Abstract
Temporal lobe epilepsy (TLE) is the most common form of medically intractable epilepsy. Advances in electrophysiology and neuroimaging have led to a more precise localization of the epileptogenic zone within the temporal lobe. Resective surgery is the most effective treatment for TLE. Despite the variability in surgical techniques and in the extent of resection, the overall outcomes of different TLE surgeries are similar. Here, we review different surgical interventions for the management of TLE.
1. Introduction
The first surgical intervention for the amelioration of epilepsy was performed by Horsley and involved a cortical resection in a patient suffering from posttraumatic epilepsy [1]. Cortical resection to treat epilepsy has since been performed by other surgeons [2–4]. Following the first human application of electroencephalography (EEG) by Berger in 1929 [5], EEG and electrocorticography (ECOG) were used by Penfield and Jasper to tailor resective surgeries for epilepsy [6, 7]; they modified cortical resections based on an extensive mapping of different cortical regions. Early in the practice of temporal lobe surgery to treat epilepsy, hippocampal preservation was advocated to avoid memory disruption [8–11]; however, Penfield observed that the failure to resect mesial temporal structures was associated with poor epilepsy control [7, 12]. Subsequently, surgery for temporal lobe epilepsy (TLE) has come to constitute the majority of resective epileptic surgical interventions.
Several modifications have been made to the surgical techniques and methods used to treat epilepsy over the last 50 years. Modifications to temporal lobe resective surgery have been based either on resection of the epileptogenic zone, assisted by the use of ECOG and cortical mapping to avoid functional deficits, or on resection of the seizure onset zone, as with selective amygdalohippocampectomy (SAH). Functional deficits following temporal resection surgeries were identified early by Penfield and Scoville [9, 13]. Since that time, neuropsychological assessment has become a standard part of the multidisciplinary approach for the treatment of epilepsy. The primary goal of temporal lobe surgery is to achieve freedom from seizures without causing neurological or cognitive dysfunction. In turn, the achievement of this goal should improve psychosocial adjustment, education and employment status, and quality of life, as well as significantly reducing the overall treatment cost for patients [14, 15]. Although surgery is effective in the majority of patients with TLE, not all show improvements. Wiebe et al. demonstrated the effectiveness of temporal resective surgery compared to medical therapy [14]. TLE can be classified as either mesial temporal lobe epilepsy (mTLE) or neocortical temporal lobe epilepsy (nTLE). It can be also classified based on the presence or absence of lesions. The term “temporal lobe epilepsy” describes numerous underlying pathological substrates and their clinical features. The term “TLE” is also nonspecific and comprises several surgical techniques and procedures. In this paper, we describe temporal lobe surgical techniques. A detailed discussion of preoperative investigations or the ECOG-based tailored approach is beyond the scope of this paper.
2. Surgical Anatomy
The temporal lobe comprises three heterogeneous cortices: a six-layered neocortex (with superior, middle, inferior, transverse, temporal, and fusiform gyri), a three-layered archicortex that includes the hippocampus, the prepiriform area, the uncal semilunar gyrus, and the parahippocampus, a transitional region between the neocortex and the archicortex [16, 17]. The lateral upper surface of the temporal lobe is separated from the frontal and parietal lobes by the sylvian fissure. Posteriorly, the temporal lobe is separated from the occipital and parietal lobes by imaginary lines. The parietotemporal line extends from the parietooccipital fissure impression to the preoccipital notch on the lateral surface. The temporooccipital line runs perpendicular to the parietooccipital line, starting at the posterior end of the sylvian fissure. The basal surface of the temporal lobe is separated from the occipital lobe by the basal parietooccipital line, which connects the preoccipital notch to the inferior end of the parietooccipital fissure. The temporal lobe is connected superiorly and medially to the insula by the temporal stem, anteromedially to the globus pallidus via the amygdala, and anterolaterally to the frontal base by the limen insulae.
The following five gyri are located on different temporal lobe surfaces: the superior (T1), middle (T2), and inferior (T3) gyri, the fusiform gyrus (T4), and the parahippocampal gyrus (T5), Figure 1. The above gyri are separated by multiple sulci, including S1, S2, S3, and S4. S1 is a deep sulcus that extends toward the temporal horn and serves as an important landmark for the identification of the temporal horn. S4 is a collateral fissure located at the edge of the lateral temporal horn wall that forms the collateral eminence. Medial to the superior surface of T1, the transverse temporal gyri, also known as Heschl's convolutions, extend to the depth of the sylvian fissure and mark the location of the primary auditory cortex. The posterior region of T1 is the planum temporale. This structure is larger on the left side in males (but not females) and is involved in the receptive language function.
Figure 1.
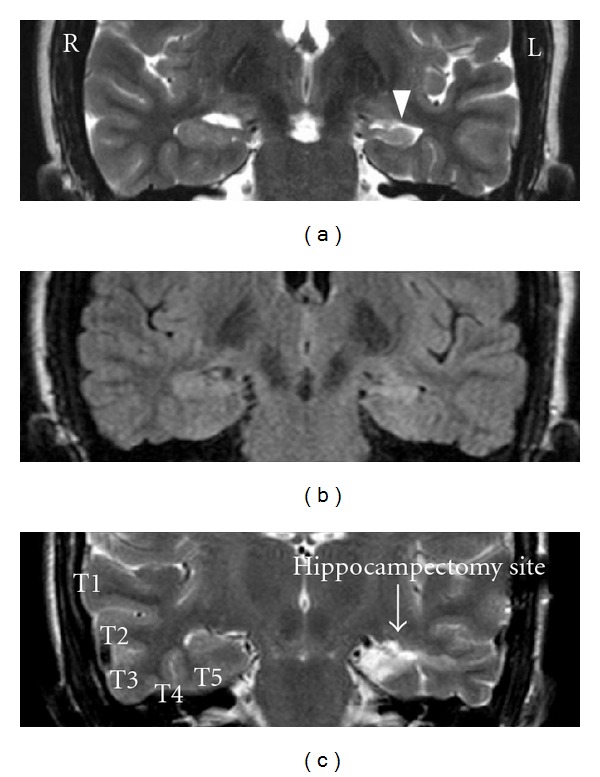
((a) and (b)) Coronal T2 and FLAIR magnetic resonance image (MRI) respectively depicting a left mesial temporal sclerosis. (c) Coronal T2 MRI depicting hippocampectomy site after selective amygdalohippocampectomy on left and temporal gyri (superior (T1), middle (T2), and inferior (T3) gyri, the fusiform gyrus (T4), and the parahippocampal gyrus (T5)) on right side.
The parahippocampus ends anteriorly at the level of the posterior uncus, approximately 2 cm from the temporal pole [18]. The anterior calcarine sulcus is located at the posterior aspect of the parahippocampus gyrus and divides the parahippocampus into superior and inferior regions. The superior parahippocampus continues along the isthmus of the cingulate gyrus, whereas the inferior region merges with the lingual gyrus near the occipital lobe.
The uncus is a conical structure partially formed by the anterior parahippocampal gyrus. The uncus extends medially and then curves posteriorly to form the uncal notch sulcus; this path inspired the name “uncus,” which means “hook.” The other region of the uncus is formed by the medial extension of the hippocampus and the dentate gyrus. There are several gyri at the surface of the uncus, including the intralimbic gyrus (posteriorly), the band of Giacomini, the uncinate gyrus, the ambient gyrus, and the semilunar gyrus (superiorly). The uncus continues along the globus pallidus at its superior surface.
Rostral to the uncus, the amygdala is occupying the depth of the medial temporal lobe. It is connected to the striatum superiorly without clear border, Figure 2 [18]. The posterior inferior border of the amygdala is bounded by the anterior temporal horn, while the anterior inferior border is related to the entorhinal area. The medial side is bounded by the uncus and the mesial cistern. From structural standpoint, the amygdala is composed of 13 nuclei divided into three main groups: central, corticomedial, and basolateral groups. Grossly, the amygdala is recognized with its relatively brownish color or the hazelnut tissue appearance, Figure 3.
Figure 2.
