Abstract
Temporal lobe epilepsy (TLE) is the most common form of adult epilepsy that is amenable to surgical treatment. In the carefully selected patient, excellent seizure outcome can be achieved with minimal or no side effects from surgery. This may result in improved psychosocial functioning, achieving higher education, and maintaining or gaining employment. The objective of this paper is to discuss the surgical selection process of a patient with TLE. We define what constitutes a patient that has medically refractory TLE, describe the typical history and physical examination, and distinguish between mesial TLE and neocortical TLE. We then review the role of routine (ambulatory/sleep-deprived electroencephalography (EEG), video EEG, magnetic resonance imaging (MRI), neuropsychological testing, and Wada testing) and ancillary preoperative testing (positron emission tomography, single-photon emission computed tomography (SPECT), subtraction ictal SPECT correlated to MRI (SISCOM), magnetoencephalography, magnetic resonance spectroscopy, and functional MRI) in selecting surgical candidates. We describe the surgical options for resective epilepsy surgery in TLE and its commonly associated risks while highlighting some of the controversies. Lastly, we present teaching cases to illustrate the presurgical workup of patients with medically refractory TLE.
1. Introduction
1.1. History of Temporal Lobe Epilepsy Surgery
Cerebral localization and electroencephalography (EEG) have together been two fundamental advances that have been paramount in the diagnosis and management of epilepsy. The clinical observations of Broca [1] and Jackson and Colman [2], along with the landmark observations of Fritsch and Hitzig [3], the electrical excitability of the human brain, and discrete localization of brain functions, began to be established. Through experiments of electrical stimulation on narcotized dogs, Fritsch and Hitzig were able to differentiate the motor from the nonmotor cortex [3]. Drawn to these findings, Sir Horsley was likely the first to attempt amelioration of epilepsy in a patient with posttraumatic seizures via a craniotomy [4]. Not long after this, in 1875, Caton was able to measure electrical activity from the cat brain [5], and this was followed by EEG recordings in humans by Berger in 1929 [6]. Thereafter, Bailey and Gibbs proceeded to operate on individuals with psychomotor epilepsy solely based on anterior temporal spikes on EEG [7]. Penfield later observed that patients failing neocortical resection could benefit from resection of the mesial temporal lobe structures such as the amygdala and the hippocampus. While many new developments have occurred since, these represent the key contributions that have remained as the fundamentals building blocks of the modern-day practice of epilepsy surgery.
1.2. Background
With approximately 1% of the world population affected by epilepsy, it is classified by the International League Against Epilepsy (ILAE) as the most common serious neurological disorder in the world [8]. The annual incidence rate is between 40 and 70 per 1000 people in developed countries [9]. Patients with epilepsy are at a threefold higher risk of cognitive decline as compared to the general population [10]. In addition, epilepsy is associated with significant psychosocial harm including social isolation, depression, and stigmatization [11]. Patients with epilepsy are less likely to complete secondary and postsecondary education translating into higher rates of unemployment [12]. In the United States, the direct average cost of epilepsy is $10,000 a year for patients with medically uncontrolled epilepsy and $2,000 a year for patients with medically controlled epilepsy [13, 14]. However, the direct medical costs comprise only 25% of the total economic impact of epilepsy [15]. For the estimated 2.3 billion people with epilepsy in the United States of America, the annual indirect cost is $12.5 billion; costs are eightfold higher in patients with medically intractable epilepsy [13, 16].
Medical intervention is the first step in the management of epilepsy. However, this fails to achieve seizure freedom in up to one-third of patients [17]. In a subset of patients who are refractory to medical management, evaluation of surgical candidacy is appropriate [18]. Temporal lobe epilepsy (TLE) is particularly common and amenable to surgery resulting in better seizure outcomes (50–70% seizure freedom at 5 years) [19] as compared to extratemporal epilepsy [20]. In addition, patients undergoing TLE surgery may benefit from improved psychosocial functioning [21], achieving higher education, maintaining or gaining employment [22], long-term seizure freedom [23], in addition to significant monetary savings by the society [9].
1.3. Objectives
The objective of this paper is to discuss the surgical selection process of a patient with TLE. We will outline the definition of a medically refractory patient with TLE, distinguish between mesial TLE (mTLE) and neocortical TLE (nTLE), review the role of routine and ancillary preoperative testing, describe surgical techniques and discuss common surgical risks. We lastly present several case studies to review the rationale for surgery.
2. Selection of Patients for Temporal LobeEpilepsy Surgery
2.1. Medically-Refractory Temporal Lobe Epilepsy
A recent consensus paper defined medically refractory epilepsy as having seizures despite being treated with 2 consecutive first-line antiepileptic medications (AEDs) over 2 years [24]. Complex partial seizures (CPSs), most commonly generated in the temporal lobe, are least likely to respond to medications [25]. Patients with CPSs, along with radiographic abnormalities in the temporal lobe or mesial temporal sclerosis (MTS), are most likely to fail medical management and are amongst the best surgical candidates [26]; hence why TLE could be considered a surgically-remediable form of epilepsy from the onset [27]. A small proportion of patients with mTLE may ultimately become seizure-free with more drug trials [20]. Furthermore, another subgroup of patients may achieve seizure freedom without treatment. This is referred to as benign mTLE in which the etiology may have an underlying genetic component [28]. There is scarce data regarding the predictors of this condition [28].
2.2. Differentiating between Mesial and Neocortical Temporal Lobe Epilepsy
From an electrical and clinical perspective, there are two subtypes of TLE: mTLE and nTLE. This distinction is made (although there is indeed overlap) as it has important implications with respect to electrophysiology, neuropsychological profile, underlying pathological substrate, and response to surgery [29]. The electroclinical and diagnostic differences are presented in Table 1.
Table 1.
Electroclinical and diagnostic differences between mTLE and nTLE.
| mTLE | nTLE | |
|---|---|---|
| Clinical aspects | Auras (simple partial seizures) (i) Not present in approximately half of TLE patients (ii) Visceral sensation/fear (or both) (iii) Déjà vu (iv) Illusions/hallucinations Complex partial seizures (i) Autonomic changes (ii) Arrest of behavior/motionless stare (iii) Oroalimentary automatism (iv) Contralateral dystonic posturing (v) Nose rubbing (vi) Dysphasia (if dominant hemisphere involved) |
Same as mTLE |
|
| ||
| Preoperative testing | MRI (i) MTS (ii) Other structural pathologies (iii) Dual pathology (iv) No lesion (“MRI normal”) TLE |
Same as mTLE |
| Neuropsychological testing (i) Lateralized memory impairment |
Neuropsychological testing (i) More likely to have naming problems |
|
| Wada test (i) Less likely to have lateralized memory dysfunction on side of seizure onset, compared with mTLE |
||
| Scalp EEG (i) “Classic” anterior temporal inter-ictal spikes |
Scalp EEG (i) No unique pattern (ii) Absence/multiple types of inter-ictal spikes |
|
|
| ||
| Intracranial recordings | Seizures originate from mesial structures | Variable with widespread electrophysiological changes |
The most common pathological substrate for TLE is MTS. This is characterized by segmental loss of pyramidal cells, dispersion of granule cells, and a resultant reactive gliosis. Other pathological entities resulting in TLE include tumors (either malignant or benign, e.g., ganglioglioma, dysembryoplastic neuroepithelial tumour, oligodendroglioma, low- or high-grade glioma, and meningiomas), infections (e.g., herpes, tuberculosis, and cysticercosis), vascular malformations (arteriovenous malformations, cavernous hemangioma, and meningioangiomatosis), migrational disorders (cortical dysplasia and hamartoma), and trauma (encephalomalacia and gliosis). The differential diagnosis of nTLE is similar to mTLE with the exception of MTS.
2.2.1. Dual Pathology
Approximately 15% of patients with partial epilepsy that have an extratemporal lesion have associated MTS; these cases are referred to as involving dual pathology [30]. The amount of hippocampal cell loss is correlated to the specific type of extra-temporal pathology with vascular lesions, gliomas, and hamartomas resulting in the least amount of cell loss while heterotopias are associated with the greatest amount of cell loss [30, 31]. While it is not clear whether it is the hippocampus alone, the extra-temporal lesion, or both that serve as the true epileptogenic lesion, it is evident that resection of both lesions generally yields the highest likelihood of attaining seizure freedom, provided that preoperative testing demonstrates concordant localization [32].
2.3. Routine Diagnostic Workup
2.3.1. Goal of Presurgical Patient Evaluation
The main goal of surgical management of epilepsy is the removal of the epileptogenic zone: the region which, if resected completely, would result in seizure freedom [33]. Hence, the preoperative workup seeks to identify this region and determine the safety of its resection. As part of the evaluation, the ictal onset zone, the symptomatogenic zone, the irritative zone, and the functional deficit zone may also be identified. The ictal onset zone is the region from which seizures arise. The symptomatogenic zone reproduces the clinical semiology of the ictal episodes upon stimulation. The irritative zone is the region in which interictal discharges can be detected; this depends highly on the method used for measurement, the level of patient awareness, and the amount of medication they are on. The functional deficit zone is correlated to neurological deficits during the interictal period. In an ideal scenario, substantial overlap is observed between the aforementioned zones, and there is congruence with the epileptogenic lesion identified on imaging. In such cases, there is a high likelihood that the patient will attain seizure freedom postoperatively [34–36].
These concepts are simplifications, and they may not be accepted amongst all epileptologists. An alternative method of conceptualizing seizure onset and propagation is the theory of cortical and subcortical neuronal networks (NNs); these are bilateral brain regions that are interconnected functionally and anatomically [37]. Individual components within the network have the ability to influence each other and the particular clinical semiology, and electrographical seizure manifestation is dependent on the particular NN involved. A well-defined NN is the medial temporal-limbic network consisting of the hippocampus, amygdala, neocortex of the lateral temporal lobe, entorhinal cortex, medial thalamus, and the inferior region of the frontal lobes. The identification of NNs typically involves the use of ictal EEG in addition to PET and fMRI [38]. Based on this premise, the identification of any perturbation within the network can be used to predict a seizure before it is clinically and electrographically manifested. Further, the use of NNs can be used to tailor treatment strategies to the particular network involved. For example, the resection of a component within the network but distant from eloquent cortex could theoretically help diminish seizure frequency without significant morbidity to the patient [37]. The primary focus of this review paper will be based on the epileptogenic and related zones and the various diagnostic modalities that can be used to identify them.
