Abstract
Despite accelerated epithelial closure, oral mucosal wounds exhibit lower levels of VEGF and a more refined angiogenic response than do skin wounds. The specific differences in angiogenesis suggest that skin and oral mucosal wounds may experience dissimilar levels of hypoxia and HIF-1α. Using a model of comparable wounds on murine dorsal skin and tongue, we determined levels of hypoxia and HIF-1α. Skin wounds were found to be significantly more hypoxic and had higher levels of HIF-1α than mucosal wounds. Furthermore, under stressed conditions, skin wounds, but not mucosal wounds, exhibited a further elevation of HIF-1α beyond that of non-stressed levels. To determine if manipulation of oxygen levels might equalize the repair response of each tissue, we exposed mice to hyperbaric oxygen treatment (HBOT) following wounding. HBOT did not significantly change HIF-1α or VEGF expression in either skin or mucosal wounds, nor did it alter wound bed vascularity. These studies suggest that skin wounds have higher levels of hypoxia than do mucosal wounds, along with a differential expression of HIF-1α. Interestingly, modulation of oxygen by HBOT does not ameliorate this difference. These results suggest that differential responses to hypoxia may underlie the distinctive wound-healing phenotypes seen in skin and oral mucosa.
Keywords: hypoxia-inducible factor-1α, wound and injuries, oxygen, stress, skin, mucous membrane
Introduction
Wounds usually cause interruption in local tissue perfusion, which results in a decline in tissue hypoxia. Hypoxia has been shown to induce critical factors that stimulate proliferation and migration of endothelial cells, keratinocytes, and fibroblasts in wounds (Tandara and Mustoe, 2004). Cellular responses to hypoxia are mediated by hypoxia-inducible factor (HIF)-1 signaling. HIF-1 is a heterodimeric transcription factor complex composed of HIF-1α and HIF-1β subunits (Manalo et al., 2005; Andrikopoulou et al., 2011). HIF-1α expression increases exponentially as oxygen tension declines (Jiang et al., 1996), whereas HIF-1β is constitutively expressed (Manalo et al., 2005). In the presence of sufficient O2, HIF-1α is hydroxylated and degraded (Semenza and Wang, 1992; Tandara and Mustoe, 2004; Manalo et al., 2005; Sen, 2009; Andrikopoulou et al., 2011). When oxygen tension falls, HIF-1α is stabilized against degradation, translocates to the nucleus, and, upon dimerization with HIF-1β, activates transcription of angiogenic factors, notably VEGF, as well as molecules involved in cell survival/proliferation, erythropoiesis/iron metabolism, vascular tone, glucose, and matrix metabolism (Semenza and Wang, 1992; Tandara and Mustoe, 2004; Manalo et al., 2005; Sen, 2009; Andrikopoulou et al., 2011).
It has been well-documented that oral mucosal wounds heal faster than skin wounds, and the parameter of wound closure is demonstrably faster in oral mucosa than in skin (Sciubba et al., 1978; Szpaderska et al., 2003; Schrementi et al., 2008; Chen et al., 2010). Our previous studies indicate that oral mucosal wounds have a lower level of VEGF and a less robust increase in wound vascularity than do skin wounds (Szpaderska et al., 2005). Expression of HIF-1α in skin wounds has been reported (Mace et al., 2007; Botusan et al., 2008; Owings et al., 2009). However, it remains unclear if the level of hypoxia and the regulation of HIF-1α are different in skin and mucosal wounds. In the present study, we examined levels of hypoxia and HIF-1α in mucosal and skin wounds, and looked at how HIF-1α is regulated under conditions of stress and hyperbaric oxygen therapy. Our studies demonstrated that skin wounds have higher levels of hypoxia and HIF-1α than do mucosal wounds. Under stressed conditions, skin wounds, but not mucosal wounds, exhibit a marked elevation of HIF-1α. HBOT did not have a significant impact on HIF-1α, VEGF, or vascularity in either acute skin or mucosal wound-healing.
