Abstract
B7-H3, a novel member of the B7 superfamily, plays a critical role during T cell activation; its functions are still unclear. In this study we obtained a novel anti-mouse B7-H3 monoclonal antibody (MAb) and characterized its biological functions. Our results demonstrated that this MAb could be used for flow cytometry and Western blot and immunohistochemistry analyses, suggesting that the performance of this MAb is much better than a commercial MAb (M3.2D7). Furthermore, data showed different expression profiles of mouse B7-H3 on various immune cells. We further showed that mouse B7-H3 protein was not expressed on normal tissues except for bladder epithelial cells using this MAb. Interestingly, the MAb could stimulate the proliferation and cytokine secretion of T cells. Taken together, this MAb might be of great value for further investigation of B7-H3 molecule.
Introduction
Also referred to as CD276, B7 homolog3 (B7-H3) has been identified both in human and mice. Murine B7-H3 gene encodes a 316 amino acid protein, which shares about 87% sequence homology with human.(1,2) Mouse B7-H3 protein is a type I transmembrane glycoprotein containing two extracellular Ig domains. Murine B7-H3 mRNA is widely expressed in multiple tissues, but B7-H3 protein is not detected in these tissues.(1–3) Until now the B7-H3 receptor had not been identified.(4,5) Previous studies showed B7-H3 stimulated the proliferation of T cells and enhanced the secretion of IFN-γ.(6) But subsequent results indicated that B7-H3 down-regulated Th1-mediated immune responses.(7,8) Luo and colleagues demonstrated that B7-H3 had antitumor activity in mice.(9) Recently, B7-H3 was shown to be uniformly aberrantly expressed in sera or tumor tissues of cancer patients.(10–14) Thus B7-H3 might be a promising target in diagnosis and therapy for malignancies.
In this study, we generated a novel rat anti-mouse B7-H3 MAb and examined the expression of B7-H3 molecule by immunostaining. Furthermore, we found that this antibody could stimulate the proliferation and enhance the cytokine secretion of T cells.
Materials and Methods
Animals, cell lines, and antibodies
SD rats were purchased from the Department of Experimental Animals, Shanghai Institute of Biological Products (Ministry of Health of China, Shanghai, China). Mouse myeloma cell line SP2/0, Chinese hamster ovary (CHO) cells, and human embryonic kidney (293) cells were originally obtained from American Type Culture Collection (Manassas, VA). These cells were cultured in RPMI 1640 or DMEM (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 25 mM HEPES buffer. PE-conjugated rat anti-mouse B7-H3 MAb (clone M3.2D7) and PE-conjugated donkey anti-rat IgG (H+L) were purchased from eBioscience (Woburn, MA). HRP-conjugated goat anti-rat IgG (H+L) and rat IgG2b were purchased from Immunotech (Marseille, France). All chemicals were obtained from Sigma-Aldrich (St. Louis, MO). All immunohistochemistry reagents were obtained from Invitrogen (Carlsbad, CA).
Construction of transfectants
The full-length cDNA encoding mouse B7-H3 was cloned from bone marrow–derived dendritic cells by reverse transcription polymerase chain reaction (RT-PCR) with specific primers and was inserted into vector pIRES2-EGFP (Clontech, Mountain View, CA). The recombinant vector was transfected into CHO and 293 cells by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The B7-H3 transfected cells (CHO/B7-H3 and 293/B7-H3) were selected by G418 (Invitrogen) and confirmed by a combination of commercial PE-conjugated anti-mouse B7-H3 MAb (M3.2D7) and GFP using flow cytometry (Beckman Coulter, Brea, CA). Empty vector-transfected CHO and 293 cell lines (CHO/mock and 293/mock, respectively) were obtained in the same manner.
Generation of anti-mouse B7-H3 MAb
Female SD rats were immunized with injections of 1×107 293/B7-H3 cells in 0.5 mL phosphate-buffered saline (PBS) per rat at 21 day intervals for a total of four times. The first subcutaneous injection was accompanied with complete Freund's adjuvant. Four days after the final boost injection, the splenocytes of immunized rats were fused with murine myeloma SP2/0 cells according to the method described previously.(15) Flow cytometry (Beckman Coulter) was performed to screen positive clones. CHO/B7-H3 cells were used as the positive control and CHO/mock cells were used as the negative control. The anti-mouse B7-H3 MAb was purified from the ascites of nude mice using protein G-sepharose CL4B affinity columns (Pharmacia, Uppsala, Sweden).
