The role of dental hygiene in caries management: a new paradigm. J Dent Hyg 84:121-129 [PubMed] [Google Scholar]