Materials & Methods
Animals and Wound Models
Six- to 8-week old female Balb/c mice (Harlan, Inc., Indianapolis, IN, USA) were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg). For skin wounds, one 5-mm dermal incisional wound was placed on the right side of the dorsal skin. For tongue wounds, the same-sized wound was placed on the anterior one-third of the dorsal surface of the tongue. Skin and tongue wounds were placed on different mice. Incisional wounds were used because of ease of identification and uniformity of preparation. Six and 24 hrs after wounding occurred, the wound and surrounding tissues were removed and stored in RNAlater (Sigma, St. Louis, MO, USA) or OCT compound for future procedures. Normal skin and tongue tissues were also collected for comparison. All animal procedures were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Restraint Stress Paradigm
Mice underwent 3 cycles of restraint stress as described previously (Mercado et al., 2002a,b; Rojas et al., 2002; Eijkelkamp et al., 2007) before skin and tongue injury. Six and 24 hrs after wounding occurred, wound samples were harvested. (For detailed methods, see the Appendix.)
Hyperbaric Oxygen Treatment (HBOT)
Mice were subjected to HBOT after being wounded. [The details of the methods are included in the Appendix and reference (Gajendrareddy et al., 2005).] Six-hour samples were obtained after 2 treatments (1 hr apart), and 24-hour samples were harvested after 4 treatments. Day 7 samples were obtained from mice that received HBOT each day on days 0 to 5 post-wounding, for a total of 10 treatments.
Hypoxyprobe Staining
Hypoxia in tissues was assessed as we previously described (Radek et al., 2008). (The details of the methods are included in the Appendix and reference.)
Microarray
Microarray analysis was performed as previously described (Chen et al., 2010). (The details of the methods are included in the Appendix and reference.)
Real-time PCR, Indirect Immunofluorescence Detection of HIF-1α, CD31, and Nuclear HIF-1α ELISA
The details of the methods are included in the Appendix.
Statistical Analyses
We used a one-way analysis of variance (ANOVA) to assess the microarray and real-time PCR analysis of HIF-1α expression. A t test was used for the remaining experiments. Statistical analysis was completed with GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA, USA). N = 5 for each group. p values less than 0.05 were considered statistically significant.
Results
Levels of Hypoxia Are Higher in Skin Wounds than in Mucosal Wounds
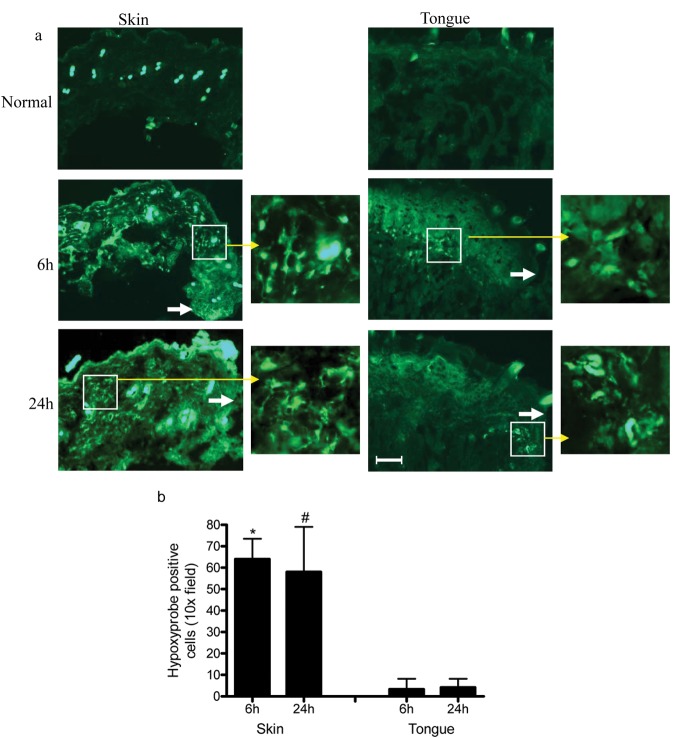
The number of hypoxyprobe-positive cells was used as an indicator of hypoxia in skin and mucosal wounds. Whereas skin wounds exhibited 64.3 ± 9.5 and 57.8 ± 21.5 hypoxyprobe-positive cells per 10x field at 6 and 24 hrs, respectively, after wounding occurred (Fig. 1a), tongue wounds exhibited significantly lower numbers of hypoxic cells (3.3 ± 4.9 and 4.2 ± 4.0 at 6 and 24 hrs, respectively; Fig. 1b, p < 0.0001 and p = 0.0005). These results indicate that skin wounds experience significantly more hypoxia than do mucosal wounds of similar size.