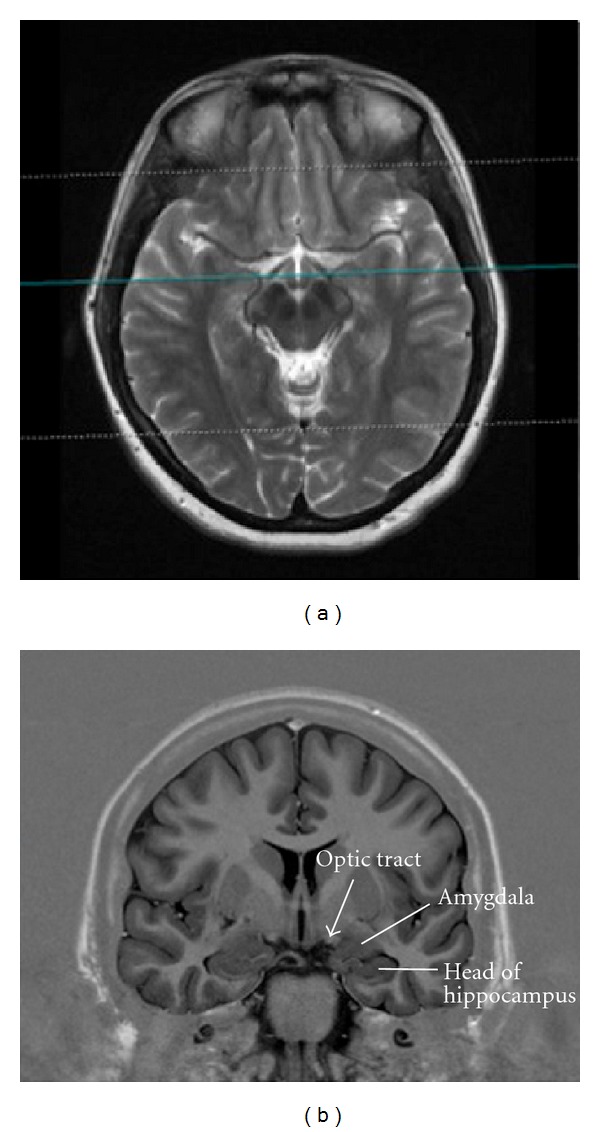
MRI coronal inversion recovery image (right) at the level of optic tract ((a), blue line) depicting the anatomical relationship of the amygdala to the optic tract (b).
Figure 3.
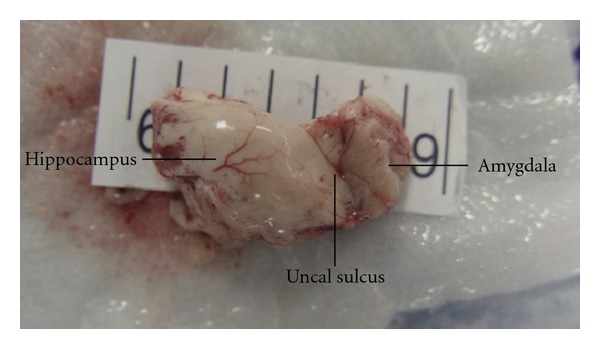
Surgical specimen photographs of the hippocampus and amygdala. The brownish color of the amygdala tissue is noted.
The hippocampus is an intraventricular structure. It has a C shape that resembles a seahorse and occupies the medial surface and the floor of the temporal horn. The hippocampus proper covers both surfaces of the hippocampal sulcus, which contains the hippocampal feeding vessels. The hippocampus is divided into three regions: the head, the body, and the tail. The head contains the largest area and extends anteriorly and medially toward the uncal recess, which is a continuation of the lateral eminence, Figure 3. The head is the only region of the hippocampus that has no choroid plexus coverage. Posteriorly, the head ends at the choroidal fissure and the beginning of the fimbria, Figure 4(a). The presence of several digitations usually characterizes the head of the hippocampus. The hippocampal body begins at the junction of the choroidal fissure and the fimbria, extending posteriorly and superiorly toward the atrium of the lateral ventricle. At the medial hippocampal body, the choroidal fissure communicates with the ambient cistern below the pulvinar of the thalamus. The tail of the hippocampus is formed at the pulvinar level of the posterior intraventricular region and fuses medially with the calcar avis, the inferior bulge on the medial wall of the atrium.
Figure 4.
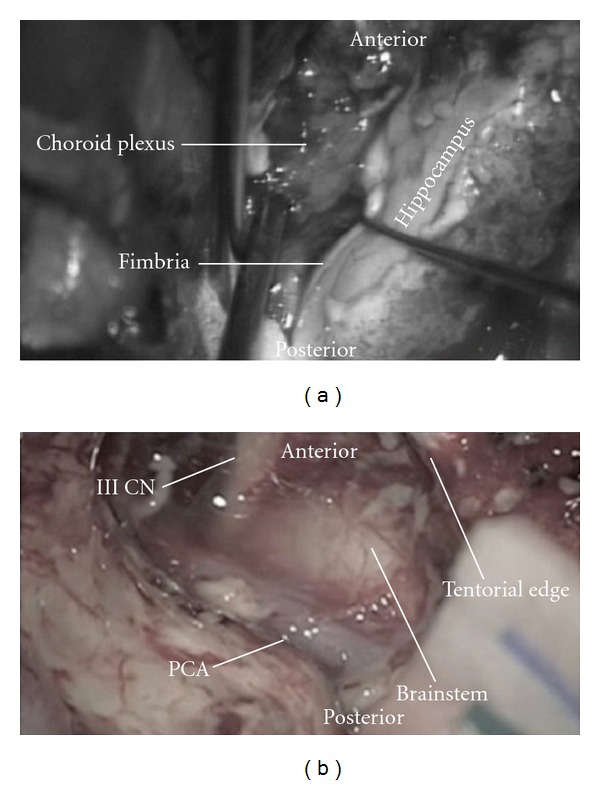
Intraoperative photographs showing (a) the dissection of the fimbria to expose the choroidal point. (b) Postresection of the uncus and amygdala showing the third cranial nerve, brainstem, PCA (posterior cerebral artery), and the tentorial edge.
The alveus, a thin layer of white matter, forms the fimbria, a structure that runs horizontally along the medial hippocampus. The fimbria is separated from the dentate gyrus by the shallow fimbriodentate sulcus. The dentate gyrus continues anteriorly along the band of Giacomini and posteriorly along the fasciolar gyrus. Above the corpus callosum, the dentate gyrus becomes the indusium griseum [16].
The entorhinal cortex is formed by the anterior portion of the parahippocampal gyrus and connects the hippocampus to the neocortex. The hippocampal efferent pathway projects through the fornix and the entorhinal cortex. Internally, the hippocampus is composed of a pyramidal cell layer called the cornu ammon (CA). The CA is divided into 4 regions: CA1–CA4. The trisynaptic circuit connects the entorhinal cortex, the dentate gyrus, and CA3 through mossy fibers. Shaffer collaterals then connect CA1 back to the entorhinal cortex. These structures are important for the pathophysiology of mTLE. Pathological findings in patients with mesial temporal sclerosis (MTS) have suggested that pyramidal cell loss occurs primarily in the CA1 region and, to a lesser extent, the CA3 and CA4 regions. There is little cell loss in the CA2 region.
3. Overview of Surgical Procedures
Surgical treatment for TLE mainly targets the mesial structures, employing a variable degree of lateral neocortical resection. This section summarizes the different temporal lobectomy surgical (TLY) techniques (Table 1).
Table 1.
Summary of different surgical approaches and techniques for TLE surgery.
| Standard anterior temporal lobectomy | |
| Electrocorticography tailored temporal lobectomy | |
| Anteromedial temporal lobectomy | |
| Transcortical selective amygdalohippocampectomy | |
| Transsylvian selective amygdalohippocampectomy | |
| Subtemporal selective amygdalohippocampectomy | |
| Temporal lobe disconnection | |
| Hippocampal transection |
4. Standard Anterior Temporal Lobectomy
Performing a standard anterior temporal lobectomy (ATL) consists of resecting the lateral temporal and mesial temporal structures, either en bloc or separately. Removal of the lateral temporal structures allows better visualization of the mesial structures, allowing en bloc removal of the hippocampus. The procedure is usually performed with the patient in the supine position, elevating the ipsilateral shoulder with a roll and rotating the head to the contralateral side. The head is tilted slightly laterally to place the zygoma at an approximately 10-degree angle from the horizontal plane of the surgical floor. There are several techniques for opening the skin and temporalis muscle. Some surgeons perform a question-mark skin incision followed by reflection of the myocutaneous flap. Others use curvilinear or straight skin incisions. To avoid injury to the frontalis branch of the facial nerve, the incision is begun 1 cm above the zygoma and 1 cm anterior to the tragus. The superficial temporal artery is dissected and preserved if possible. A subperiosteal dissection is used to remove the muscle from the bone. Extensive cauterization is avoided to minimize the subsequent atrophy of the temporalis muscle. A craniotomy is performed on small portion of the frontal bone posterior to the pterion. Some surgeons tend to expose the pterion at the frontal bone. Venous oozing from the sphenoid ridge can usually be controlled using bone wax or gelfoam. Bleeding from the middle meningeal artery branches is controlled by bipolar coagulation. A U-shaped durotomy is often preformed with the base reflected anteriorly. A cruciate durotomy can also be used.