2.3.2. History and Physical Examination
The presurgical workup requires a detailed history and physical exam. Specific components of the history include a detailed account of seizure semiology, past medical history, family history, and attempted AED. Having a family member or friend who has witnessed the episodes can provide useful information, as the individual may not have any recollection of the events. A complete neurological examination can have localization value and, together with the history, can help identify the functional deficit zone.
2.3.3. Ambulatory and Sleep Deprived Electroencephalogram
Scalp EEG is an essential component of the initial patient evaluation. This test is often performed on an outpatient basis both for convenience and its noninvasive nature. For outpatient analysis, a 30-minute awake/sleep-deprived analysis may suffice if there is a typical clinical history and obvious imaging findings, especially if ictal recording with video-EEG telemetry in a monitoring unit is not possible [39]. However, there are situations where this may not be sufficient, for example, bilateral TLE with unilateral hippocampal sclerosis (HS). Repeated EEG, especially if performed within 48 hours of a seizure, increases the sensitivity of detecting an abnormality [40]. Sleep deprivation or cessation of AEDs can also be used to induce seizures [41]. Most patients with mTLE have unilateral anterior temporal inter-ictal spikes on surface EEG. However, some patients with unilateral mTLE may have bilateral independent spikes in the anterior temporal lobes [20]. Some authors report that unilateral temporal rhythmic theta activity less than 30 seconds after electrical seizure onset is associated with ipsilateral mTLE [42]. Scalp EEG analysis is an invariable test performed at all comprehensive epilepsy centers. By detecting ictal and inter-ictal epileptic discharges, it enables the approximate delineation of the ictal onset and the irritative zones. At most centers, however, surgery is only undertaken after documentation of seizure onsets after long-term video monitoring in an epilepsy monitoring unit (EMU) [43].
2.3.4. Video Electroencephalography
Admission to the EMU for continuous scalp EEG and video monitoring is the final common pathway and is usually considered a necessary step in determining surgical candidacy. This provides localizing value for both inter-ictal and ictal onset zones, allowing for correlation of the clinical manifestation of the epileptic event to ictal and inter-ictal EEG activity. The patient may be subjected to provocative measures such as medication reduction, sleep deprivation, hyperventilation, or photic stimulation to increase the likelihood of capturing epileptiform activity [44]. In certain situations, invasive electrodes may be necessary to provide better localization (see below).
2.3.5. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) scanning has significantly aided the diagnosis and management of epilepsy, and it has been established as the key imaging modality of choice [45]. If neocortical epilepsy is suspected, imaging protocol should include a whole head thin-sectioned high-resolution 3D T1- and T2-weighted images as well as a gradient echo T2 sequence to investigate the presence of blood products. Gadolinium administration is not necessary unless a mass lesion or tumour is found. If mTLE is suspected, high-resolution coronal T1, T2 and fluid-attenuated inversion recovery (FLAIR) sequences through the hippocampus should be obtained, preferably with a 3 Tesla scanner [46]. HS is identified through volume acquisition T1-weighted MR images along with FLAIR sequences [45]. T2-weighted imaging can identify increased T2 signal in the mesial temporal lobe and atrophy of the hippocampus, both key features of MTS [47, 48]. The presence of hippocampal atrophy on preoperative MRI has been associated with good seizure outcomes following temporal lobectomy (TLY) [20]. Thus, MR imaging allows for the identification of the postulated epileptogenic lesion, which can be used in parallel with other diagnostic modalities to help localize the epileptogenic zone.
2.3.6. Neuropsychological Assessment
A comprehensive neuropsychological evaluation can identify preoperative functional deficits and predict postoperative neuropsychological outcomes [49]. The most important cognitive domains to be tested in TLE are memory and language [49]. Patients with dominant lobe TLE typically display verbal memory deficits, whereas those with nondominant TLE display visuospatial memory deficits. Word-finding difficulties (a neocortical function) are also common in patients with language dominant TLE [50, 51].
Memory decline is the most common deficit following TLE surgery. The relationship between verbal memory decline following left sided surgery is more robust compared to the relationship between visuospatial memory decline following right-sided surgery [52–54]. Patients with average or above average memory and language function are at a higher risk for developing postoperative deficits [55, 56]; therefore, a comprehensive preoperative discussion is necessary with such patients before offering surgical management. Conversely those individuals with histologically proven MTS are least likely to show significant memory decline postoperatively [57].
2.3.7. Wada Test
The Wada test has been traditionally used to assess language and memory function of the two cerebral hemispheres independently [58, 59]. The agent most commonly used is amobarbital, but other agents such as methohexital, propofol, and etomidate have also been used [60, 61]. Recently amobarbital has become unavailable in some countries. Cerebral angiography is used to assess the vasculature and extent of cross-over flow to contralateral arteries. Baseline memory function is typically assessed a day before the actual test. Prior to injection of the intra-arterial anesthetic agent, the patient is asked to elevate both arms (to monitor the development of contralateral hemiplegia as a surrogate for adequate anesthesia) and count out loud. Language and memory are assessed while hemiplegia persists. Efforts are made to evaluate the side harboring the postulated epileptogenic zone first; the contralateral hemisphere is usually tested 30 minutes after the initial injection although some centers choose a one-day delay.
Global aphasia develops upon the injection of the dominant hemisphere. The duration of speech arrest can also be used to identify the language-dominant hemisphere. However, some suggest that if the difference in time to development of speech arrest is less than 30 seconds among the two hemispheres, the patient may have bilateral cortical language representation. Other parameters such as dysarthria and paraphasias may also be used to assess language dominance. Recent studies suggest that language lateralization is a continuum between both hemispheres, and that language unilaterality may be secondary to a lesion in the contralateral hemisphere [62].
For memory evaluation, the patient is required to correctly identify items shown during hemiparesis. An overall passing score is assigned based on the ability of the contralateral side in supporting memory upon injection of the side ipsilateral to the epileptogenic focus. Scores ranging from 50 to 67% have been deemed as a pass [63, 64]. While there is no gold standard to compare the Wada test results to, a passing score has been associated with a decreased likelihood of postoperative amnesia [2]. Based on the same premise, the Wada test can also be used to lateralize the epileptogenic zone in TLE patients. Injection of the side contralateral to the seizure focus would be expected to result in a greater loss of memory function with the correlation being stronger if profound amnesia is observed.
Despite the high accuracy of the Wada test in lateralizing language and memory function, this test is associated with false negatives and false positives [65]. These have important clinical implications. For example, some patients may be deemed unsuitable surgical candidates when in fact they would benefit from surgery. Also, less hippocampal resection may be performed resulting in poorer seizure control postoperatively [57].
The Wada test results can be affected by a variety of factors such as drug dose, unblinding of test assessors, and patient cooperation. Furthermore, the Wada test is associated with risks such as seizures, contrast allergy, catheter site hematoma, dissection, stroke, and infection [66, 67]. The risk of arterial dissection or stroke is estimated at 1% [68]. As a result many centers selectively use the WADA test [68] for certain clinical situations only, for example, a nonconcordant neuropsychological profile (memory deficit contralateral to the site of MTS) or patients who have bilateral memory deficits. Others restrict its use to left-handed individuals or those with ictal/postictal aphasia [69].
2.3.8. Invasive EEG Monitoring
Scalp EEGs are unable to lateralize the epileptogenic side in up to one-third of patients with TLE [70]. Even in cases where noninvasive tests are lateralizing, up to 10% could be falsely localizing [71]. In addition, synchronous activity across a cortical region of at least 6 cm2 is necessary for detection of an abnormality on scalp EEG [72]. Thus the indications for invasive recordings that stem from the limitations of scalp recordings include discordance amongst the various preoperative tests, seemingly multifocal epilepsy which includes bitemporal epilepsy, MRI-negative TLE that requires discrimination between nTLE and mTLE and as well to determine the extent of resection [73], situations where scalp recorded fields exceed the spatial involvement that would be expected in either lesional epilepsy or MTS, and proximity of neocortical lesions to eloquence are amongst the most common indication, but this by no means represents an exhaustive list. In patients with scalp EEG suggestive of bitemporal abnormalities, depth electrodes can be placed bilaterally within the mesial temporal lobe structures to lateralize the seizure focus. Certain TLE patients can present with dual pathology wherein it is unclear whether the hippocampus alone, the extra-hippocampal pathology, or a combination of the two is the epileptogenic lesion [31]. If the analysis shows concordant localization, then removal of both lesions results in the highest likelihood of seizure freedom postoperatively [32]. In certain situations, such as tuberous sclerosis, cortical dysplasia, or head trauma, invasive EEG may be necessary as the epileptogenic zone may extend beyond the visible lesion [74, 75]. Seizures that do not present with classic mesial temporal IEDs attributed to mTLE are likely to be of neocortical origin. If there is concern regarding proximity to eloquent cortex, subdural or depth electrodes can be used to better map the epileptogenic and functional areas, thus identifying a safe resection margin for the patient [76].
With invasive recordings, the characteristic ictal EEG pattern of mTLE includes periodic spiking activity from the hippocampus followed by episodes of high-voltage rhythms, which can last up to one minute. Subsequently, a regular 5–9 Hz rhythm is commonly observed [77]. In nTLE, ictal rhythms show high variability but a low voltage, high frequency discharge is commonly observed. Sharp waves of low frequency are also highly specific for seizures of a neocortical origin [69]. Patients with focal cortical dysplasia may demonstrate well-localized fast rhythms or repetitive fast spikes.
Upon the completion of scalp/invasive EEG video monitoring, some patients will have epilepsy that not amenable to surgery. This can be attributed to a myriad of causes including psychogenic nonepileptic seizures (PNESs), multifocal epilepsy, patients having a generalized seizure disorder, or the inability to accurately localize the ictal focus. However, almost half of the patients that flow through an adult EMU will have a distinctively identifiable symptomatogenic zone or will warrant intracranial recordings to determine surgical candidacy.