Figure 1.
Hypoxia is more apparent in skin wounds than in mucosal wounds. Five-mm incisional wounds were made on tongue or dorsal skin. Mice were injected intraperitoneally with hypoxyprobe-1α 10 min before and 6 and 24 hrs after samples were harvested. Hypoxyprobe was detected by indirect immunofluorescence. Positively stained cells were counted in 10x field. (a) Representative microphotographs of hypoxyprobe staining of normal, 6- and 24-hour-wounded skin and tongue tissues. White arrows indicate the location of the original wound edges. Yellow arrows indicate higher magnification of the boxed areas on adjacent photos. (b) Summary of numbers of hypoxyprobe-positive stained cells. n = 5, *p < 0.0001 between 6-hour skin and tongue wounds, #p = 0.0005 between 24-hour skin and tongue wounds. Results demonstrate that there is more hypoxia in skin than in tongue wounds. Scale bar: 200 µm.
HIF-1α Is Significantly Up-regulated in Skin Wounds Compared with Mucosal Wounds
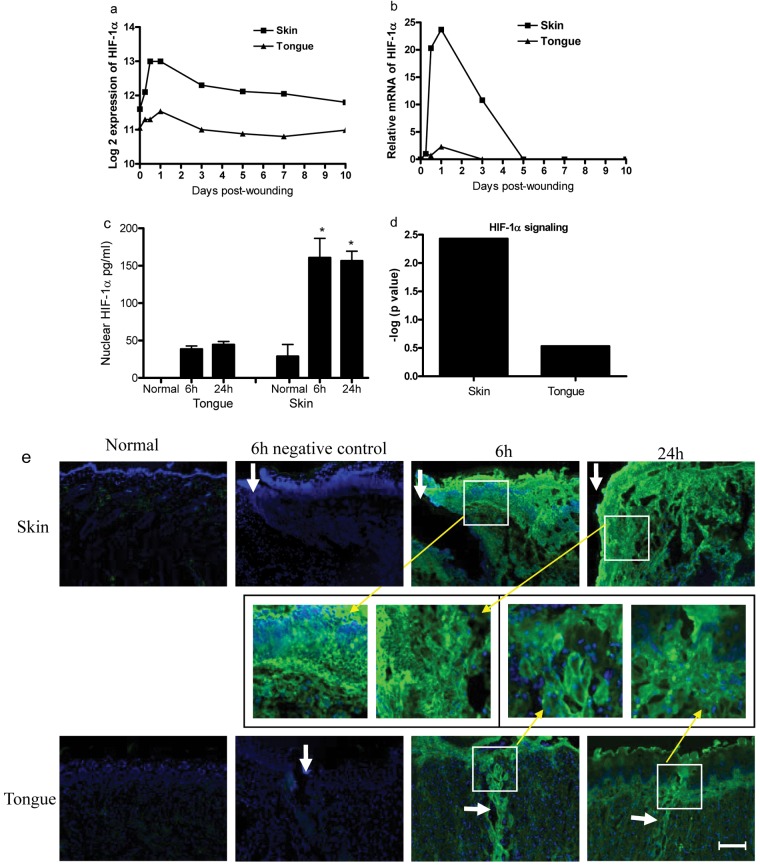
Having demonstrated that hypoxia is more severe in skin than in mucosal wounds, we investigated if HIF-1α, a major transcriptional regulator of VEGF, was differentially expressed at these 2 locations. Analysis of our existing microarray database (Chen et al., 2010) demonstrated that HIF-1α expression was dramatically elevated at the early time-points after skin-wounding occurred (p = 2.56e-07). HIF-1α expression was also significantly increased over baseline in tongue wounds at these time-points, but on a much smaller scale (p = 0.03, Fig. 2a). To verify the results obtained from the microarray, we also performed relative mRNA expression using real-time PCR. This analysis confirmed the patterns (Fig. 2b) demonstrated by the microarray. To compare the levels of HIF-1α protein in skin and tongue wound tissues, we used indirect immunofluorescence. As shown in Fig. 2e, HIF-1α staining was much stronger at the edges of skin wounds than in tongue wounds at both 6 and 24 hrs after wounding occurred. Both epithelial and mesenchymal cells were observed to express HIF-1α. Since functional HIF-1α is located in the nucleus, we