A posterior cortical incision at the lateral temporal gyri begins approximately 5.5 cm from the temporal tip on the nondominant hemisphere and 4.5 cm from the temporal tip on the dominant side at the level of T2, Figure 5. A number 1 Penfield dissector is used to measure the length from the temporal tip. The posterior resection is slanted anteriorly across T1 to avoid the primary auditory cortex. The pia mater at the upper border of T1 is coagulated and divided. A subpial dissection is performed to elevate T1 from the sylvian fissure using bipolar cauterization and controlled suction, an ultrasonic aspirator, or a dissector technique. The pia and middle cerebral artery (MCA) branches are protected. Oozing from the pia can be controlled using cottonoid packing or Surgicel. The insula is exposed, and dissection extending to the lateral uncus is performed. The temporal pole is reflected laterally after the coagulation and division of the anterior leptomeninges. The posterior resection line is extended from the T1 through T2 and into T3. This line is then extended medially through the fusiform gyrus to the collateral sulcus. The temporal horn is entered through the white matter above the fusiform gyrus. The wall of the temporal horn can be identified by the bluish ependyma. Subsequently, opening of the ventricle anteriorly exposes the hippocampal head. The temporal stem is resected at the inferior circular sulcus. The temporal neocortex is removed by dividing the basal leptomeninges lateral to the temporal horn exposure. If en bloc temporal resection is intended, further resection of the mesial structures is performed. During the resection of the mesial structures, an ultrasonic aspirator is used at a low setting to avoid injury to the arachnoid that overlay the posterior cerebral artery (PCA), the basal vein of Rosenthal, the third cranial nerve, and the midbrain.
Figure 5.
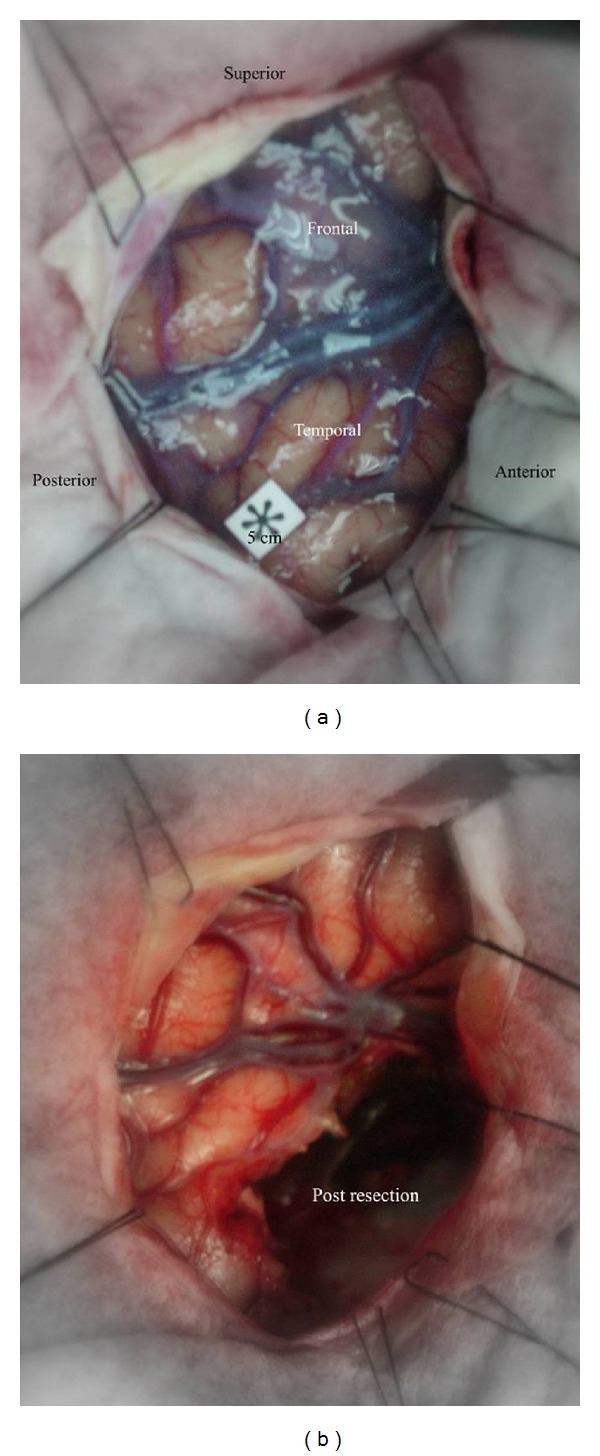
Intraoperative photographs demonstrating pre and post resection for right anterior temporal lobectomy.
Different surgical techniques have been used to resect the mesial temporal structures. In general, the areas of the uncus that extends to the level of the limen insulae and the parallel M1 segment of the MCA are removed with an ultrasonic aspirator. The amygdala is resected at the line that connects the choroidal point and the limen insulae, Figure 4(b). The choroidal point is located at the anterior portion of choroid plexus. Care should be taken to not extend the resection superior and medial into the globus pallidus. Due to the absence of clear demarcation between the amygdala and globus pallidus, the anatomic landmarks for amygdala resection are variable among different surgeons. Wieser and Yazargil advocate using the insular circular sulcus and uncus to avoid entry into globus pallidus [35]. Based on anatomical dissection study, Wen et al. found that a line interconnecting the inferior choroidal point and the proximal MCA can define the superior limit of amygdalar resection [16]. Recently, Tubbs et al. examined the line connecting the anterior choroidal artery and the MCA bifurcation in 20 sides cadavers [36]. In this study, no damage to the striatum was found using this line for upper amygdala removal. The entorhinal cortex is resected to the anterior portion of the parahippocampal gyrus. At this stage, the fimbria can be dissected laterally from the arachnoid attachment, exposing the hippocampal sulcus that carries the Ammon's horn arteries, Figure 4(a). Next, the subpial dissection of the parahippocampal gyrus exposes the hippocampal sulcus. This step will allow the lateral reflection of the hippocampal body. The hippocampal feeders are coagulated and divided at the hippocampus edge, and the tissues of the hippocampus and parahippocampus are removed en bloc. The posterior portion of the hippocampus is removed using an ultrasonic aspirator to the level of the midbrain tectum, as identified by image guidance. Next, hemostasis is secured, and wound closure is performed in a standard manner.
5. Anteromedial Temporal Resection
The anteromedial temporal resection technique was developed by Spencer to preserve the function of lateral temporal cortex and to access the mesial temporal structures through the temporal pole corridor [37]. Approximately 5 to 6 cm of the temporal lobe is exposed in this technique.
The cortical incision begins in the T2, 3 to 3.5 cm from the temporal tip, and curves toward T3 and temporal base. The T1 is usually spared. The temporal tip is removed lateral to the temporal horn. At this stage, the mesial temporal structures are removed using an ultrasonic aspirator. The temporal horn is entered, followed by resection of the uncus and amygdala. Resection of the hippocampus and parahippocampal gyrus is performed from anterior to posterior. The parahippocampal gyrus is removed as it curves medially posterior to the brainstem. The hippocampus is removed posterior to the tail region. After mesial temporal resection, hemostasis is achieved, and the wound is closed in a standard manner.
6. Transcortical Selective Amygdalohippocampectomy
Transcortical SAH was introduced in 1958 by Niemeyer and was originally referred to as “transventricular amygdalohippocampectomy” [38]. Niemeyer used a cortical incision through the T2 to reach the mesial temporal structures. Subsequently, Olivier modified this technique to include resection of the anterior portion of T1 [33, 40].