Characterization of MAb
The Ig isotype was identified with multiplex fluorescent bead assay (SouthernBiotech, Birmingham, LA) according to the manufacturer's instructions. To analyze the expression of mouse B7-H3 molecule on cells, including T cells, DCs, monocytes, NK cells, and B cells, 1×106 cells were incubated with MAb 18F9 for 30 min at 4°C. After washing with PBS, the cells were stained with PE-conjugated donkey anti-rat IgG for another 30 min, and analyzed using flow cytometry. Western blotting was performed to analyze the binding capacity of the two MAbs (18F9 and M3.2D7) to recombinant B7-H3-Ig. Briefly, 5 μg of purified B7-H3-Ig were mixed with loading buffer and boiled at 95–100°C for 5 min followed by separation on 10% SDS-polyacrylamide gels, transferred onto a nitrocellulose membrane, which was incubated with biotinylated anti-mouse B7-H3 MAbs or rat IgG2b isotype control for 1 h. After washing, the membrane was stained with horseradish peroxidase (HRP)-conjugated goat anti-rat IgG for 2 h. Results were observed utilizing the Chemiluminescence Western Blotting Kit from Boehringer (Mannheim, Germany) according to the manufacturer's instructions.
Immunohistochemical staining
The paraffin sections of mouse tissues were collected for immunohistochemical staining. In brief, after dewaxing, sections were incubated with 3% H2O2 in PBS for 10 min to block endogenous peroxidase. Sections were treated with EDTA working dilution for antigen retrieval. After cross-reactivity was blocked with normal non-immune rat serum, the sections were incubated at 37°C for 1 h with biotinylated MAb 18F9 (10 μg/mL). The sections were incubated with streptavidin-HRP for 30 min, then DAB solution for 3 min. Negative controls were established by replacing the primary antibody (MAb 18F9) with rat IgG2b isotype control. Positive staining of B7-H3 was defined as the presence of brown color in cytoplasm and on cell membrane.
Cell proliferation assay
T cells were cultured in 96-well cell culture plate in triplicate (1×105 cells/well) in complete RPMI 1640 at 37°C, 5% CO2. The wells were coated with agonistic anti-CD3 MAb (0.5 μg/mL). T cells were incubated with various concentration of MAb 18F9 for up to 3 days. Cell proliferation was analyzed by cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) at 2, 6, 12, 24, 48, and 72 h, respectively. The cytokine levels in the supernatants of T cell cultures were assessed by two commercial ELISA kits, IFN-γ and IL-2 (Invitrogen/Biosource, Carlsbad, CA), according to the manufacturer's instructions.
Statistical analysis
The statistical analysis was performed using two-tailed t-test. A p value of<0.05 was considered significant.
Results
Establishment of novel rat anti-mouse B7-H3 MAb
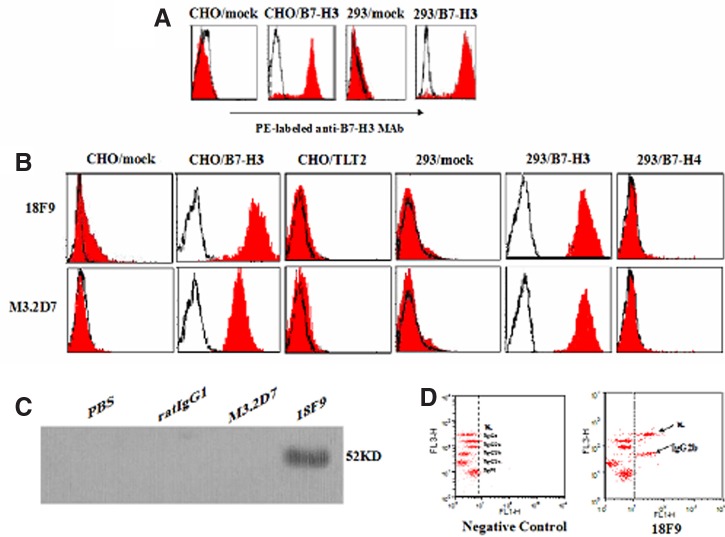
We cloned the full length of mouse B7-H3 cDNA from mouse DCs and transfected it into 293 cells and CHO cells, respectively. To generate MAbs specific for mouse B7-H3, the splenocytes of rats immunized with 293/B7-H3 cells were fused with myeloma SP2/0 cells. After few cycles of screenings and sub-clonings, one stable hybridoma cell line named 18F9 was obtained (Fig. 1A). As shown in Figure 1B, the MAb produced by this clone could recognize transfected cells, CHO/B7-H3 and 293/B7-H3, but not CHO/mock, CHO/TLT2, 293/mock, and 293/B7-H4 cells, indicating that the MAb is specific for B7-H3. Western blot analysis was performed to confirm the binding ability of 18F9 to recombinant B7-H3-Ig. The data in Figure 1C showed that, unlike commercial MAb M3.2D7, MAb 18F9 specifically bound to B7-H3-Ig. Thus we prepared one specific rat anti-mouse MAb that could be used for flow cytometry and Western blot analyses. The isotype of the MAb was IgG2b subclass with a κ light chain (Fig. 1D).