determined the nuclear levels of HIF-1α by ELISA. Levels of nuclear HIF-1α were significantly greater in skin vs. tongue wounds at both 6 and 24 hrs after wounding occurred (160.5 ± 26.7 and 156.4 ± 13.4 pg/mL in skin vs. 38.4 ± 4.4 and 44.4 ± 4.3 pg/mL in the tongue at 6 and 24 hrs, respectively, p < 0.0001, Fig. 2c). Nuclear HIF-1α was undetectable in normal tongue tissue (Fig. 2c). Our previous microarray analysis (Chen et al., 2010) identified 1,479 and 502 differentially expressed genes in skin and tongue wounds, respectively, by using criteria of absolute fold change ≥ 2 and FDR corrected p value < 1E-5 as compared with normal tissue. Utilizing IPA software (Ingenuity® Systems, www.ingenuity.com, Redwood City, CA, USA), we found that skin wounds expressed a high level of genetic elements involved in HIF-1α signaling. Skin expressed 15 molecules (SLC2A1, PIK3R1, MMP10, MMP13, HIF1α, NOS3, SLC2A3, PGF, MMP23B, RRAS2, MMP8, EGLN3, PIK3R2, MMP9, and MMP1) involved in HIF-1α signaling (–log p value of 2.4), while tongue wounds exhibited only 4 associated genes (SLC2A1, PIK3R1, PIK3R5, MMP10) involved in the same pathway ( –log p value of 0.5) (Fig. 2d, Appendix Fig.). Together, the results suggest that skin injury induces significantly higher expression of HIF-1α and the HIF-1α signaling pathway than does tongue injury. This differential activity may partially explain the more robust angiogenesis seen in skin vs. mucosal wounds (Szpaderska et al., 2005).
Figure 2.
Up-regulated HIF-1α gene expression in wounds. (a) Microarray analysis of HIF-1α gene expression kinetics in skin and tongue wounds. (b) Validation of HIF-1α mRNA by real-time PCR. (c) Levels of HIF-1α nuclear protein in skin and tongue wounds examined by ELISA. *p < 0.0001 compared with unwounded skin, 6 hrs and 24 hrs in tongue. (d) -log p values of HIF-1α signaling pathway involved in wound healing. (e) Indirect immunofluorescence staining of HIF-1α in 6- and 24-hour 5-mm incisional wounds. White arrows indicate the location of the original wound edges or wounds. Yellow arrows indicate higher magnification of the marked parts. Scale bar: 200 µm.