Furthermore, as deep seated or even certain superficial epileptiform activities may be missed by scalp EEG due to the filtering effect of the skull on higher frequency signals [78], intracranial recordings and in particular depth electrodes are of utility in recording from these electrographically occult lesions. While “ripples” (100–200 Hz) are associated with normal hippocampal electrical activity, fast ripples (150–500 Hz frequency) have a high likelihood of being associated with the ictal onset zone in the epileptogenic hippocampus and parahippocampal regions in patients with MTS [79–81]. While “fast ripple” detection holds great potential for the identification of the epileptogenic zone, its testing is invasive and is therefore restricted to seizure patterns originating from the hippocampus and hence less applicable to nTLE [81].
Although in extratemporal epilepsy detection of residual interictal epileptiform activity at the margins of resection can assist in deciding whether further resection is necessary, this approach appears to have little utility in the temporal lobe [27]. Disadvantages of intraoperative electrode recordings include the additional cost of equipment and extra operating room time, the need for an experienced neurophysiologist, and the rare occurrence of ictal recordings. Furthermore, with improvements in preoperative invasive monitoring, the need for intra-operative monitoring has decreased. Even though the use of invasive recording in general has diminished over time, it is nonetheless a valuable tool in select cases. Regardless, before embarking on invasive monitoring, the clinical question must be clear and the answer derived from the test should aid in the surgical evaluation of the patient.
2.4. Ancillary Testing
In situations where the standard presurgical assessment does not provide definitive seizure lateralization and/or localization (e.g., when the seizure focus appears to be bilateral, temporal, and extratemporal, mTLE with a larger field of activity than would otherwise be expected from standard mTLE), or there is discrepancy between the presurgical tests, the following ancillary investigations can be performed.
2.4.1. Positron Emission Tomography
Positron emission tomography (PET) is an imaging modality that uses radioactive isotopes linked to metabolically active molecules (such as glucose) to analyze functionality in various regions of the body depending on metabolic activity. The nuclei of these tracers emit positrons which generate photons upon collision with electrons in the surrounding environment. The concentration of radioactive glucose, and hence amount of photon emission, within a region depends on the relative metabolic activity. Hypometabolism is not correlated with the amount of cell loss or hippocampal atrophy. In the investigation of TLE, this test seeks to identify the region of interictal hypometabolism which is slightly larger than the ictal onset zone. Occasionally in TLE, hypometabolism can be detected in regions other than the temporal lobe. This may reflect the extratemporal connections of the seizure focus [82].
Although obtaining a truly ictal PET study is rare, it can be valuable in identifying the seizure focus, by demonstrating a marked area of hypermetabolism [45, 83]. Accordingly, EEG recording during PET acquisition is important to ensure hypometabolism detected in one hemisphere is not secondary to an active seizure on the contralateral side resulting in hypermetabolism [84].
Fluorodeoxyglucose (FDG) is the most commonly used isotope in PET. The inter-ictal FDG-PET has a high specificity for mTLE (MTS is associated with hypometabolism localized to the hippocampus, amygdala, entorhinal cortex, and temporal pole) [20, 85]. In addition, hypometabolic regions identified by FDG-PET correlate well with predicted lateralization when compared to depth electrodes [86]. The sensitivity of the test is increased when the metabolic activity of both temporal lobes is sampled to quantify hypometabolism on one side in relation to the other.
PET is generally utilized in the evaluation of symptomatic (formerly referred to as cryptogenic) cases and for identifying seizure-spread patterns, thus guiding the placement of intracranial electrodes. If PET and MRI are concordant, there is prognostic utility as better seizure outcomes are predicted following surgery. However, PET does not usually provide any additional information if MTS is demonstrated on MRI [87, 88]. Therefore, it is not commonly used at all centers for presurgical evaluation.
2.4.2. Single Photon Emission Computed Tomography
Cerebral blood flow is increased within regions of the brain undergoing epileptic seizures to match the increased metabolic demand. Single photon emission computed tomography (SPECT) measures local cerebral perfusion using either technetium-99m hexamethyl propelene amine oxime or technetium-99m bicisate. These can be maximally extracted into the neurons within seconds of injection and remain within the cell for several hours [89]. Therefore, injection of radiotracers immediately following a seizure can help identify the ictal onset zone. The sensitivity of this test is increased further if inter-ictal SPECT studies are used for comparison to determine the relative change in cerebral perfusion during seizures. SPECT can be used as an important adjunct for localization of seizure onset, particularly in MRI-normal cases or when EEG is non-localizing [90]. While the spatial and temporal resolutions of SPECT are not as high as PET, it is less costly and more widely available.
When independent seizure foci reside in the temporal lobes bilaterally, ictal SPECT studies must be interpreted with caution. Furthermore, SPECT may provide falsely lateralizing information if the epileptiform activity has terminated in the temporal lobe of origin but is ongoing in the contralateral temporal lobe. In certain cases of nTLE, the regional cerebral blood flow cannot be accurately identified by inter-ictal SPECT; therefore, SPECT is overall less sensitive for nTLE. Currently, SPECT imaging can only be used to provide information that is complementary to EEG. However, modifications to the SPECT analysis (as discussed below) can increase its utility in identifying the ictal zone.
2.4.3. Subtraction Ictal SPECT Correlated to MRI
With a higher accuracy than SPECT, subtraction ictal SPECT correlated to MRI (SISCOM) is another imaging modality that can be used to localize the epileptogenic zone, especially for those with nonlesional MRI or extensive focal cortical dysplasia [91]. In SISCOM, normalized coregistered inter-ictal SPECT images are subtracted from ictal images, and the resultant difference in cerebral blood flow (only those with intensities greater than 2 standard deviations above zero) is matched to high-resolution corresponding MR images to identify the epileptogenic zone [89]. Spiral CT images of implanted subdural electrodes can also be coregistered with SISCOM images to correlate changes in cerebral perfusion with the ictal onset zone [92]. SISCOM can also be used to guide intracranial EEG electrode placement [92]. Concordance of SISCOM with other preoperative studies identifying the epileptogenic focus may have prognostic value in postoperative seizure outcomes [91].
To improve the diagnostic yield of SISCOM, injection of radiotracers should be performed within 45 seconds of seizure onset and ideally the seizure lasting greater than 5–10 seconds [93]. Furthermore, for accurate correlation to the epileptogenic zone, continuous EEG (cEEG) recordings are required. In addition, the cost of the radioisotopes is relatively high as well. Therefore, despite SISCOM's clinical utility, its use is limited to certain comprehensive epilepsy centers.
2.4.4. Magnetoencephalography
The neurophysiologic process that generates the magnetoencephalogram (MEG) signal is identical as to what produces the EEG [94]. The fluctuation of the dendritic membrane potential is observed as a current dipole perpendicular to the cortical surface [95]. A certain volume of excitable cortex is required to generate a “brain wave” which is detected by MEG or EEG. MEG spike localization does not necessarily identify the epileptogenic zone or seizure onset zone. However, it does detect inter-ictal epileptiform discharges (IEDs) generated within the neocortex [96, 97].
The current indication for MEG in TLE is unknown, and its potential advantage must be weighed against the high cost and limited availability. In a retrospective study, it was found that MEG utilized in the presurgical evaluation did not provide any additional information in over half of the patients [98]. Its utility in mTLE is suspect given its inability to detect deep sources and in particular hippocampally generated IEDs [99, 100]. Its benefit is likely larger in neocortical epilepsy or in those with equivocal findings following other testing modalities [98, 101]. Its greatest utility is perhaps in non-lesional TLE cases where a strong correlation has been established between MEG spike patterns and the seizure onset zone [102]. MEG may at the very least provide more support for the recommended treatment strategy, whether for or against surgery. The main advantage of MEG over scalp EEG is its improved accuracy in spike source localization. Although it must be borne in mind that MEG provides complimentary information to EEG, the sources that generate MEG signals are thought to arise from the sulci, whereas those generating the EEG signals arise from the crowns of the gyri [100]. As well, it can also be superimposed on other functional imaging modalities and guide surgical resections as part of the neuronavigational system [103, 104].
2.4.5. Magnetic Resonance Spectroscopy
N-Acetylaspartate (NAA) is primarily found in neurons, and its decrease is often indicative of neuronal loss or dysfunction. In contrast, creatinine (Cr) and choline (Cho) are present at higher concentrations within glial cells. By studying the levels of NAA, Cr, and Cho, 1H magnetic resonance spectroscopy (MRS) can also be helpful in localizing the epileptogenic zone. A decrease in the ratio of NAA to Cr + Cho has been suggested to be correlated with HS with correct seizure lateralization in greater than 90% of cases [105]. A proportion of patients may demonstrate bilateral metabolic abnormalities with 1H MRS; this may correlate with a higher likelihood of surgical failure [73]. 1H MRS can aid in the placement of intracranial grid and strip electrodes as well [73]. However, due to its technical challenges and lack of widespread availability, this tool has yet to be established in the presurgical evaluation of epilepsy although it may have an expanded utility in the future [106].
2.4.6. Functional MRI
Functional MRI (fMRI) studies neural activity by measurement of alteration in the MRI signal due to changes in oxygenation levels (an increase in T2 signal is observed during epileptiform activity) [107]. The main indications for this imaging modality are for the identification of eloquent cortical regions such as motor and language areas. In addition, when coupled with EEG analysis, it can also be used to help identify the irritative zone and potentially the ictal onset zone [108]. Significant improvements in EEG-fMRI analysis (e.g., MRI-compatible EEG electrodes, higher strength magnets, and offline signal processing using mathematical tools) [109] have increased the application of this imaging modality in the evaluation of patients with epilepsy. Amongst its many advantages, fMRI has a spatial resolution of a few millimeters and it is a noninvasive alternative for the Wada test for language lateralization and localization of cortical speech regions [110]. Increased signal activation on fMRI during memory and language tasks on the side ipsilateral to the ictal focus has been suggested to be associated with greater deficits post resection [111, 112]. This correlation may be an even stronger predictor than neuropsychological testing [112]. While fMRI is a sensitive tool for the evaluation of the irritative zone, its sensitivity to patient motion, including changes in patients' cardiac and respiratory parameters, makes it difficult to fully evaluate the ictal onset zone. However, the development of specific algorithms to adjust for these artifacts may allow fMRI to become a standard component of the presurgical evaluation.