FIG. 1.
Preparation of mouse B7-H3 transfectants and characterization of B7-H3 MAb. (A) CHO/B7-H3 and 293/B7-H3 transfectants were selected and identified by flow cytometry using PE-labeled M3.2D7 (red histograms) against PE-conjugated rat IgG2b (open histograms). (B) Reactivity of the MAbs with the transfectants CHO/mock, CHO/B7-H3, CHO/TLT2, 293/mock, 293/B7-H3, and 293/B7-H4. Cells were stained with MAbs 18F9 and M3.2D7 (red histograms) or negative control rat IgG2b (open histograms). (C) Purified B7-H3-Ig was separated on 10% SDS-polyacrylamide gels, transferred to NC membrane, and stained with PBS, rat IgG2b, M3.2D7, and 18F9. (D) The Ig isotype of the MAb was identified with carboxyl blue-based bead assay. Negative control and 18F9 staining were shown in figure at left (without hybridoma supernatant) and figure at right (with hybridoma supernatant), respectively. The figure is representative of four independent experiments.
Expression of mouse B7-H3 molecule by immune cells
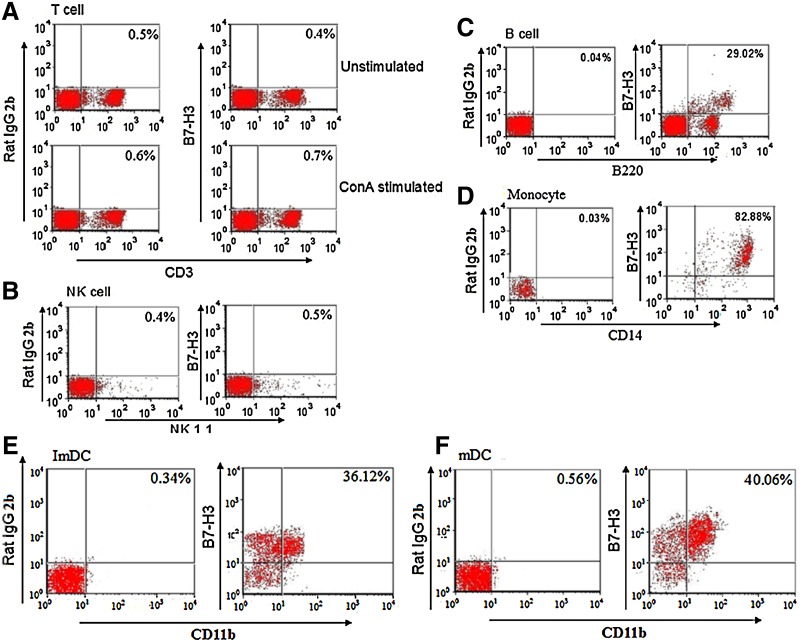
The expression of mouse B7-H3 was examined on immune cells by flow cytometry analysis. In spleen, B7-H3 was not expressed on NK cells and resting and activated T cells (Fig. 2A, B). In contrast, 29.02% of B220+ B cells (Fig. 2C) and 82.88% of CD14+ monocytes (Fig. 2D) constitutively expressed B7-H3. Bone marrow-derived immature and mature dendritic cells both expressed B7-H3 (Fig. 2E, F), suggesting that mouse B7-H3 was expressed on antigen-presenting cells (APCs).
FIG. 2.
Expression of mouse B7-H3 on immune cells. Expression of B7-H3 by spleen cells (A–D) and bone marrow-derived dendritic cells (E, F) were examined by 18F9 in conjunction with other indicated markers. Rat IgG2b was used as an isotype control. The figure is representative of three independent experiments.