Stress Induces Significant Expression of HIF-1α in Skin Wounds
Since skin and mucosal wounds had different levels of hypoxia and HIF-1α, we wondered if wound levels of HIF-1α would be regulated differently in these 2 tissues under specific physiological or pathological conditions. HIF-1α mRNA expression was compared in skin and tongue wounds in mice subjected to an established restraint stress protocol for 3 days prior to being wounded. As shown in Fig. 3, HIF-1α expression in the skin wounds was up-regulated 15-fold at 6 hrs after wounding in stressed mice compared with control mice (p < 0.01). The elevation disappeared 24 hrs after wounding occurred. However, in tongue wounds, only a slight, but not significant, increase of HIF-1α was seen at 6 hrs after wounding in stressed vs. control non-stressed mice (p > 0.05). The results indicate that the response to stress affects HIF-1α expression differently in skin and mucosal wounds.
Figure 3.
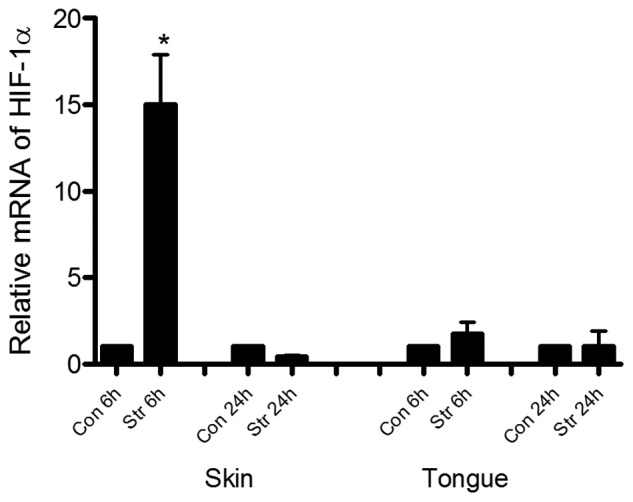
Stress up-regulates HIF-1α expression in skin but not in tongue wounds. Mice were subjected to restraint stress for 3 cycles as described in Materials & Methods. Six and 24 hrs after wounding occurred, wounded tissues from skin and tongue were harvested, and real-time PCR was utilized to analyze mRNA expression of HIF-1α. *p < 0.001 compared with control group. Con: control. Str: stressed.
HBOT Does Not Alter HIF-1α or VEGF Expression in Either Skin or Tongue Wounds
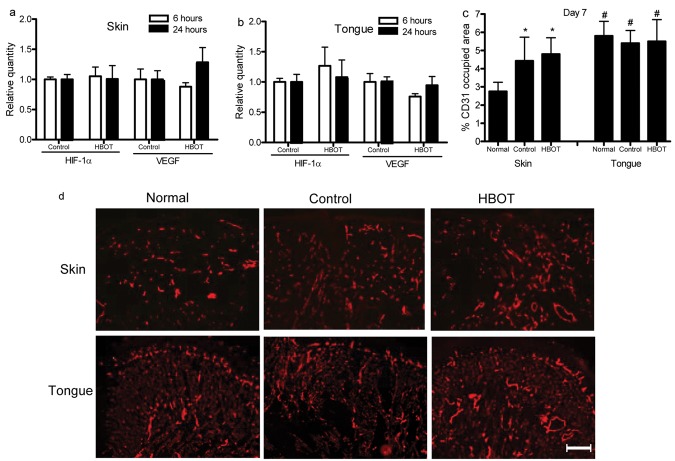
The above results suggested that skin and tongue wounds differ in levels of hypoxia (Fig. 1) and in the production of HIF-1α (Fig. 2). Moreover, when mice were exposed to stress, skin wounds responded with a marked up-regulation of HIF-1α as compared with mucosal wounds (Fig. 3). Given these observations, we hypothesized that HBOT, a treatment known to alter tissue oxygen levels, might modify both HIF-1α and VEGF expression in wounds. In particular, we reasoned that HBOT might have a beneficial effect on skin wound-healing, perhaps converting it to a more mucosal-like phenotype. To examine this concept, we subjected mice to HBOT, and HIF-α and VEGF mRNA expression was examined in wounds. HBOT did not significantly alter HIF-1α or VEGF mRNA expression in either skin or tongue wounds (Figs. 4a, 4b). The effect of HBOT on capillary density in wounds was also examined. In baseline uninjured tissues, the percentage dermal area in skin occupied by vessels was 2.75 ± 0.5%. In contrast, the tongue was significantly more vascular at baseline, with 5.8 ± 0.8% of the subepithelial lamina propria occupied by vessels (p < 0.001). At day 7 after wounding occurred, skin wounds of both HBOT-treated and control groups showed similar levels of vascularity which were significantly higher than those of normal skin (p < 0.05) (Figs. 4c, 4d). Tongue wounds, however, in both HBOT-treated and control mice, exhibited levels of vascularity that were similar to those of the normal uninjured tongue (Figs. 4c, 4d). These results suggest that HBOT has no effect on HIF-1α or VEGF expression and vascularity in skin and tongue wounds.