3. Surgical Strategy
3.1. Extent of Lateral Resection
The extent of lateral resection is variable and commonly dependent on strategies to avoid postoperative language deficits and whether or not the patient has mTLE or nTLE.
3.1.1. mTLE
One approach to mTLE is to resect a predetermined amount of neocortex according to language dominance: 4.5 cm and 5 cm along the Sylvian fissure in the dominant and nondominant sides, respectively [113, 114]. Resections beyond this length may be associated with postoperative aphasia in the dominant hemisphere. In the dominant hemisphere, others spare a greater amount of superior temporal gyrus (STG) with a minimal resection combined with a 4.5 cm resection of the middle temporal gyrus (MTG) [115]. An even more conservative approach is to spare the entire STG and only resect 3.5 cm of the MTG [116]. Alternatively, the lateral resection can be tailored based on stimulation mapping of the essential language sites and avoiding resections within 2 cm of these sites [117].
The most conservative approach to the resection of the mesial structures can be accomplished by various selective approaches through a transcortical-transventricular [118] or a transsylvian approach [119]. The selective approach was based on a concept from Hughlings Jackson's description of an uncal lesion causing psychomotor seizures and the role of the mesial temporal lobe in epilepsy [58]. Subsequent experiments provided evidence that these structures play an important role in mTLE [120, 121]. This generated surgical interest in attempting to achieve the best results for seizure outcomes while sparing resection of brain tissue that is not believed to be involved in the generation of seizures. In theory, this approach is thought by some authors to have neuropsychological advantages compared to a more aggressive neocortical resection [122].
3.1.2. nTLE
The amount of neocortex to be resected in nTLE should include the epileptogenic zone as determined by preoperative testing and possibly intra-operative ECOG which seeks to identify the irritative zone through recording pre-resection IEDs. In the dominant hemisphere, the extent of posterior resection is limited by language areas. Complete removal of a radiographically identified lesion usually results in cessation of seizures when lesions are well circumscribed (e.g., benign tumors or cavernous hemangiomas) [123, 124]. However, in lesions with ill-defined borders such as cortical dysplasia and posttraumatic gliosis, the likelihood of operative success is lower as microscopic damage surrounding the visible boundaries of the lesion may be present [125].
3.2. Extent of Mesial Resection
Since the introduction of the en bloc ATL and the subsequent advent of selective procedures, there is much debate regarding the identity of the critical structures that should be removed to achieve seizure freedom in a temporal resection.
3.2.1. Hippocampal Resection
The general consensus is that the hippocampus should be included in resective procedures for TLE; however, the degree of hippocampal resection is controversial. Wyler et al.'s randomized trial demonstrated that patients that underwent a total hippocampectomy (extending to the superior colliculus) were more likely to be seizure free at 1-year followup compared to patients that underwent a partial hippocampectomy (extending to the lateral edge of the cerebral peduncle) [57]. Undergoing a partial hippocampectomy is controversial especially if the epileptogenic zone has been localized to the hippocampus. In addition, a partial resection of the hippocampus will result in its deafferentation from the entorhinal cortex and thus render it ineffective for memory storage and recall. Therefore, a partial resection is not an effective strategy.
3.2.2. Parahippocampal Resection
The parahippocampal gyrus (PHG) is generally removed along with the hippocampus. There is evidence from depth electrode studies to suggest that epileptiform activity originating from the PHG and amygdala is more likely to manifest clinically than activity from the hippocampus [126]. Furthermore, a retrospective study by YaSargil et al. had demonstrated that the volume of PHG resected had a greater impact on seizure outcome than the volume of any other mesial temporal lobe structure [119].
3.2.3. Amygdalar Resection
The amygdala has intricate connections with both limbic and neocortical structures and a great propensity to generate seizures as demonstrated following kindling experiments [127]. The combination of focal epileptic discharges from the periamygdaloid region and stimulation mapping able to reproduce automatisms and amnesia in this region indicated the importance of including the amygdala in TLE resections [128, 129]. Interestingly, some studies suggest that amygdalar sclerosis may in fact occur in isolation from the hippocampus [130].
3.3. Risks Associated with Surgery for TLE
Despite the potential to achieve excellent seizure control, TLE surgery is associated with several risks specific to the procedure: motor, visual field, cranial nerve, language, memory, cognitive, and psychiatric deficits. The cumulative morbidity for TLE surgery, not considering adverse psychiatric outcomes, is approximately 11% with permanent deficits in approximately 3% [24, 131].
3.3.1. Motor Deficits
Contralateral hemiplegia is a well-described complication of TLE surgery. It is thought to result due to manipulation of the anterior choroidal artery with subsequent infarction of the posterior limb of the internal capsule. This is estimated to occur in 2% of the cases with the majority of patients improving over the course of several months to a year [132, 133].
3.3.2. Cranial Nerve Deficit
Cranial nerve morbidity is mainly associated with the oculomotor (CNIII) and the trochlear (CNIV) nerves. The oculomotor nerve traverses the ambient cistern bordering the medial aspect of the temporal lobe on route to the cavernous sinus. The trochlear nerve travels lateral to the cerebral peduncles and between the posterior cerebral and superior cerebellar arteries lateral to the oculomotor nerve prior to entering the cavernous sinus. Cranial nerve injury occurs most commonly due to traction, is estimated at 1.5–3%, and is usually transient [132, 134].
3.3.3. Visual Field Deficits
The most common visual field deficit following TLE is a superior quadrantanopsia, resulting from damage to the optic radiations comprising the most lateral aspect of Meyer's loops as they course inferomedially. However, visual deficits can range from small triangular defects to a complete homonymous hemianopsia. A more extensive hemianopsia has been attributed to a greater amount of resection as well as individual variance on the course of the optic radiations. A randomized trial of temporal lobe epilepsy surgery found quadtrantic visual field defects in 55% of the patients [35]. However, in the vast majority of cases, this is diagnosed on formal visual field testing and the patient is unaware of this deficit [35]. A selective surgical approach does not appear to offer an advantage [135]. Damage to the optic radiations in these cases has been attributed to suction devices and retractors being driven through the optic radiations en route to the mesial temporal lobe structures.
3.3.4. Language Deficit
Dominant TLE surgery is associated with a language risk due to the close proximity of Broca's and Wernicke's area localized to the inferior frontal gyrus and the posterior STG, respectively. However, the most common language deficit is a transient anomia [136, 137]. Some surgeons routinely perform a tailored resection by conducting intra-operative language mapping and/or avoid resection of the STG, while others argue that this does not provide a benefit [136]. In a large multicenter study comparing a tailored resection utilizing intra-operative mapping, tailored resection without intra-operative mapping, a standard approach sparing the STG, and a standard approach not sparing the STG, a similar decline in visual confrontational naming as assessed by the Boston Naming Test (BNT) was observed in all groups with no differences between groups [138]. Although there is variability between centers, most do not perform tailored resections according to language mapping, and they routinely spare the STG except the first centimeter or so [116]. A multicenter trial demonstrated that early age of seizure onset was a protective factor for postoperative anomia, perhaps due to the early collateralization of language [139].
3.3.5. Memory Deficit
While the Wada test is an important adjunct that assesses the ability of the contralateral hemisphere in supporting memory function, carefully selected patients may still suffer significant memory deficits following TLE surgery. The lateral neocortical temporal lobe is associated with naming and short-term working memory while the mesial temporal lobe is implicated in long-term consolidation of memory and retrieval [140]. In individuals with typical language dominance, visuospatial and verbal memory is commonly associated with the right and left hippocampi, respectively [141]. High ipsilateral memory function and lack of radiographic features of MTS on preoperative MRI are associated with a greater degree of postoperative memory decline. Patients with contralateral hippocampal dysfunction are generally not candidates for an ipsilateral mesial temporal lobe resection as bilateral hippocampal lesions can result in a severe anterograde amnesia [140, 142].
3.3.6. Psychiatric Risks
TLE has been associated with a high risk (almost 50%) of depression [143]. In particular, a preoperative history of depression is a strong predictor of postoperative depression [143]. In addition, suicide rates are 5 times greater than the general population. While most patients improve following surgery as a result of greater seizure control and increased independence, others are at risk of developing further psychiatric illnesses. In a cohort of 28 patients undergoing ATL, impairments of facial recognition of expression of fear, anger, disgust, and sadness were identified [144]. Although rare, some patients may develop a psychotic-type illness similar to schizophrenia [145]. Therefore, there must be a lowthreshold to refer a patient for psychiatric assessment.
4. Case Examples
4.1. Typical MTS
Ms. A is a 34-year-old, right-hand-dominant female who presented with her first convulsive seizure at the age of 27 years although a detailed past history suggested that she may have been suffering from brief partial seizures without loss of awareness for many years prior to that. These seizures were confirmed on EEG. Initial drug therapy, with 400 mg per day of carbamazepine, maintained her seizure free for 7 years until she presented again with a generalized tonic-clonic seizure (GTCS) during sleep. Subsequently her dose was increased to 800 mg per day, but this did not fully prevent the GTCSs. Also, she had been suffering from simple partial seizures as well as up to 7 CPSs per month. She described auras of nausea and a “funny feeling” up her spine. She also felt that she tried to remember something that had not happened. This would then tend to be followed by a blank stare and lip smacking. From a neuropsychological point of view, she complained of blunted emotions and poor memory.
Ms. A was admitted to the EMU where 7 seizures from the right temporal lobe, all with maximal onset over the anterior/mid and basolateral structures were detected. One of the seizures secondarily generalization towards the end of this event ictal discharges was recorded over the left posterior temporal structures. MRI demonstrated sclerosis of the right mesial temporal lobe (Figure 1). Neuropsychological testing demonstrated deficits in non-verbal memory. Given that all testing was concordant with a right mTLE, a right selective amygdalohippocampectomy was recommended. The procedure was carried out without complications. At 6-month postoperative followup, Ms. A was free of seizures including auras. She had been maintained on her preoperative medications. She noted significant improvement of memory and concentration.
Figure 1.