The head position in this procedure is similar to that used for ATL. A linear or slightly curvilinear skin incision is made anterior to the tragus and above the zygoma. Neuronavigation is a helpful intraoperative tool to tailor the surgical approach, Figure 6. It is applied to navigate the optimal bony exposure over the cortical entry point. Through out the procedure, neuronavigation helps in guiding the surgical pathway to the temporal horn and the posterior extent of mesial temporal resection. However, van Roost et al. found that neuronavigation can overestimate the extent of posterior hippocampal resection, which is related mainly to brain shift during the procedure [41]. While neuronavigation is a useful adjunct, a thorough understanding of the anatomy is essential. On the other hand, intraoperative MRI was found to be helpful to ensure the completeness of hippocampal resection [42].
Figure 6.
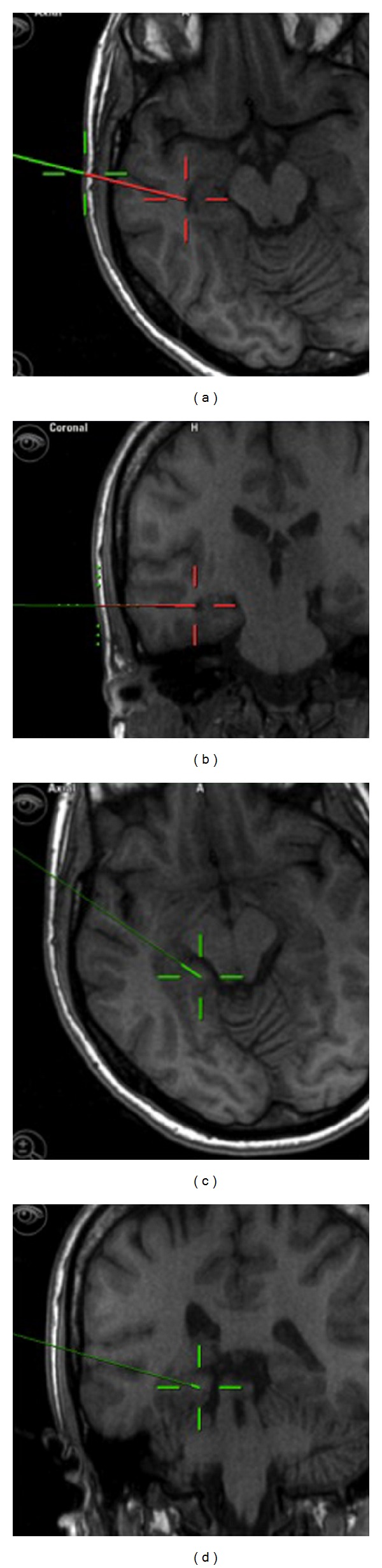
Snapshot from neuronavigation showing the entry point through the middle temporal gyrus and the trajectory toward the temporal horn ((a) and (b)). ((c) and (d)) showed the posterior extent of mesial temporal structures resection at the level of quadrigeminal plate.
After exposure of the bone, neuronavigation can guide to center the craniotomy over the middle temporal gyrus, Figures 6 and 7. Olivier used image guidance to place the cortical incision at the T2, anterior to the central sulcus on the nondominant hemisphere and anterior to the precentral sulcus on the dominant side [33]. The pathway to the ventricle traverses the white matter. The lateral ventricular wall is usually found 2 mm above the fusiform gyrus. The white matter over the ventricle is resected from anterior to posterior in a slit-like fashion, Figure 8. Exposure of the intraventricular structures is performed by applying a retractor that elevates the upper ventricular wall and choroid plexus, Figure 7. This movement exposes the fimbrial attachment to the ambient cistern arachnoid. An ultrasonic aspirator is used at a low setting to remove the parahippocampal gyrus using the endopial technique. The hippocampus is resected at the junction between the body and tail regions, followed by dissection of the fimbria from the arachnoid to allow the lateral elevation of the hippocampus. This procedure exposes the hippocampal sulcus and allows the coagulation of the hippocampal feeders. The uncus is removed beginning with the apex and followed by the regions of the amygdala that are posterior to the M1 segment of the MCA. The residual posterior hippocampus is resected extending to the level of the tectal plate. In this approach, Meyer's loop fibers can be affected by removal of the white matter located lateral to the temporal horn.
Figure 7.
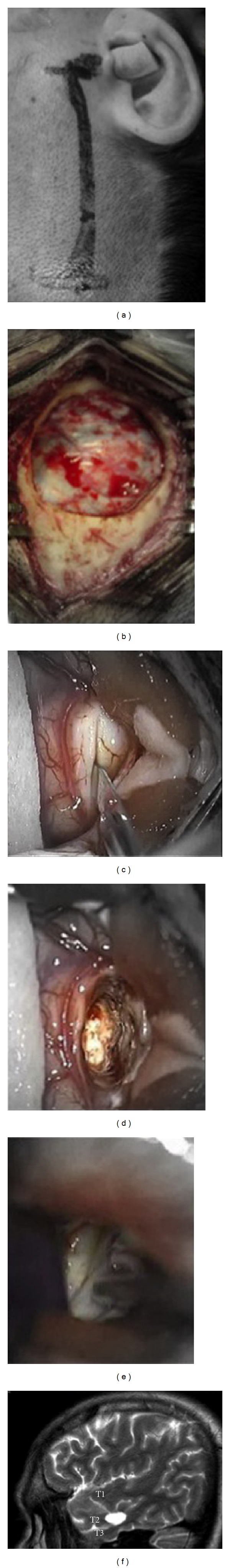
(a) Intraoperative photograph showing site of skin incision for selective amygdalohippocampectomy. (b) Minicraniotomy and dura exposure. (c) Corticectomy at middle temporal gyrus (T2). (d) Transcortical access to temporal horn. (e) Hippocampus exposure. (f) Postoperative sagittal T2 MRI depicting the transcortical access through middle temporal gyrus.
Figure 8.
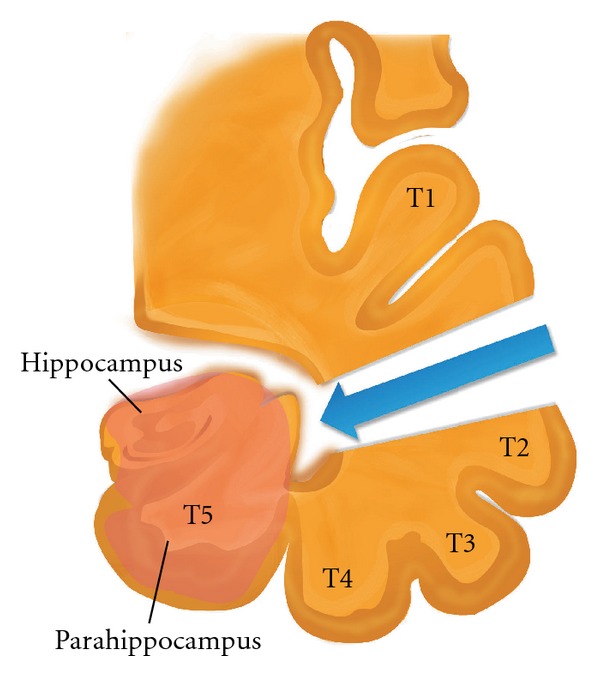
Diagram representing transcortical selective amygdalohippocampectomy approach.
7. Transsylvian Selective Amygdalohippocampectomy
Wieser and Yasargil introduced the transsylvian SAH approach for resecting the mesial temporal structures through the sylvian fissure corridor without compromising the adjacent temporal neocortex [35, 43]. The patient's position is different from that in other temporal procedures: the head is tilted such that the malar eminence is the highest point. A curvilinear skin incision exposes the frontal and temporal bones above and below the sylvian fissure. The sphenoid ridge is flattened to the anterior clinoid process. The dura is opened in a curvilinear fashion and reflected onto the sphenoid ridge. Next, the sylvian fissure is opened from the level of the carotid artery bifurcation through the bifurcation of the MCA, exposing the anterior insular cortex, limen insulae, mesial uncus, and temporal pole. A 15-mm incision is made in the temporal stem at the level of the limen insulae. The temporal horn is entered, and the uncus is removed using an ultrasonic aspirator. This step is followed by the removal of the amygdala, anterior parahippocampus, and entorhinal cortex. The choroid plexus and choroidal point are identified, and the hippocampus is disconnected from the lateral regions in an anterior to posterior manner using (preferably) an ultrasonic aspirator until the collateral sulcus is reached. The fimbria is dissected from the mesial arachnoid using a dissector. The hippocampus is dissected laterally, exposing the hippocampal sulcus, followed by the coagulation of the hippocampal feeders. Finally, a posterior hippocampal resection is performed to remove the hippocampal tissue, hemostasis is secured, and closure is performed.