Figure 4.
Hyperbaric oxygen therapy (HBOT) does not change HIF-1α and VEGF expression in skin and tongue wounds. After skin and tongue injury, mice were subjected to HBOT as described in Materials & Methods. Six and 24 hrs later, wounds were collected, and mRNA levels of HIF-1α and VEGF were examined by real-time PCR. Seven days after wounding occurred, wounded sections were stained for CD31 by indirect immunofluorescence. (a) mRNA expression of HIF-1α and VEGF in skin wounds. (b) mRNA expression of HIF-1α and VEGF in tongue wounds. (c) Percentage of CD31-occupied area in wound bedsat day 7. *p < 0.05, # p < 0.01 compared with normal skin. (d) Representative photomicrographs of CD31 staining in normal skin/tongue, control, and HBOT groups. Scale bar: 200 µm.
Discussion
To the best of our knowledge, the present study is the first demonstration of differences in the levels of hypoxia in comparable skin and oral mucosal wounds. Our experiments show that expression of HIF-1α, a critical transcription factor involved in the maintenance of oxygen homeostasis, differs in skin and mucosal wounds. Moreover, HIF-1α was differently regulated under conditions of stress. Interestingly, HBOT, a treatment that would seem likely to ameliorate wound hypoxia, did not markedly effect HIF-1α or VEGF production in either the skin or mucosal acute wounds.
An injury to the skin or mucosa damages the integrity of tissues and disturbs the oxygen supply. Oxygen is an essential element for successful wound healing (Tandara and Mustoe, 2004; Sen, 2009), since reparative processes such as cell proliferation, bacterial defense, angiogenesis, and collagen synthesis demand oxygen (Tandara and Mustoe, 2004; Sen, 2009). Hypoxia sensing and the tissue response to hypoxia are primarily controlled through the HIF system (Sen, 2009). The HIF system is a critical regulator of many other pathophysiological processes, including angiogenesis in wound healing and tumor development (Rey and Semenza, 2010; Schreml et al., 2010). HIF-1α dysregulation has been shown in poorly healing chronic wounds, especially diabetic wounds (Mace et al., 2007; Botusan et al., 2008; Thangarajah et al., 2010). HIF-1α is significantly decreased in diabetic wounds, and its sustained expression or stabilization can improve diabetic wound-healing (Mace et al., 2007; Botusan et al., 2008). These studies have been interpreted to suggest that high levels of HIF-1α are critical to optimal healing.