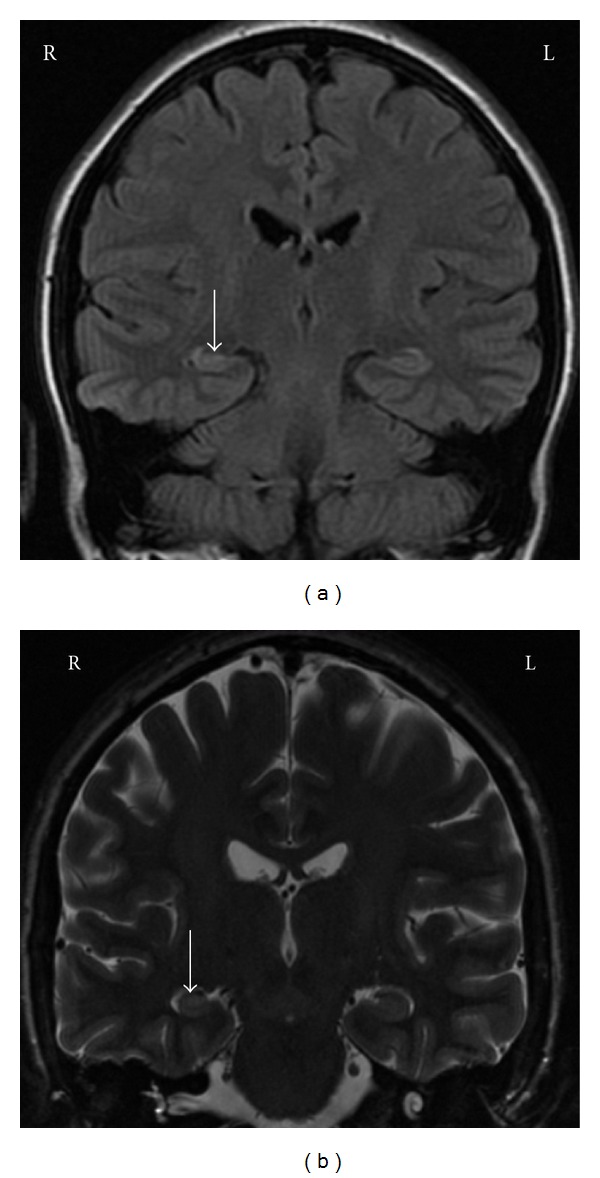
Ms. A—FLAIR and T2-weighted MR demonstrating right MTS as can be identified based on the loss of architecture and high signal of flair images.
4.2. MRI Normal nTLE
Mr. B is a 28-year-old, right-hand dominant who was first seen at the age of 22 for evaluation of a long-standing seizure disorder. He had been suffering from complex partial seizures from the age of 10, which were described as periods of disorientation, twitching, lip smacking, picking at his shirt, and difficulties with speech lasting 1-2 minutes. He also described auras of epigastric discomfort and fear. He had not experienced any GTCSs seizures or secondary generalization of his seizures. Carbamazepine, valproic acid, and phenytoin had been attempted without significant benefit. Previous MRI with supplementary detailed views of the temporal lobes was normal (Figure 2). Mr. B was subsequently admitted to the EMU, with scalp EEG monitoring.
Figure 2.
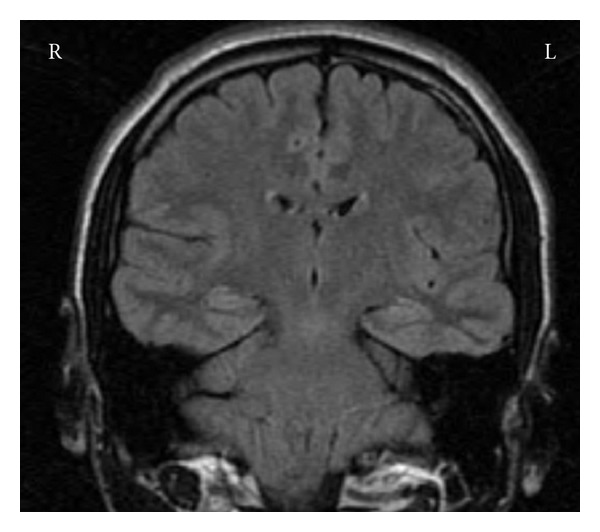
Mr. B—normal MR.
Abnormalities, concentrated in the left anterior quadrant of the head, consisted of continuous dysrhythmia with spread to the frontal regions in the form of long-lasting 4-5 Hz, monorhythmic trains of activity with abrupt onset and offset without clinical accompaniment. He demonstrated interictal slow wave activity localizing to the left mesial temporal as well as left temporal region. Furthermore, distinctive phase reversals were identified in electrodes approximating Wernicke's area and inferior. Ictal activity always began on the left side starting anteriorly and then proceeding posteriorly. Main source imaging spikes all localized to the mesial temporal region. No inter-ictal activity was noted in the posterior temporal region.
Neuropsychological evaluation demonstrated diminished verbal functioning with a pattern most consistent with left-sided neocortical dysfunction rather than mesial temporal (verbal learning and retention were excellent). fMRI revealed left hemispheric language dominance. As a result of these investigations, the benefit of a surgical resection was unknown. He was discharged on 100 mg per day of topiramate, which also failed to decrease his seizures. Therefore, to better delineate the site of seizure onset and for functional mapping, intracranial monitoring was recommended.
A large square grid was placed at the end of the distal sylvian fissure and overlying the inferior and superior parietal lobules. Three subtemporal strip electrodes (labeled as frontal, middle, and posterior temporal) were also placed. Subsequent monitoring in the EMU demonstrated the middle temporal subdural strip electrode to be most epileptogenic. MRI correlated these leads to the left inferior temporal and fusiform gyri.
Surgical resection, guided by ECOG and language mapping, was performed. The mesial temporal structures were spared to avoid memory deficits. Pathological examination revealed mild cortical and subcortical gliosis. Postoperatively, he experienced a few very brief auras (similar to ones experienced in the past) but no progression to CPSs. He also complained of poor memory and reading ability, but spoken language was intact. He was maintained on 400 mg per day of topiramate. At 2 years postoperative followup, Mr. B was seizure free although he did complain of intermittent sensations of his typical aura. He also complained of mild word finding difficulties which did not interfere with daily life. He maintained a full-time job without any difficulties.
4.3. Dual Pathology
Mr. C is a 34-year-old, left-hand-dominant man who started having seizures at 25 years of age. His family described his episodes as starting with a few minutes of increased rate and volume of speech followed by fatigue, slowed speech, and occasional automatisms. Postictally, he would fall asleep and rarely remember these episodes. Seizures occurred approximately twice a week. He presented to the hospital following his first episode of a GTCS.
EMU studies at a peripheral hospital had been able to record eight seizures of similar clinical semiology. Two were electrographically of left temporal origin while the remaining six were poorly lateralized, appearing bi-hemispheric and perhaps even right hemispheric predominancy at onset followed by rhythmic activity localized to the left temporal head regions within 3-4 seconds. An ictal SPECT scan during one of these episodes demonstrated left temporal activation. MRI at that point had been interpreted as normal. Conservative medical management with trials of phenytoin, topiramate, and pregabalin was attempted without success.
For further clarification, he was monitored in the EMU at our institution where bilateral inter-ictal abnormalities from both the left anterior temporal regions as well as the right midlateral or midposterior temporal regions were demonstrated. On certain days, seizures, of a 3 : 1 ratio, favoring the right hemisphere was observed. He also had multiple electrographic seizures that were either poorly lateralized or not lateralized at onset. Subsequent MRI demonstrated left HS in addition to signal abnormalities in the inferior right temporal region as well, likely representing cortical dysplasia (Figure 3). Neuropsychological testing suggested a full-scale IQ of 119 with only a slight relative weakness in verbal memory; otherwise, the tests were nonlateralizing. At this point, he had worsening depression, loss of motivation, and problems with short-term memory and concentration, all contributing to him quitting his graduate degree. To better delineate the epileptogenic focus/foci, anterior and posterior temporal strip electrodes, subtemporal strip electrodes, along with hippocampal depth electrodes, were placed bilaterally for EMU monitoring.
Figure 3.
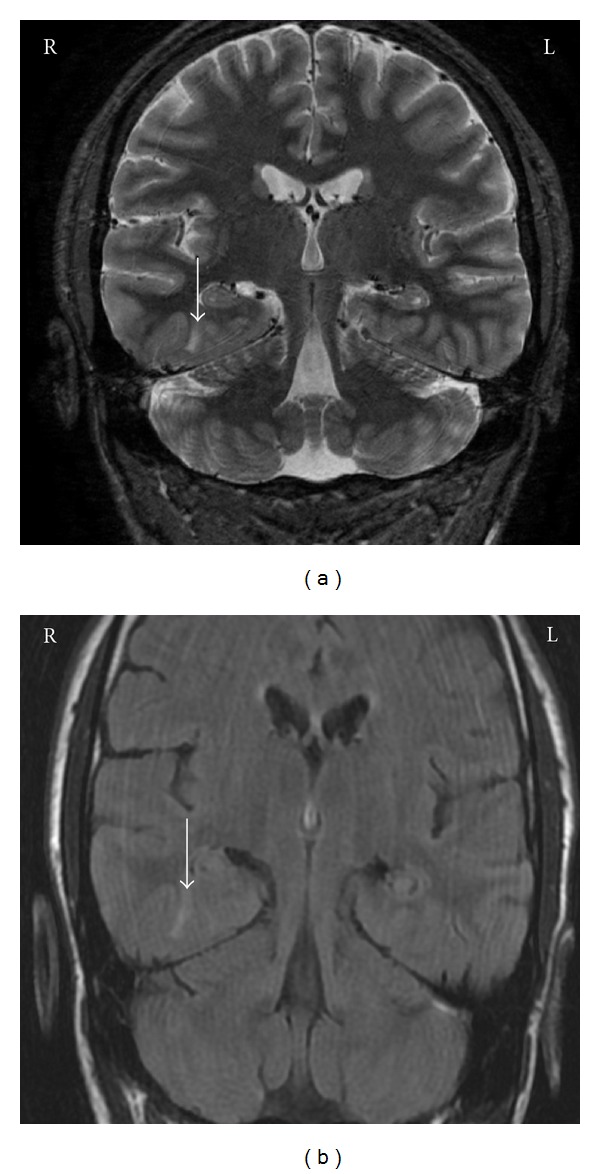
Mr. C—T2-weighted and FSTIR sequence MR demonstrating a right inferior temporal lobe lesion in addition to left MTS.