8. Subtemporal Selective Amygdalohippocampectomy
Subtemporal SAH was first described in 1993 by Hori et al. [44, 45]. This technique involves removing the fusiform gyrus to access the temporal horn and cutting the tentorium to minimize retraction onto the temporal lobe. Later, the same group modified the subtemporal approach, opting for retrolabyrinthine presigmoid transpetrosal access to resect the mesial temporal structures [46]. Shimizu et al. described the removal of the zygomatic arch and the minimal resection of the T3 to access the mesial temporal structures using a zygomatic approach [47]. Park et al. reported a modification of the subtemporal approach that employed transparahippocampal access, thus preserving the fusiform gyrus [48, 49]. Miyamoto and colleagues performed an amygdalohippocampectomy using a combined subtemporal and transventricular-transchoroidal fissure approach [50]. In general, the rationale for using this approach is the avoidance of an incison into the temporal stem and the preservation of the temporal neocortex. This approach, however, risks damaging to the vein of Labbe caused by temporal retraction. Moreover, the limited exposure of the amygdala and uncus limits resection.
9. Other Procedures
Several other surgical procedures have been used to treat TLE. Temporal disconnection has been advocated as an alternative surgical procedure to avoid certain complications while providing a level of seizure control comparable to that of traditional surgery [51]. A study by Chabardes et al. described 47 patients with nonlesional TLE who underwent the temporal disconnection procedure [51]. Of those, 85% were seizure-free 2 years after surgery. Hippocampal transection has been advocated to minimize memory dysfunction following hippocampectomy [52, 53]. Stereotactic ablation and resection of the hippocampus have been reported by several authors [54–60]. Stereotactic radiosurgery has also been used and may be useful for the treatment of MTS related to epilepsy [61, 62]. Neuromodulation, another treatment, involves a combination of neurostimulation, drug delivery, neuronal tissue transplants, and gene therapy. The FDA has approved neurostimulation of the vagus nerve for the treatment of refractory epilepsy; however, the only effective use of this technique in temporal lobe epilepsy remains palliative [63]. Recently, anterior thalamic stimulation was shown to be promising for the treatment of TLE [64]. Hippocampal stimulation performed by the London Ontario group also showed some long-term benefits with no significant negative impact on memory [65, 66]. Recently, responsive cortical stimulation was shown to provide a reduction in seizure frequency in a multicenter, double blind, randomized controlled trial [67].
10. Outcomes and Complications of Resective Surgery
It is difficult to compare the success of various surgical techniques because of the lack of standardized outcome criteria. Overall, 50–70% of patients report no seizures 5 years after surgery [14, 35, 38, 39, 68]. Table 2 summarizes the outcomes of selected studies that have utilized different surgical techniques. It has been suggested that the amount of mesial temporal tissue resected is correlated with successful surgery [69–73]. Residual tissue is a known risk factor for seizure recurrence, and a second operation should be considered in patients who continue to experience seizures. The success in achieving a seizure-free state after a second operation is approximately 50% [74–77]. The effectiveness of residual hippocampal resection and the positive outcomes following SAH suggest that a thorough resection of the hippocampus may be necessary for optimal seizure control. The neuropsychological state and quality of life of patients are most improved when a seizure-free state is achieved [14].
Table 2.
Summary of the surgical outcome from selected studies.
| Author | Year of publication | Follow-up period (years) | Number of patients | Outcome measure | Type of surgery | Percentage of best outcome |
|---|---|---|---|---|---|---|
| Blume and Girvin [19] | 1997 | 5 | 100 | 2-year seizure freedom | ATL | 58% |
| Spencer et al. [20] | 2005 | 5 | 339 | Seizure freedom ± auras for 2 years | AMTL | 69% |
| Jeong et al. [21] | 2005 | 5 | 227 | Engel I | ATL | 75% |
| Urbach et al. [22] | 2004 | 2 | 209 | Engel IA | SAH | 73% |
| Wiebe et al. [14] | 2001 | 1 | 80 | Freedom from seizures that impair awareness | ATL | 58% |
| Mihara et al. [23] | 1996 | 5 | 132 | Engel I | ATL or SAH | 70% |
| Zentner et al. [24] | 1995 | 3 | 178 | Engel I | ATL or SAH | 62% |
| Sperling et al. [25] | 1996 | 5 | 89 | Engel I | ATL | 70% |
| Wieser et al. [26] | 2001 | 7 | 369 | Engel I | SAH | 62% at 5-year follow-up |
| McIntosh et al. [27] | 2004 | 10 | 325 | Engel I | ATL | 41% |
| Paglioli et al. [28] | 2004 | 5 | 135 | Engel IA | ATL or SAH | 74% at 5-year follow-up |
ATL: anterior temporal lobectomy, AMTL: anteromedial temporal lobectomy, SAH: selective amygdalohippocampectomy, Engel: Engel's classification for seizure outcome after surgery.
Operative complications from temporal lobe resective procedures are variable but uncommon. These complications include the following: death (<1%) [78]; infection [79]; mild contralateral superior quadrantanopsia caused by the resection of Meyer's loop fibers in the roof of the temporal horn [78]; hemianopsia caused by injuries to the optic tract or by posterior extension of the white matter (optic radiation fibers) dissection during ATL [80]; postsurgical hematoma [79, 81]; oculomotor and trochlear nerve palsy [82]; rarely, facial nerve palsy [83]. Hemiparesis can occur as the result of the manipulation or thrombosis of the anterior choroidal, MCA or perforators of the PCA. Moreover, hemiparesis can occur from direct injury to the cerebral peduncle and brain stem, or neuroparalytic edema, as described by Penfield et al. [79, 84–86]. Girvin described only one postoperative hemiplegia caused by an internal capsule infarction in a series of 300 cases of ATL [87]. Resection of the dominant temporal lobe rarely produces permanent dysphasia; however, it more frequently causes transient dysphasia [78]. Postoperative dysnomia or aphasia is observed following approximately 30% of dominant temporal lobe resective surgeries; however, most of symptoms usually disappear gradually over a few weeks [88]. Language deficits occur even after cortical language mapping [89, 90]. The causes of transient language dysfunctions are not clear; however, they are more common when resection is performed within 1-2 cm of the language area [91, 92]. Other possible causes include edema caused by brain retraction, the deafferentation of white matter pathways, and ischemia [84, 93].
Global memory deficits are rare following temporal lobe resection, but verbal memory dysfunction occurs more frequently. Postoperative de novo psychiatric disorders have been reported in some cases. A survey of various reports indicates that de novo psychosis occurs in 0.5% to 21% of patients [15, 94–96]. Affective disorders have also been described in the literature: transient mood elevation and emotional changes can occur in the first year after surgery [97, 98], whereas postoperative depression occurs in approximately 10% of patients [99, 100]. Resection of the nondominant temporal lobe may carry a greater risk for depression [101]. Recent systemic review demonstrates that most of the studies showed improvement or no change in the psychiatric outcome after epilepsy surgery [102]. Table 3 summarizes reported complications from selected studies.
Table 3.