The current findings suggest that, in certain tissues, hypoxia and the resultant increase in HIF-1α are not required for adequate tissue repair. The present study demonstrates that mucosal wounds, a tissue known to heal rapidly and with minimal scar formation, heal under conditions of significantly less hypoxia than do skin wounds. Some of this difference may derive from baseline differences, since the normal tongue has a higher baseline vascularity than does skin. The lower level of hypoxia in mucosal wounds may explain why HIF-1α expression is lower in mucosal than in skin wounds. Decreased hypoxia may also explain why the expression of VEGF, a factor downstream of HIF-1α, is reduced in tongue vs. skin wounds (Szpaderska et al., 2005).
Stress has been extensively reported to impair both skin and mucosal wound-healing (Marucha et al., 1998; Gajendrareddy et al., 2005; Eijkelkamp et al., 2007). Restraint stress impairs wound-healing in mice, partially through stress-induced release of glucocorticoid and catecholamines, and alterations in cutaneous pro-inflammatory cytokine and growth factor gene expression (Mercado et al., 2002a; Eijkelkamp et al., 2007). Our results show that restraint stress significantly up-regulated HIF-1α expression in skin, but had little effect in tongue wounds. Since HIF-1α is an oxygen-sensitive molecule, stress may increase hypoxia, a suggestion that needs further study.
Hypoxia is present in both acute and chronic wounds and has been assumed to be a driving force in the healing process. Yet compelling evidence supports the idea that tissue hypoxia has a negative net impact on wound closure (Modarressi et al., 2010). Our studies support the emerging concept that hypoxia is not required for successful repair (Sen, 2003; Hunt et al., 2004; Gajendrareddy et al., 2005; Gordillo et al., 2008). However, hypoxia may play a supporting role in wound-healing by generating growth and repair factors that support wound closure in an acute wound environment (Sen, 2009; Sen and Roy, 2010). Supplemental oxygen treatment has been extensively studied and has been proven beneficial for wound repair (Sen, 2003; Hunt et al., 2004; Gajendrareddy et al., 2005; Gordillo et al., 2008). Sheikh and colleagues demonstrated that hyperoxia rendered by HBOT increased VEGF production in day 5 skin wounds in a rat model, although not at day 2 (Sheikh et al., 2000). The same group found that HBOT improves microvascular perfusion at day 7 and day 10 after wounding in a murine wound model (Sheikh et al., 2005). HBOT was able to ameliorate the effect of impaired wound-healing in stressed mice. However, HBOT did not affect the wounds of control animals (Gajendrareddy et al., 2005). Our findings demonstrate that HBOT does not significantly change wound HIF-1α or VEGF gene expression, or vascularity, in either tongue or skin. Although this lack of effect differs from studies of chronic or ischemic wounds, the results are consistent with a large amount of evidence to suggest that additional or supplemental treatments of any kind have minimal impact on the healing of acute wounds in healthy individuals.
In conclusion, a significantly higher level of hypoxia occurs in skin wounds than in mucosal wounds at the early phase of wound-healing, which may lead to differential expression of HIF1α. Stress further up-regulates HIF-1α in skin wounds but not in tongue wounds. Finally, HBOT does not influence HIF-1α gene expression, its downstream effector molecule VEGF, or vascularity in either skin or mucosal wounds.
Acknowledgments
The authors thank Dr. Wendy Cerny for her critical review of the manuscript.