During this stay, many CPSs, all stereotypically involving the right temporal mesial and neocortical structures before spreading to involve the left temporal mesial and neocortical structures, were noted. The exact localization within the right temporal lobe was not clear given that the first electrographic changes were subtle and comprising of an attenuation of background activity over the right hippocampal depth and RMT electrode contacts. Occasional low-amplitude 20 Hz rhythms at right hippocampal depth electrode 2 prior to subsequent spread were also detected. Left temporal spiking, occurring more frequently than right temporal spiking, raised the concern regarding the role of the left temporal lobe being involved; however, brief ictal rhythmic discharges appeared solely from the right temporal lobe structures which correlated well with the patient's clinically relevant seizures. Given that the seizures were primarily right-sided but that he also demonstrated left-sided HS, a WADA test was performed which showed left-sided memory dominance. He has been scheduled for a right TLY.
5. Conclusion
Once a patient has been deemed medically refractory, the main requirement to determine surgical candidacy is the ability to accurately localize the epileptogenic zone [146]. There are tools in the armamentarium of the epilepsy team to help localize the epileptogenic zone and ensure that resection can be done in a safe manner to minimize any neurologic deficit. All ancillary testing is not employed simultaneously; rather they are tailored to the anatomical, electrical, and clinical features of each patient [147]. The best patients for surgical resection are those with concordance in localization of their seizures electrographically, radiographically, and semiologically.
TLE is the most common epilepsy syndrome that is responsive to surgical treatment. Although various pathologies can give rise to TLE including cortical dysplasia, tumours, and vascular malformations, HS remains the most common entity. Surgical patient selection is made after a thorough discussion of each case in a multidisciplinary conference including epileptologists, epilepsy surgeons, neuroradiologists, neuropsychologists, clinical psychologists, EEG technologists, and nurses. In the appropriately selected patients, seizure freedom can be achieved with no or manageable neurological deficits following surgery.
Authors' Contribution
A. Mansouri and A. Fallah should be considered co-first authors as they equally contributed to preparing the first draft of the paper. A. Fallah was responsible for several revisions of the paper. T. A. Valiante was responsible for the final editing of the paper.
Disclosure
There are no sources of support for this paper. It has not been published or presented in any form.
References
- 1.Broca P. Remarques sur le siege de la faculte du language articule, suives d'une observation d'aphemie (perte del la parole) Bulletin de la Société Anatomique. 1861;280:834–843. [Google Scholar]
- 2.Jackson JH, Colman WS. Case of epilepsy with tasting movements and “dreamy state”-very small patch of softening in the left uncinate gyrus. Brain. 1898;21(4):580–590. [Google Scholar]
- 3.Fristsch G, Hitzig E. Ueber die elektrische erregbarkeit des grosshirns. Arch Anat Physiol Wiss Med. 1870:300–302. [Google Scholar]
- 4.Horsley V. Brain surgery. BMJ. 1886:670–675. [Google Scholar]
- 5.Caton R. The electrical currents of the brain. BMJ. 1875;2, article 278 [Google Scholar]
- 6.Berger H. Uber das Elektrenkephlogram des Menshen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- 7.Bailey P, Gibbs FA. The surgical treatment of psychomotor epilepsy. JAMA. 1951;145(6):365–370. doi: 10.1001/jama.1951.02920240001001. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds EH. The ILAE/IBE/WHO epilepsy global campaign history. Epilepsia. 2002;43(supplement 6):9–11. doi: 10.1046/j.1528-1157.43.s.6.5.x. [DOI] [PubMed] [Google Scholar]
- 9. Epilepsy fact sheet, 2011, http://www.who.int/mediacentre/factsheets/fs999/en/index.html.
- 10.Dodson WE, Kinsbourne M, Hitbrunner B, editors. The Assessment of Cognitive Function in Epilepsy. New York, USA: Demos; 1991. [Google Scholar]
- 11.Baker GA, Camfield C, Camfield P, et al. Commission on outcome measurement in epilepsy, 1994–1997: final report. Epilepsia. 1998;39(2):213–231. doi: 10.1111/j.1528-1157.1998.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiebe S, Bellhouse DR, Fallahay C, Eliasziw M. Burden of epilepsy: the Ontario Health Survey. Canadian Journal of Neurological Sciences. 1999;26(4):263–270. doi: 10.1017/s0317167100000354. [DOI] [PubMed] [Google Scholar]
- 13.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41(3):342–351. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 14.Beghi E, Frigeni B, Beghi M, De Compadri P, Garattini L. A review of the costs of managing childhood epilepsy. PharmacoEconomics. 2005;23(1):27–45. doi: 10.2165/00019053-200523010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Cockerell OC, Hart YM, Sander JWAS, Shorvon SD. The cost of epilepsy in the United Kingdom: an estimation based on the results of two population-based studies. Epilepsy Research. 1994;18(3):249–260. doi: 10.1016/0920-1211(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 16.King JT, Jr., Sperling MR, Justice AC, O'Connor MJ. A cost-effectiveness analysis of anterior temporal lobectomy for intractable temporal lobe epilepsy. Journal of Neurosurgery. 1997;87(1):20–28. doi: 10.3171/jns.1997.87.1.0020. [DOI] [PubMed] [Google Scholar]
- 17.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. The Lancet Neurology. 2008;7(6):514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 18.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. The Lancet Neurology. 2008;7(6):525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 19.Spencer SS. Long-term outcome after epilepsy surgery. Epilepsia. 1996;37(9):807–813. doi: 10.1111/j.1528-1157.1996.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 20.Spencer SS. When should temporal-lobe epilepsy be treated surgically? The Lancet Neurology. 2002;1(6):375–382. doi: 10.1016/s1474-4422(02)00163-1. [DOI] [PubMed] [Google Scholar]
- 21.Jacoby A. Epilepsy and the quality of everyday life. Findings from a study of people with well-controlled epilepsy. Social Science and Medicine. 1992;34(6):657–666. doi: 10.1016/0277-9536(92)90193-t. [DOI] [PubMed] [Google Scholar]
- 22.Sperling MR, Saykin AJ, Roberts FD, French JA, O’Connor MJ. Occupational outcome after temporal lobectomy for refractory epilepsy. Neurology. 1995;45(5):970–977. doi: 10.1212/wnl.45.5.970. [DOI] [PubMed] [Google Scholar]
- 23.Bien CG, Schulze-Bonhage A, Soeder BM, Schramm J, Elger CE, Tiemeier H. Assessment of the long-term effects of epilepsy surgery with three different reference groups. Epilepsia. 2006;47(11):1865–1869. doi: 10.1111/j.1528-1167.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 24.Engel J, Jr., Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003;44(6):741–751. doi: 10.1046/j.1528-1157.2003.48202.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattson RH, Cramer JA, Collins JF. Prognosis for total control of complex partial and secondarily generalized tonic clonic seizures. Neurology. 1996;47(1):68–76. doi: 10.1212/wnl.47.1.68. [DOI] [PubMed] [Google Scholar]
- 26.Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England Journal of Medicine. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 27.Valiante TA. Selective amygdalohypocampectomy. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. Springer; 2009. [Google Scholar]
- 28.Labate A, Gambardella A, Andermann E, et al. Benign mesial temporal lobe epilepsy. Nature Reviews Neurology. 2011;7(4):237–240. doi: 10.1038/nrneurol.2010.212. [DOI] [PubMed] [Google Scholar]
- 29.Weiser HG, editor. Surgically Remediable Temporal Lobe Syndromes. New York, NY, USA: Raven Press; 1991. [Google Scholar]
- 30.Cendes F, Cook MJ, Watson C, et al. Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology. 1995;45(11):2058–2064. doi: 10.1212/wnl.45.11.2058. [DOI] [PubMed] [Google Scholar]
- 31.Levesque MF, Nakasato N, Vinters HV, Babb TL. Surgical treatment of limbic epilepsy associated with extrahippocampal lesions: the problem of dual pathology. Journal of Neurosurgery. 1991;75(3):364–370. doi: 10.3171/jns.1991.75.3.0364. [DOI] [PubMed] [Google Scholar]
- 32.Li LM, Cendes F, Andermann F, et al. Surgical outcome in patients with epilepsy and dual pathology. Brain. 1999;122, part 5:799–805. doi: 10.1093/brain/122.5.799. [DOI] [PubMed] [Google Scholar]
- 33.Carreno M, Luders HO, editors. General Principles of Pre-Surgical Evaluation. London, UK: Informa Healthcare; 2007. [Google Scholar]
- 34.McIntosh AM, Wilson SJ, Berkovic SF. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia. 2001;42(10):1288–1307. doi: 10.1046/j.1528-1157.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- 35.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. The New England Journal of Medicine. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 36.de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. The Lancet. 2011;378(9800):1388–1395. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- 37.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43(3):219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Tokoglu F, Negishi M, et al. Social network theory applied to resting-state fMRI connectivity data in the identification of epilepsy networks with iterative feature selection. Journal of Neuroscience Methods. 2011;199(1):129–139. doi: 10.1016/j.jneumeth.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cascino GD, Trenerry MR, So EL, et al. Routine EEG and temporal lobe epilepsy: relation to long-term EEG monitoring, quantitative MRI, and operative outcome. Epilepsia. 1996;37(7):651–656. doi: 10.1111/j.1528-1157.1996.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 40.Hoppe M, Wennberg R, Tai P, Pohlmann-Eden B. EEG in epilepsy. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. 2nd edition. Berlin, Germany: Springer; 2009. pp. 2575–2585. [Google Scholar]