Summary of reported temporal lobe surgery complications from selected studies.
| Author (year) | Number of patients | Type of surgery (Number of procedures) | Complications (%) |
|---|---|---|---|
| Clusmann et al. (2002)ϕ [29] | 321 | ATL (98) | Meningitis (1.5%) |
| Transsylvian SAH (138) | Subdural hematoma (0.6%) | ||
| Lesionectomy and AH (27) | Thrombosis (1.2%) | ||
| Lesionectomy/corticectomy (58) | Neurological complications (5.2%) | ||
|
| |||
| Rydenhag and Silander (2001) [30] | 247 | SAH (5) | One mortality (0.4%) |
| ATL (168) | Hemiparesis (2%) | ||
| Neocortical resection (74) | Trochlear nerve palsy (0.8%) | ||
| Oculomotor nerve palsy (0.8%) | |||
|
| |||
| Acar et al. (2008) [31] | 39 | Transcortical SAH (39) | Visual field defect (10%) |
| Fourth nerve palsy (2.5%) | |||
| Hemiparesis (2.5%) | |||
| Aphasia (2.5%) | |||
| Hemotympanum (7.5%) | |||
| Memory difficulty (5%) | |||
| Frontalis nerve palsy (2.5%) | |||
|
| |||
| Jensen (1975)* [32] | 858 | All temporal lobe resective | Persistent hemiparesis (2.4%) |
| surgical procedures (858) | Transient hemiparesis (4.2%) | ||
| Partial hemianopia (46%) | |||
| Complete hemianopia (4%) | |||
| Cranial nerve paresis (3.5%) | |||
| Dysphasia (5%) | |||
| Infection (1.5%) | |||
|
| |||
| Olivier (2000) [33] | 164 | Transcortical SAH (164) | Transient dysphasia (1.8%) |
| Wound infection (0.6%) | |||
| Brain swelling (0.6%) | |||
| Subgaleal effusion (0.6%) | |||
| Abscess (0.6%) | |||
| Third-nerve palsy (0.6%) | |||
| Otitis (3.6%) | |||
|
| |||
| Sindou et al. (2006) [34] | 100 | ATL (76) | Motor deficit (2%) |
| TTL (18) | Hydrocephalus (2%) | ||
| Transsylvian SAH (6) | Postsurgical hematoma (3%) | ||
| Temporary third cranial nerve | |||
| palsy (5%) | |||
| Bacterial meningitis (3%) | |||
| Pulmonary embolism (1%) | |||
ATL: anterior temporal lobectomy; TTL: total temporal lobectomy; AH: amygdalohippocampectomy; SAH: selective amygdalohippocampectomy.
*This data was taken from a survey covering 2282 temporal lobe surgeries worldwide between the period of 1928 and 1973.
ϕNo difference in the complications incidence between different surgical techniques was identified in this study.
11. Conclusion
There are a variety of surgical techniques employed for temporal lobe epilepsy that provide an effective treatment with significant preservation of neurological function and acceptable surgical risks. Regardless, a highly localized epileptic focus predicts the best surgical outcome. Future research should evaluate the etiology and pathology of late epilepsy recurrence.
Acknowledgment
The authors thank Monirah Albloushi, RN, MSN, for the assistance in figures and paper preparation.
References
- 1.Horsley V. Brain surgery. British Medical Journal. 1886;2:670–675. [Google Scholar]
- 2.Macewen W. An address on the surgery of the brain and spinal cord. British Medical Journal. 1888;2(1441):302–309. doi: 10.1136/bmj.2.1441.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause F. Die operative behandlung der epilepsia. Medizinische Klinik. 1909;5:1418–1422. [Google Scholar]
- 4.Foerster O. Zur pathogenese und chirurgischen behandlung der epilepsia. Zentralblatt für Chirurgie. 1925;52:531–549. [Google Scholar]
- 5.Berger H. Über das elektrenkephalogramm des menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929;87(1):527–570. [Google Scholar]
- 6.Penfield W. Temporal lobe epilepsy. The British Journal of Surgery. 1954;41(168):337–343. doi: 10.1002/bjs.18004116802. [DOI] [PubMed] [Google Scholar]
- 7.Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Little Brown and Company; 1954. [Google Scholar]
- 8.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. Archives of Neurology and Psychiatry. 1958;79(5):475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- 10.Penfield W, Flanigin H. Surgical therapy of temporal lobe seizures. Archives of Neurology and Psychiatry. 1950;64(4):491–500. doi: 10.1001/archneurpsyc.1950.02310280003001. [DOI] [PubMed] [Google Scholar]
- 11.Penfield W, Flanigin H. The surgical therapy of temporal lobe seizures. Transactions of the American Neurological Association. 1950;51:146–149. [PubMed] [Google Scholar]
- 12.Penfield W, Baldwin M. Temporal lobe seizures and the technic of subtotal temporal lobectomy. Annals of Surgery. 1952;136(4):625–634. [PMC free article] [PubMed] [Google Scholar]
- 13.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 15.Simmel MI. Clinical and psychological results of anterior temporal lobectomy in patients with psychomotor epilepsy. In: Baily B, editor. Temporal Lobe Epilepsy. Springfield, Ill, USA: Charles C Thomas; 1958. pp. 530–550. [Google Scholar]
- 16.Wen HT, Rhoton AL, de Oliveira E, et al. Microsurgical anatomy of the temporal lobe: part 1: mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45(3):549–592. doi: 10.1097/00006123-199909000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Yasargil MG. A CNS Tumors: Surgical Anatomy, Neuropathology, Neuroradiology, Neurophysiology, Clinical Considerations, Operability, Treatment Options. Vol. 4. Stuttgart, Germany: Thieme; 1996. Microneurosurgery. [Google Scholar]
- 18.Gloor P. The Temporal Lobe and Limbic System. New York, NY, USA: Oxford University Press; 1997. [Google Scholar]
- 19.Blume WT, Girvin JP. Altered seizure patterns after temporal lobectomy. Epilepsia. 1997;38(11):1183–1187. doi: 10.1111/j.1528-1157.1997.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 20.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65(6):912–918. doi: 10.1212/01.wnl.0000176055.45774.71. [DOI] [PubMed] [Google Scholar]
- 21.Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia. 2005;46(8):1273–1279. doi: 10.1111/j.1528-1167.2005.33504.x. [DOI] [PubMed] [Google Scholar]
- 22.Urbach H, Hattingen J, von Oertzen J, et al. MR imaging in the presurgical workup of patients with drug-resistant epilepsy. American Journal of Neuroradiology. 2004;25(6):919–926. [PMC free article] [PubMed] [Google Scholar]
- 23.Mihara T, Inoue Y, Matsuda K, et al. Recommendation of early surgery from the viewpoint of daily quality of life. Epilepsia. 1996;37(supplement 3):33–36. doi: 10.1111/j.1528-1157.1996.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 24.Zentner J, Hufnagel A, Wolf HK, et al. Surgical treatment of temporal lobe epilepsy: clinical, radiological, and histopathological findings in 178 patients. Journal of Neurology Neurosurgery and Psychiatry. 1995;58(6):666–673. doi: 10.1136/jnnp.58.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling MR, O’Connor MJ, Saykin AJ, Plummer C. Temporal lobectomy for refractory epilepsy. Journal of the American Medical Association. 1996;276(6):470–475. [PubMed] [Google Scholar]
- 26.Wieser HG, Blume WT, Fish D, et al. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–286. [PubMed] [Google Scholar]
- 27.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GCA, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(9):2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 28.Paglioli E, Palmini A, Paglioli E, et al. Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. Epilepsia. 2004;45(11):1383–1391. doi: 10.1111/j.0013-9580.2004.22204.x. [DOI] [PubMed] [Google Scholar]
- 29.Clusmann H, Schramm J, Kral T, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. Journal of Neurosurgery. 2002;97(5):1131–1141. doi: 10.3171/jns.2002.97.5.1131. [DOI] [PubMed] [Google Scholar]
- 30.Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990–1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49(1):51–57. doi: 10.1097/00006123-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Acar G, Acar F, Miller J, Spencer DC, Burchiel KJ. Seizure outcome following transcortical selective amygdalohippocampectomy in mesial temporal lobe epilepsy. Stereotactic and Functional Neurosurgery. 2008;86(5):314–319. doi: 10.1159/000160154. [DOI] [PubMed] [Google Scholar]