Footnotes
This publication was supported by NIH Grants RO1-GM50875 (LAD) and P20-GM078426 (LAD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
The author(s) declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Andrikopoulou E, Zhang X, Sebastian R, Marti G, Liu L, Milner SM, et al. (2011). Current insights into the role of HIF-1 in cutaneous wound healing. Curr Mol Med 11:218-235 [DOI] [PubMed] [Google Scholar]
- Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, et al. (2008). Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 105:19426-19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. (2010). Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 11:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Engeland CG, Gajendrareddy PK, Marucha PT. (2007). Restraint stress impairs early wound healing in mice via alpha-adrenergic but not beta-adrenergic receptors. Brain Behav Immun 21:409-412 [DOI] [PubMed] [Google Scholar]
- Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. (2005). Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun 19:217-222 [DOI] [PubMed] [Google Scholar]
- Gordillo GM, Roy S, Khanna S, Schlanger R, Khandelwal S, Phillips G, et al. (2008). Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp Pharmacol Physiol 35:957-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Ellison EC, Sen CK. (2004). Oxygen: at the foundation of wound healing—introduction. World J Surg 28:291-293 [DOI] [PubMed] [Google Scholar]
- Jiang BH, Semenza GL, Bauer C, Marti HH. (1996). Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271(4 Pt 1):C1172-C1180 [DOI] [PubMed] [Google Scholar]
- Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. (2007). Sustained expression of HIF-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen 15:636-645 [DOI] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. (2005). Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659-669 [DOI] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. (1998). Mucosal wound healing is impaired by examination stress. Psychosom Med 60:362-365 [DOI] [PubMed] [Google Scholar]
- Mercado AM, Padgett DA, Sheridan JF, Marucha PT. (2002a). Altered kinetics of IL-1 alpha, IL-1 beta, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav Immun 16:150-162 [DOI] [PubMed] [Google Scholar]
- Mercado AM, Quan N, Padgett DA, Sheridan JF, Marucha PT. (2002b). Restraint stress alters the expression of interleukin-1 and keratinocyte growth factor at the wound site: an in situ hybridization study. J Neuroimmunol 129:74-83 [DOI] [PubMed] [Google Scholar]
- Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, Hinz B. (2010). Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol 130:2818-2827 [DOI] [PubMed] [Google Scholar]
- Owings RA, Boerma M, Wang J, Berbee M, Laderoute KR, Soderberg LS, et al. (2009). Selective deficiency of HIF-1alpha in myeloid cells influences secondary intention wound healing in mouse skin. In Vivo 23:879-884 [PubMed] [Google Scholar]
- Radek KA, Kovacs EJ, Gallo RL, DiPietro LA. (2008). Acute ethanol exposure disrupts VEGF receptor cell signaling in endothelial cells. Am J Physiol Heart Circ Physiol 295(1):H174-H184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Semenza GL. (2010). Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86:236-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas IG, Padgett DA, Sheridan JF, Marucha PT. (2002). Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun 16:74-84 [DOI] [PubMed] [Google Scholar]
- Schrementi ME, Ferreira AM, Zender C, DiPietro LA. (2008). Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen 16:80-86 [DOI] [PubMed] [Google Scholar]
- Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. (2010). Oxygen in acute and chronic wound healing. Br J Dermatol 163:257-268 [DOI] [PubMed] [Google Scholar]
- Sciubba JJ, Waterhouse JP, Meyer J. (1978). A fine structural comparison of the healing of incisional wounds of mucosa and skin. J Oral Pathol 7:214-227 [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. (1992). A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447-5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK. (2003). The general case for redox control of wound repair. Wound Repair Regen 11:431-438 [DOI] [PubMed] [Google Scholar]
- Sen CK. (2009). Wound healing essentials: let there be oxygen. Wound Repair Regen 17:1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Roy S. (2010). Oxygenation state as a driver of myofibroblast differentiation and wound contraction: hypoxia impairs wound closure. J Invest Dermatol 130:2701-2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AY, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK. (2000). Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg 135:1293-1297 [DOI] [PubMed] [Google Scholar]
- Sheikh AY, Rollins MD, Hopf HW, Hunt TK. (2005). Hyperoxia improves microvascular perfusion in a murine wound model. Wound Repair Regen 13:303-308 [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. (2003). Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 82:621-626 [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. (2005). Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res 84:309-314 [DOI] [PubMed] [Google Scholar]
- Tandara AA, Mustoe TA. (2004). Oxygen in wound healing—more than a nutrient. World J Surg 28:294-300 [DOI] [PubMed] [Google Scholar]
- Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, et al. (2010). HIF-1alpha dysfunction in diabetes. Cell Cycle 9:75-79 [DOI] [PubMed] [Google Scholar]