- 41.Ellingson RJ, Wilken K, Bennett DR. Efficacy of sleep deprivation as an activation procedure in epilepsy patients. Journal of Clinical Neurophysiology. 1984;1(1):83–101. doi: 10.1097/00004691-198401000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Sperling MR, O’Connor MJ, Saykin AJ, et al. A noninvasive protocol for anterior temporal lobectomy. Neurology. 1992;42(2):416–422. doi: 10.1212/wnl.42.2.416. [DOI] [PubMed] [Google Scholar]
- 43.Engel J Jr., editor. Surgical Treatments of the Epilepsies. New York, NY, USA: Raven Press; 1993. [Google Scholar]
- 44.Hamer HM, editor. Noninvasive Electroencephalography Evaluation of the Irritative Zone. London, UK: Informa Healthcare; 2008. [Google Scholar]
- 45.Duncan JS. Imaging and epilepsy. Brain. 1997;120, part 2:339–377. doi: 10.1093/brain/120.2.339. [DOI] [PubMed] [Google Scholar]
- 46.Howe KL, Dimitri D, Heyn C, Kiehl TR, Mikulis D, Valiante TA. Histologically confirmed hippocampal structural features revealed by 3T MR imaging: potential to increase diagnostic specificity of mesial temporal sclerosis. American Journal of Neuroradiology. 2010;31(9):1682–1689. doi: 10.3174/ajnr.A2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinner DS, Lüders HO, Klem G. Chronic electrocorticography: cleveland clinic experience. Electroencephalography and Clinical Neurophysiology. Supplement. 1998;48:58–69. [PubMed] [Google Scholar]
- 48.Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia. 1994;35(supplement 6):S72–S89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- 49.Jones-Gotman M. Neuropsychological testing for localizing and lateralizing the epileptogenic region. In: Engel J Jr., editor. Surgical Treatment of the Epilepsies. New York, NY, USA: Raven Press; 1993. pp. 203–211. [Google Scholar]
- 50.Jokeit H, Schacher M. Neuropsychological aspects of type of epilepsy and etiological factors in adults. Epilepsy and Behavior. 2004;5(1):S14–S20. doi: 10.1016/j.yebeh.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Malow BA, Blaxton TA, Sato S, et al. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37(3):245–252. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 52.Hermann BP, Wyler AR, Richey ET, Rea JM. Memory function and verbal learning ability in patients with complex partial seizures of temporal lobe origin. Epilepsia. 1987;28(5):547–554. doi: 10.1111/j.1528-1157.1987.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 53.Sass KJ, Westerveld M, Buchanan CP, Spencer SS, Kim JH, Spencer DD. Degree of hippocampal neuron loss determines severity of verbal memory decrease after left anteromesiotemporal lobectomy. Epilepsia. 1994;35(6):1179–1186. doi: 10.1111/j.1528-1157.1994.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee TMC, Yip JTH, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43(3):283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 55.Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41(3):399–404. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- 56.Helmstaedter C, Elger CE. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37(2):171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 57.Wyler AR, Hermann BP, Somes G, Spencer DD, Roberts DW, Engel J., Jr. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37(5):982–991. doi: 10.1227/00006123-199511000-00019. [DOI] [PubMed] [Google Scholar]
- 58.Milner B, Branch C, Rasmussen T. Study of short-term memory after intracarotid injection of sodium Amytal. Transactions of the American Neurological Association. 1962;87:224–226. [Google Scholar]
- 59.Wada JA. A new method for determination of the side of cerebral dominance: a preliminary report on the intra-carotid injection of sodium amytal in man. Igaku Seibutsugaki. 1949;14:221–222. [Google Scholar]
- 60.Bazin JE, Picard P, Gabrillargues J, Dordain M. Propofol administered via the carotid artery to achieve a Wada test. Canadian Journal of Anaesthesia. 1998;45(7):707–708. doi: 10.1007/BF03012106. [DOI] [PubMed] [Google Scholar]
- 61.Gilmore RL, Heilman KM, Schmidt RP, Fennell EM, Quisling R. Anosognosia during Wada testing. Neurology. 1992;42(4):925–927. doi: 10.1212/wnl.42.4.925. [DOI] [PubMed] [Google Scholar]
- 62.Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50(6):1505–1516. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- 63.Dodrill CB, Ojemann GA. An exploratory comparison of three methods of memory assessment with the intracarotid amobarbital procedure. Brain and Cognition. 1997;33(2):210–223. doi: 10.1006/brcg.1997.0893. [DOI] [PubMed] [Google Scholar]
- 64.Rausch R, Babb TL, Engel J, Jr., Crandall PH. Memory following intracarotid amobarbital injection contralateral to hippocampal damage. Archives of Neurology. 1989;46(7):783–788. doi: 10.1001/archneur.1989.00520430077022. [DOI] [PubMed] [Google Scholar]
- 65.Simkins-Bullock J. Beyond speech lateralization: a review of the variability, reliability, and validity of the intracarotid amobarbital procedure and its nonlanguage uses in epilepsy surgery candidates. Neuropsychology Review. 2000;10(1):41–74. doi: 10.1023/a:1009044630227. [DOI] [PubMed] [Google Scholar]
- 66.Loddenkemper T, Morris HH, Perl J. Carotid artery dissection after the intracarotid amobarbital test. Neurology. 2002;59(11):1797–1798. doi: 10.1212/01.wnl.0000035635.30367.b6. [DOI] [PubMed] [Google Scholar]
- 67.Loddenkemper T, Moddel G, Morris HH. Complications during the intracarotid amobarbital test. Neurology. 2004;62(supplement 5):A248–A249. [Google Scholar]
- 68.Dinner DS, Loddenkemper T. In: Wada Test and Epileptogenic Zone. 1st edition. Luders HO, editor. London, UK: Informa Healthcare; 2008. [Google Scholar]
- 69.Jung WY, Pacia SV, Devinsky O. Neocortical temporal lobe epilepsy: intracranial EEG features and surgical outcome. Journal of Clinical Neurophysiology. 1999;16(5):419–425. doi: 10.1097/00004691-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Spencer SS, Williamson PD, Bridgers SL. Reliability and accuracy of localization by scalp ictal EEG. Neurology. 1985;35(11):1567–1575. doi: 10.1212/wnl.35.11.1567. [DOI] [PubMed] [Google Scholar]
- 71.Lee SK, Kim JY, Hong KS, Nam HW, Park SH, Chung CK. The clinical usefulness of ictal surface EEG in neocortical epilepsy. Epilepsia. 2000;41(11):1450–1455. doi: 10.1111/j.1528-1157.2000.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 72.Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46(5):669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- 73.Madhavan D, Kuzniecky R. Temporal lobe surgery in patients with normal MRI. Current Opinion in Neurology. 2007;20(2):203–207. doi: 10.1097/WCO.0b013e328042baba. [DOI] [PubMed] [Google Scholar]
- 74.Kloss S, Pieper T, Pannek H, Holthausen H, Tuxhorn I. Epilepsy surgery in children with focal cortical dysplasia (FCD): results of long-term seizure outcome. Neuropediatrics. 2002;33(1):21–26. doi: 10.1055/s-2002-23595. [DOI] [PubMed] [Google Scholar]
- 75.Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995;118, part 3:629–660. doi: 10.1093/brain/118.3.629. [DOI] [PubMed] [Google Scholar]
- 76.Luders H, Lesser RP, Dinner DS, Morris HH, Wylie E, Godoy J. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia. 1988;29(supplement 2):S56–S65. doi: 10.1111/j.1528-1157.1988.tb05799.x. [DOI] [PubMed] [Google Scholar]
- 77.Pacia SV, Ebersole JS. Intracranial EEG in temporal lobe epilepsy. Journal of Clinical Neurophysiology. 1999;16(5):399–407. doi: 10.1097/00004691-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127, part 7:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 79.Staba RJ, Wilson CL, Bragin A, Fried I. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. Journal of Neurophysiology. 2002;88(4):1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 80.Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45(9):1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 81.Bragin A, Staba RJ, Engel J Jr., editors. The Significance of Interictal Fast Ripples in the Evaluation of the Epileptogenic Zone. London, UK: Informa Healthcare; 2008. [Google Scholar]
- 82.Henry TR, Mazziotta JC, Engel J., Jr. Interictal metabolic anatomy of mesial temporal lobe epilepsy. Archives of Neurology. 1993;50(6):582–589. doi: 10.1001/archneur.1993.00540060022011. [DOI] [PubMed] [Google Scholar]
- 83.Salanova V, Markand O, Worth R. Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurologica Scandinavica. 2004;109(2):126–131. doi: 10.1034/j.1600-0404.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 84.Chugani HT, Shewmon DA, Khanna S, Phelps ME. Interictal and postictal focal hypermetabolism on positron emission tomography. Pediatric Neurology. 1993;9(1):10–15. doi: 10.1016/0887-8994(93)90003-u. [DOI] [PubMed] [Google Scholar]
- 85.Ryvlin P, Bouvard S, Le Bars D, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998;121, part 11:2067–2081. doi: 10.1093/brain/121.11.2067. [DOI] [PubMed] [Google Scholar]
- 86.Engel J, Jr., Kuhl DE, Phelps ME, Crandall PH. Comparative localization of epileptic foci in partial epilepsy by PCT and EEG. Annals of Neurology. 1982;12(6):529–537. doi: 10.1002/ana.410120605. [DOI] [PubMed] [Google Scholar]
- 87.Gaillard WD, Bhatia S, Bookheimer SY, Fazilat S, Sato S, Theodore WH. FDG-PET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology. 1995;45(1):123–126. doi: 10.1212/wnl.45.1.123. [DOI] [PubMed] [Google Scholar]
- 88.Salanova V, Markand O, Worth R, et al. FDG-PET and MRI in temporal lobe epilepsy: relationship to febrile seizures, hippocampal sclerosis and outcome. Acta Neurologica Scandinavica. 1998;97(3):146–153. doi: 10.1111/j.1600-0404.1998.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 89.O’Brien TJ, So EL, Mullan BP, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50(2):445–454. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- 90.Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. Journal of Nuclear Medicine. 1999;40(5):730–736. [PubMed] [Google Scholar]
- 91.O’Brien TJ, So EL, Mullan BP, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000;55(11):1668–1677. doi: 10.1212/wnl.55.11.1668. [DOI] [PubMed] [Google Scholar]
- 92.So EL. Integration of EEG, MRI, and SPECT in localizing the seizure focus for epilepsy surgery. Epilepsia. 2000;41(supplement 3):S48–S54. doi: 10.1111/j.1528-1157.2000.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 93.Lachhwani D, Cascino GD, editors. Ictal SPECT in the Definition of the Seizure Onset Zone. London, UK: Informa Healthcare; 2008. [Google Scholar]