- 32.Jensen I. Temporal lobe surgery around the world. Results, complications, and mortality. Acta Neurologica Scandinavica. 1975;52(5):354–373. doi: 10.1111/j.1600-0404.1975.tb05831.x. [DOI] [PubMed] [Google Scholar]
- 33.Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Canadian Journal Neurological Sciences. 2000;27(supplement 1):S68–S96. doi: 10.1017/s031716710000069x. [DOI] [PubMed] [Google Scholar]
- 34.Sindou M, Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguière F. Temporo-mesial epilepsy surgery: outcome and complications in 100 consecutive adult patients. Acta Neurochirurgica. 2006;148(1):39–45. doi: 10.1007/s00701-005-0644-x. [DOI] [PubMed] [Google Scholar]
- 35.Wieser HG, Yasargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surgical Neurology. 1982;17(6):445–457. doi: 10.1016/s0090-3019(82)80016-5. [DOI] [PubMed] [Google Scholar]
- 36.Tubbs RS, Miller JH, Cohen-Gadol AA, et al. Intraoperative anatomic landmarks for resection of the amygdala during medial temporal lobe surgery. Neurosurgery. 2010;66(5):974–977. doi: 10.1227/01.NEU.0000368105.64548.71. [DOI] [PubMed] [Google Scholar]
- 37.Spencer DD, Spencer SS, Mattson RH, et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15(5):667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Niemeyer P. The transventricular amygdalohippocapmectomy in temporal lobe epilepsy. In: Bailey P, Baldwin M, editors. Temporal Lobe Epilepsy. Springfield, Ill, USA: Charles C. Thomas; 1958. pp. 461–482. [Google Scholar]
- 39.Tanriverdi T, Olivier A, et al. Long term seizure outcome after mesial temporal lope epilepsy surgery: corticalamegdalohipectomy virus selective amegdalohipectomy. Journal of Neurosurgery. 2008;108(3):517–524. doi: 10.3171/JNS/2008/108/3/0517. [DOI] [PubMed] [Google Scholar]
- 40.Olivier A. Surgical techniques in temporal lobe epilepsy. Clinical Neurosurgery. 1997;44:211–241. [PubMed] [Google Scholar]
- 41.van Roost D, Meyer B, Schramm J, Schaller C. Can neuronavigation contribute to standardization of selective amygdalohippocampectomy? Stereotactic and Functional Neurosurgery. 1997;69(1–4):239–242. doi: 10.1159/000099881. [DOI] [PubMed] [Google Scholar]
- 42.Kaibara T, Myles ST, Lee MA, Sutherland GR. Optimizing epilepsy surgery with intraoperative MR imaging. Epilepsia. 2002;43(4):425–429. doi: 10.1046/j.1528-1157.2002.32401.x. [DOI] [PubMed] [Google Scholar]
- 43.Yasargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Advances and Technical Standards in Neurosurgery. 1985;12:93–123. doi: 10.1007/978-3-7091-7008-3_2. [DOI] [PubMed] [Google Scholar]
- 44.Hori T, Tabuchi S, Kurosaki M, et al. Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery. 1993;33(1):50–57. [PubMed] [Google Scholar]
- 45.Kondo S, Takenobu A, Tabuchi S, Kurosaki M, Okamoto H, Hori T. Subtemporal amygdalohippocampectomy for medically intractable temporal lobe epilepsy. Japanese Journal of Psychiatry and Neurology. 1993;47(2):273–274. doi: 10.1111/j.1440-1819.1993.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 46.Hori T, Kondo S, Takenobu A, et al. Retrolabyrinthine presigmoid transpetrosal approach for selective subtemporal amygdalohippocampectomy. Neurologia Medico-Chirurgica. 1999;39(3):214–225. doi: 10.2176/nmc.39.214. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu H, Suzuki I, Ishijima B. Zygomatic approach for resection of mesial temporal epileptic focus. Neurosurgery. 1989;25(5):798–801. doi: 10.1097/00006123-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Park TS, Bourgeois BFD, Silbergeld DL, Dodson WE. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy: technical note. Journal of Neurosurgery. 1996;85(6):1172–1176. doi: 10.3171/jns.1996.85.6.1172. [DOI] [PubMed] [Google Scholar]
- 49.Robinson S, Park TS, Blackburn LB, Bourgeois BFD, Arnold ST, Dobson WE. Transparahippocampal selective amygdalohippocampectomy in children and adolescents: efficacy of the procedure and cognitive morbidity in patients. Journal of Neurosurgery. 2000;93(3):402–409. doi: 10.3171/jns.2000.93.3.0402. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto S, Kataoka H, Ikeda A, et al. A combined subtemporal and transventricular/transchoroidal fissure approach to medial temporal lesions. Neurosurgery. 2004;54(5):1162–1169. doi: 10.1227/01.neu.0000119234.61432.e8. [DOI] [PubMed] [Google Scholar]
- 51.Chabardes S, Minotti L, Hamelin S, et al. Temporal disconnection as an alternative treatment for intractable temporal lobe epilepsy: techniques, complications and results. Neurochirurgie. 2008;54(3):297–302. doi: 10.1016/j.neuchi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu H, Kawai K, Sunaga S, Sugano H, Yamada T. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. Journal of Clinical Neuroscience. 2006;13(3):322–328. doi: 10.1016/j.jocn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 53.Sunaga S, Morino M, Kusakabe T, et al. Efficacy of hippocampal transection for left temporal lobe epilepsy without hippocampal atrophy. Epilepsy and Behavior. 2011;21(1):94–99. doi: 10.1016/j.yebeh.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Kitchen ND, Thomas DGT, Thompson PJ, Shorvon SD, Fish DR. Open stereotactic amygdalohippocampectomy—clinical, psychometric, and MRI follow-up. Acta Neurochirurgica. 1993;123(1-2):33–38. doi: 10.1007/BF01476282. [DOI] [PubMed] [Google Scholar]
- 55.Parrent AG. Stereotactic radiofrequency ablation for the treatment of gelastic seizures associated with hypothalamic hamartoma: case report. Journal of Neurosurgery. 1999;91(5):881–884. doi: 10.3171/jns.1999.91.5.0881. [DOI] [PubMed] [Google Scholar]
- 56.Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia. 1999;40(10):1408–1416. doi: 10.1111/j.1528-1157.1999.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 57.Miyagi Y, Shima F, Ishido K, et al. Inferior temporal sulcus approach for amygdalohippocampectomy guided by a laser beam of stereotactic navigator. Neurosurgery. 2003;52(5):1117–1124. [PubMed] [Google Scholar]
- 58.Kratimenos GP, Pell MF, Thomas DGT, Shorvon SD, Fish DR, Smith SJN. Open stereotactic selective amygdalo-hippocampectomy for drug resistant epilepsy. Acta Neurochirurgica. 1992;116(2–4):150–154. doi: 10.1007/BF01540868. [DOI] [PubMed] [Google Scholar]
- 59.Kelly PJ, Sharbrough FW, Kall BA, Goerss SJ. Magnetic resonance imaging-based computer-assisted stereotactic resection of the hippocampus and amygdala in patients with temporal lobe epilepsy. Mayo Clinic Proceedings. 1987;62(2):103–108. doi: 10.1016/s0025-6196(12)61877-1. [DOI] [PubMed] [Google Scholar]
- 60.Parrent AG, Lozano AM. Stereotactic surgery for temporal lobe epilepsy. The Canadian Journal of Neurological Sciences. 2000;27(supplement 1):S79–S96. doi: 10.1017/s0317167100000718. [DOI] [PubMed] [Google Scholar]
- 61.Regis J, Bartolomei F, Rey M, et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40(11):1551–1556. doi: 10.1111/j.1528-1157.1999.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 62.Regis J, Roberts DW. Gamma Knife radiosurgery relative to microsurgery: epilepsy. Stereotactic and Functional Neurosurgery. 1999;72(supplement 1):11–21. doi: 10.1159/000056434. [DOI] [PubMed] [Google Scholar]
- 63.Kuba R, Brazdil M, Novak Z, Chrastina J, Rektor I. Effect of vagal nerve stimulation on patients with bitemporal epilepsy. European Journal of Neurology. 2003;10(1):91–94. doi: 10.1046/j.1468-1331.2003.00547.x. [DOI] [PubMed] [Google Scholar]
- 64.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 65.Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006;66(10):1490–1494. doi: 10.1212/01.wnl.0000209300.49308.8f. [DOI] [PubMed] [Google Scholar]