- 94.Barth DS. The neurophysiological basis of epileptiform magnetic fields and localization of neocortical sources. Journal of Clinical Neurophysiology. 1993;10(1):99–107. doi: 10.1097/00004691-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Benke T, Köylü B, Visani P, et al. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada test. Epilepsia. 2006;47(8):1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 96.Sutherling WW, Barth DS. Neocortical propagation in temporal lobe spike foci on magnetoencephalography and electroencephalography. Annals of Neurology. 1989;25(4):373–381. doi: 10.1002/ana.410250409. [DOI] [PubMed] [Google Scholar]
- 97.Leijten FSS, Huiskamp GJM, Hilgersom I, Van Huffelen AC. High-resolution source imaging in mesiotemporal lobe epilepsy: a comparison between MEG and simultaneous EEG. Journal of Clinical Neurophysiology. 2003;20(4):227–238. doi: 10.1097/00004691-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Pataraia E, Simos PG, Castillo EM, et al. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004;62(6):943–948. doi: 10.1212/01.wnl.0000115122.81621.fe. [DOI] [PubMed] [Google Scholar]
- 99.Wennberg R, Valiante T, Cheyne D. EEG and MEG in mesial temporal lobe epilepsy: where do the spikes really come from? Clinical Neurophysiology. 2011;122(7):1295–1313. doi: 10.1016/j.clinph.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 100.Halgren E. How can intracranial recordings assist MEG source localization? Neurology and Clinical Neurophysiology. 2004;2004, article 86 [PubMed] [Google Scholar]
- 101.Otsubo H, Chitoku S, Ochi A, et al. Malignant rolandic-sylvian epilepsy in children: diagnosis, treatment, and outcomes. Neurology. 2001;57(4):590–596. doi: 10.1212/wnl.57.4.590. [DOI] [PubMed] [Google Scholar]
- 102.Knowlton RC, Laxer KD, Aminoff MJ, Roberts TPL, Wong STC, Rowley HA. Magnetoencephalography in partial epilepsy: clinical yield and localization accuracy. Annals of Neurology. 1997;42(4):622–631. doi: 10.1002/ana.410420413. [DOI] [PubMed] [Google Scholar]
- 103.Ganslandt O, Fahlbusch R, Nimsky C, et al. Functional neuronavigation with magnetoencephalography: outcome in 50 patients with lesions around the motor cortex. Journal of Neurosurgery. 1999;91(1):73–79. doi: 10.3171/jns.1999.91.1.0073. [DOI] [PubMed] [Google Scholar]
- 104.Gross RE, Dean A, Lewine J, et al. The relationship of magnetic source imaging to ictal electrocorticography in a neuronavigational workspace. Stereotactic and Functional Neurosurgery. 2000;73(1–4):109–114. doi: 10.1159/000029765. [DOI] [PubMed] [Google Scholar]
- 105.Capizzano AA, Vermathen P, Laxer KD, et al. Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. American Journal of Neuroradiology. 2002;23(8):1359–1368. [PMC free article] [PubMed] [Google Scholar]
- 106.Carreno M, Luders HO, editors. General Principles of Presurgical Evaluation. London, UK: Informa Healthcare; 2008. [Google Scholar]
- 107.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mirsattari SM, Ives JR, Leung LS, Menon RS. EEG monitoring during functional MRI in animal models. Epilepsia. 2007;48(supplement s4):37–46. doi: 10.1111/j.1528-1167.2007.01240.x. [DOI] [PubMed] [Google Scholar]
- 109.Mirsattari SM, Ives JR, Bihari F, Leung LS, Menon RS, Bartha R. Real-time display of artifact-free electroencephalography during functional magnetic resonance imaging and magnetic resonance spectroscopy in an animal model of epilepsy. Magnetic Resonance in Medicine. 2005;53(2):456–464. doi: 10.1002/mrm.20357. [DOI] [PubMed] [Google Scholar]
- 110.Medina LS, Bernal B, Ruiz J. Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: a Bayesian analysis. Radiology. 2007;242(1):94–100. doi: 10.1148/radiol.2421050677. [DOI] [PubMed] [Google Scholar]
- 111.Janszky J, Jokeit H, Kontopoulou K, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005;46(2):244–250. doi: 10.1111/j.0013-9580.2005.10804.x. [DOI] [PubMed] [Google Scholar]
- 112.Sabsevitz DS, Swanson SJ, Hammeke TA, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- 113.Penfield W, Baldwin M. Temporal lobe seizures and the technic of subtotal temporal lobectomy. Annals of Surgery. 1952;136(4):625–634. [PMC free article] [PubMed] [Google Scholar]
- 114.Olivier A. Surgery of epilepsy: methods. Acta Neurologica Scandinavica. 1988;78(supplement 117):103–111. doi: 10.1111/j.1600-0404.1988.tb08011.x. [DOI] [PubMed] [Google Scholar]
- 115.Crandall PH. Standard en Bloc Anterior Temporal Lobectomy. Boston, Mass, USA: Blackwell Scientific; 1991. [Google Scholar]
- 116.Spencer DD, Spencer SS, Mattson RH. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15(5):667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 117.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery. 1989;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 118.Niemeyer P. In: The Transventricular Amygdalo-Hippocampectomy in Temporal Lobe Epilepsy. Baldwin M, Bailey P, editors. Springfield, Ill, USA: Charles Thomas; 1958. [Google Scholar]
- 119.Yaşargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Advances and Technical Standards in Neurosurgery. 1985;12:93–123. doi: 10.1007/978-3-7091-7008-3_2. [DOI] [PubMed] [Google Scholar]
- 120.Morris AA. Temporal lobectomy with removal of uncus, hippocampus, and amygdala; results for psychomotor epilepsy three to nine years after operation. A. M. A. Archives of Neurology and Psychiatry. 1956;76(5):479–496. doi: 10.1001/archneurpsyc.1956.02330290023003. [DOI] [PubMed] [Google Scholar]
- 121.Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiologica Scandinavica. Supplementum. 1951;24(83):1–262. [PubMed] [Google Scholar]
- 122.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia. 2004;45(8):960–962. doi: 10.1111/j.0013-9580.2004.42203.x. [DOI] [PubMed] [Google Scholar]
- 123.Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. Journal of Neurosurgery. 1995;83(2):237–242. doi: 10.3171/jns.1995.83.2.0237. [DOI] [PubMed] [Google Scholar]
- 124.Awad IA, Rosenfeld J, Ahl J, Hahn JF, Luders H. Intractable epilepsy and structural lesions of the brain: mapping, resection strategies, and seizure outcome. Epilepsia. 1991;32(2):179–186. doi: 10.1111/j.1528-1157.1991.tb05242.x. [DOI] [PubMed] [Google Scholar]
- 125.Carreno M, Luder HO. In: General Principles of Pre-Surgical Evaluation. Luder HO, editor. London, UK: Informa Healthcare; 2008. [Google Scholar]
- 126.Wennberg R, Arruda F, Quesney LF, Olivier A. Preeminence of extrahippocampal structures in the generation of mesial temporal seizures: evidence from human depth electrode recordings. Epilepsia. 2002;43(7):716–726. doi: 10.1046/j.1528-1157.2002.31101.x. [DOI] [PubMed] [Google Scholar]
- 127.Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214(5092):1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- 128.Feindel W, Penfield W. Localization of discharge in temporal lobe automatism. A. M. A. Archives of Neurology and Psychiatry. 1954;72(5):603–630. [PubMed] [Google Scholar]
- 129.Feindel W, Penfield W, Jasper H. Localization of epileptic discharge in temporal lobe automatism. Transactions of the American Neurological Association. 1952;56:14–17. [PubMed] [Google Scholar]
- 130.Hudson LP, Munoz DG, Miller L, McLachlan RS, Girvin JP, Blume WT. Amygdaloid sclerosis in temporal lobe epilepsy. Annals of Neurology. 1993;33(6):622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- 131.Engel J, Jr., Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy—report of the quality standards subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 132.Behrens E, Schramm J, Zentner J, König R. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41(1):1–10. doi: 10.1097/00006123-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 133.Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990–1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49(1):51–57. doi: 10.1097/00006123-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 134.Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43(2):170–174. doi: 10.1046/j.1528-1157.2002.33800.x. [DOI] [PubMed] [Google Scholar]
- 135.Egan RA, Shults WT, So N, Burchiel K, Kellogg JX, Salinsky M. Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology. 2000;55(12):1818–1822. doi: 10.1212/wnl.55.12.1818. [DOI] [PubMed] [Google Scholar]
- 136.Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. Journal of Neurosurgery. 1991;74(4):560–566. doi: 10.3171/jns.1991.74.4.0560. [DOI] [PubMed] [Google Scholar]
- 137.Davies KG, Risse GL, Gates JR. Naming ability after tailored left temporal resection with extraoperative language mapping: increased risk of decline with later epilepsy onset age. Epilepsy and Behavior. 2005;7(2):273–278. doi: 10.1016/j.yebeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 138.Hermann BP, Chelune GJ, Loring DW, et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13(1):3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- 139.Stafiniak P, Saykin AJ, Sperling MR, et al. Acute naming deficits following dominant temporal lobectomy: prediction by age at 1st risk for seizures. Neurology. 1990;40(10):1509–1512. doi: 10.1212/wnl.40.10.1509. [DOI] [PubMed] [Google Scholar]
- 140.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clinical Neurosurgery. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 142.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. A. M. A. Archives of Neurology and Psychiatry. 1958;79(5):475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- 143.Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(supplement 4):45–49. doi: 10.1111/j.1528-1167.2005.463009.x. [DOI] [PubMed] [Google Scholar]
- 144.Brierley B, Medford N, Shaw P, David AS. Emotional memory and perception in temporal lobectomy patients with amygdala damage. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75(4):593–599. doi: 10.1136/jnnp.2002.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Blumer D. Psychiatric aspects of intractable epilepsy. Advances in Experimental Medicine and Biology. 2002;497:133–147. doi: 10.1007/978-1-4615-1335-3_15. [DOI] [PubMed] [Google Scholar]
- 146.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124(9):1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 147.Morris H, Najm I, Kahane P. In: Epilepsy Surgery: Patient Selection. 1st edition. Luders HO, editor. London, UK: Informa Healthcare; 2008. [Google Scholar]