- 66.McLachlan RS, Pigott S, Tellez-Zenteno JF, Wiebe S, Parrent A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. Epilepsia. 2010;51(2):304–307. doi: 10.1111/j.1528-1167.2009.02332.x. [DOI] [PubMed] [Google Scholar]
- 67.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 68.Bien CG, Kurthen M, Baron K, et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42(11):1416–1421. doi: 10.1046/j.1528-1157.2001.43300.x. [DOI] [PubMed] [Google Scholar]
- 69.Awad IA, Katz A, Hahn JF, Kong AK, Ahl J, Luders H. Extent of resection in temporal lobectomy for epilesy. I. Interobserver analysis and correlation with seizure outcome. Epilepsia. 1989;30(6):756–762. doi: 10.1111/j.1528-1157.1989.tb05335.x. [DOI] [PubMed] [Google Scholar]
- 70.Bonilha L, Kobayashi E, Mattos JPV, Honorato DC, Li LM, Cendes F. Value of extent of hippocampal resection in the surgical treatment of temporal lobe epilepsy. Arquivos de Neuro-Psiquiatria. 2004;62(1):15–20. doi: 10.1590/s0004-282x2004000100003. [DOI] [PubMed] [Google Scholar]
- 71.Nayel MH, Awad IA, Luders H. Extent of mesiobasal resection determines outcome after temporal lobectomy for intractable complex partial seizures. Neurosurgery. 1991;29(1):55–61. doi: 10.1097/00006123-199107000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Renowden SA, Matkovic Z, Adams CBT, et al. Selective amygdalohippocampectomy for hippocampal sclerosis: postoperative MR appearance. American Journal of Neuroradiology. 1995;16(9):1855–1861. [PMC free article] [PubMed] [Google Scholar]
- 73.Wyler AR, Hermann BP, Somes G, Spencer DD, Roberts DW, Engel J. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37(5):982–991. doi: 10.1227/00006123-199511000-00019. [DOI] [PubMed] [Google Scholar]
- 74.Awad IA, Nayel MH, Luders H. Second operation after the failure of previous resection for epilepsy. Neurosurgery. 1991;28(4):510–518. doi: 10.1097/00006123-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Germano IM, Poulin N, Olivier A. Reoperation for recurrent temporal lobe epilepsy. Journal of Neurosurgery. 1994;81(1):31–36. doi: 10.3171/jns.1994.81.1.0031. [DOI] [PubMed] [Google Scholar]
- 76.Hennessy MJ, Elwes RDC, Binnie CD, Polkey CE. Failed surgery for epilepsy: a study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123, part 12:2445–2466. doi: 10.1093/brain/123.12.2445. [DOI] [PubMed] [Google Scholar]
- 77.Wyler AR, Hermann BP, Richey ET. Results of reoperation for failed epilepsy surgery. Journal of Neurosurgery. 1989;71(6):815–819. doi: 10.3171/jns.1989.71.6.0815. [DOI] [PubMed] [Google Scholar]
- 78.Pilcher WH, Rusyniak WG. Complications of epilepsy surgery. Neurosurgery Clinics of North America. 1993;4(2):311–325. [PubMed] [Google Scholar]
- 79.Olivier A. Risk and benefit in the surgery of epilepsy: complications and positive results on seizures tendency and intellectual function. Acta Neurologica Scandinavica. 1988;78(supplement 117):114–121. doi: 10.1111/j.1600-0404.1988.tb08012.x. [DOI] [PubMed] [Google Scholar]
- 80.Katz A, Awad IA, Kong AK, et al. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30(6):763–771. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 81.Sasaki-Adams D, Hader EJ. Temporal lope epilepsy surgery: sugical complications. In: Luders H, editor. Textbook of Epilepsy Surgery. London, UK: Informa Healthcare; 2008. pp. 1288–1298. [Google Scholar]
- 82.Van B. Complications of surgical procedures in the diagnosis and treatment of epilepsy. In: Engel J, editor. Surgical Treratment of the Epilepsies. New York, NY, USA: Raven Press; 1987. pp. 465–475. [Google Scholar]
- 83.Anderson J, Awad IA, Hahn JF. Delayed facial nerve palsy after temporal lobectomy for epilepsy: report of four cases and discussion of possible mechanisms. Neurosurgery. 1991;28(3):453–456. doi: 10.1097/00006123-199103000-00022. [DOI] [PubMed] [Google Scholar]
- 84.Penfield W. Pitfalls and success in surgical treatment of focal epilepsy. British Medical Journal. 1958;1(5072):669–672. doi: 10.1136/bmj.1.5072.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Penfield R. Manipulation hemiplegia. Journal of Neurosurgery. 1961;18:760–776. [Google Scholar]
- 86.Penfield W, Paine K. Results of surgical therapy for focal epileptic seizures. Canadian Medical Association journal. 1955;73(7):515–531. [PMC free article] [PubMed] [Google Scholar]
- 87.Girvin JP. Complications of epilepsy surgery. In: Luders H, editor. Epilepsy Surgery. New York, NY, USA: Raven Press; 1991. pp. 653–660. [Google Scholar]
- 88.Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Archives of Neurology. 1996;53(1):72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- 89.Stafiniak P, Saykin AJ, Sperling MR, et al. Acute naming deficits following dominant temporal lobectomy: prediction by age at 1st risk for seizures. Neurology. 1990;40(10):1509–1512. doi: 10.1212/wnl.40.10.1509. [DOI] [PubMed] [Google Scholar]
- 90.Wyllie E, Luders H, Morris HH, et al. Clinical outcome after complete or partial cortical resection for intractable epilepsy. Neurology. 1987;37(10):1634–1641. doi: 10.1212/wnl.37.10.1634. [DOI] [PubMed] [Google Scholar]
- 91.Ojemann GA. Intraoperative techniques for reducing language and memory deficits with left temporal lobectomy. In: Ojemann GA, editor. Advances in Epileptology. New York, NY, USA: Raven Press; 1987. pp. 327–330. [Google Scholar]
- 92.Ojemann GA. Surgical therapy for medically intractable epilepsy. Journal of Neurosurgery. 1987;66(4):489–499. doi: 10.3171/jns.1987.66.4.0489. [DOI] [PubMed] [Google Scholar]
- 93.Helgason CM, Bergen D, Bleck TP, Morrell F, Whisler W. Infarction after surgery for focal epilepsy: manipulation hemiplegia revisited. Epilepsia. 1987;28(4):340–345. doi: 10.1111/j.1528-1157.1987.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 94.Mace CJ, Trimble MR. Psychosis following temporal lobe surgery: a report of six cases. Journal of Neurology Neurosurgery and Psychiatry. 1991;54(7):639–644. doi: 10.1136/jnnp.54.7.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trimble MR. Behavior changes following temporal lobectomy, with special reference to psychosis. Journal of Neurology Neurosurgery and Psychiatry. 1992;55(2):89–91. doi: 10.1136/jnnp.55.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson SM. Postoperative psychiatric changes. In: Engel J, editor. Surgical Treatment of the Epilepsies. New York, NY, USA: Raven Press; 1993. pp. 649–661. [Google Scholar]
- 97.Krahn LE, Rummans TA, Peterson GC. Psychiatrie implications of surgical treatment of epilepsy. Mayo Clinic Proceedings. 1996;71(12):1201–1204. doi: 10.4065/71.12.1201. [DOI] [PubMed] [Google Scholar]
- 98.Fraser RT. Improving functional rehabilitation outcome following epilepsy surgery. Acta Neurologica Scandinavica. 1988;78(supplement 117):122–128. doi: 10.1111/j.1600-0404.1988.tb08013.x. [DOI] [PubMed] [Google Scholar]
- 99.Naylor AS, Rogvi-Hansen B, Kessing L, Kruse-Larsen C. Psychiatric morbidity after surgery for epilepsy: short term follow up of patients undergoing amygdalohippocampectomy. Journal of Neurology Neurosurgery and Psychiatry. 1994;57(11):1375–1381. doi: 10.1136/jnnp.57.11.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bruton CJ. The Neuropathology of Temporal Lobe Epilepsy. New York, NY, USA: Oxford University Press; 1988. [Google Scholar]
- 101.Fenwick P. Presurgical psychiatric assessment. In: Engel J, editor. Surgical Treatment of the Epilepsies. New York, NY, USA: Raven Press; 1993. pp. 649–661. [Google Scholar]
- 102.Macrodimitris S, Sherman EMS, Forde S, et al. Psychiatric outcomes of epilepsy surgery: a systematic review. Epilepsia. 2011;52(5):880–890. doi: 10.1111/j.1528-1167.2011.03014.x. [DOI] [PubMed] [Google Scholar]