MAb 18F9 recognizes B7-H3 molecule in tissues
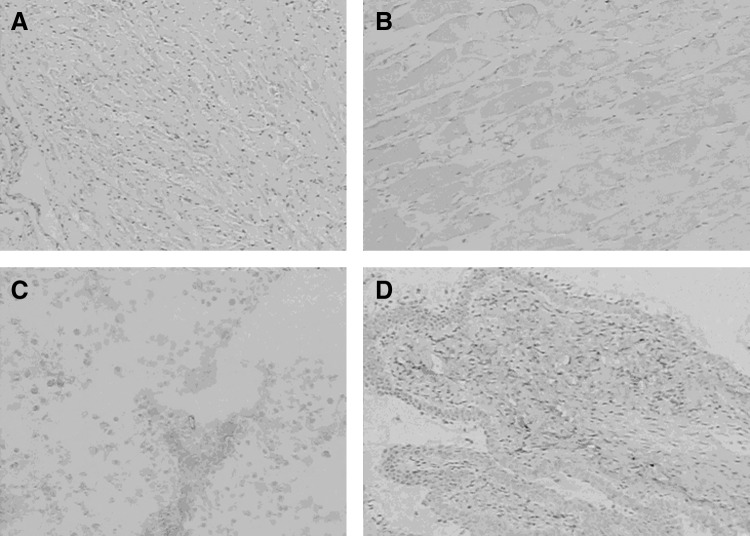
The expression of murine B7-H3 on various tissues was investigated by immunohistochemistry staining with MAb 18F9. Results showed that murine B7-H3 protein was not expressed in normal tissues, except for bladder epithelial cells (Fig. 3). Thus, MAb 18F9 performed well during immunohistochemical staining and may have great value for the study of bladder cancers.
FIG. 3.
Immunohistochemistry analysis of B7-H3 in mouse tissues by MAb 18F9. Heart (A, ×40), muscle (B, ×40), liver (C, ×100), and bladder (D, ×40). Brown color was defined as positive staining. All these experiments used rat IgG2b as isotype control. The figure is representative of three independent experiments.
Effect of MAb 18F9 on T cell proliferation and cytokine production
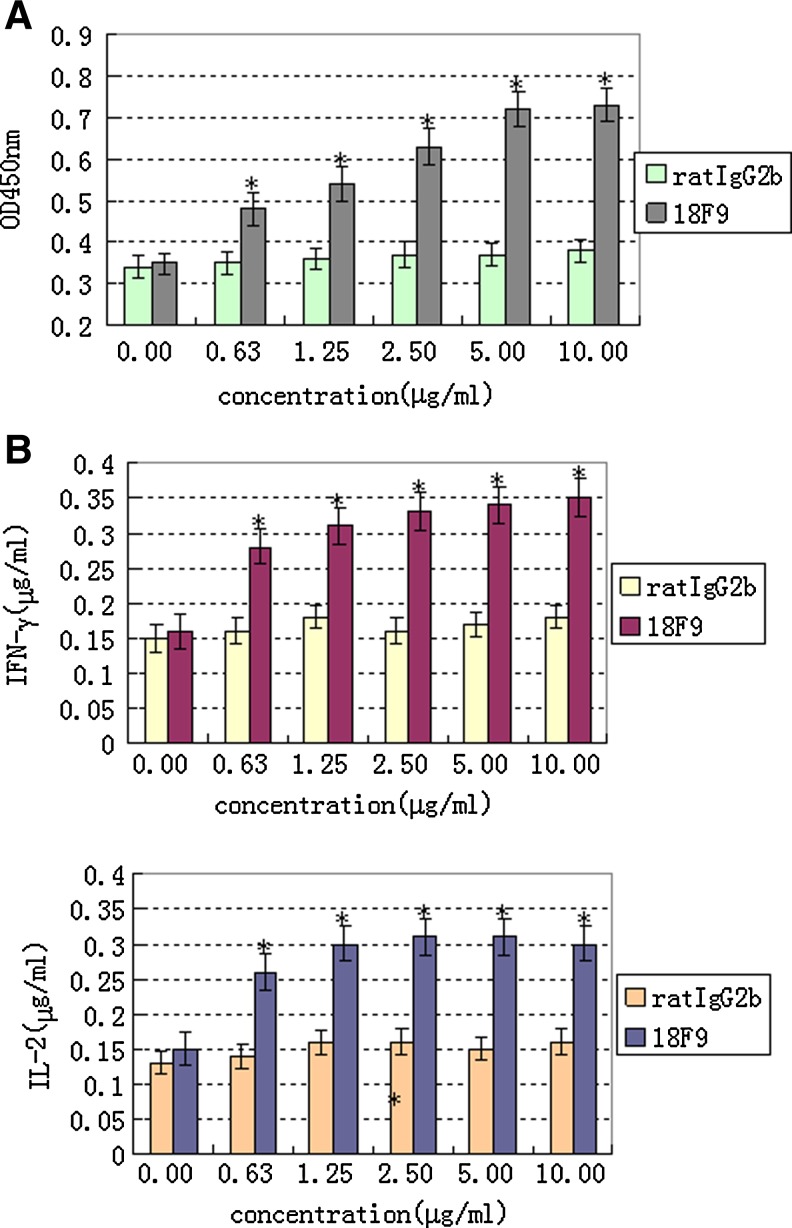
In this study, the biological effect of MAb 18F9 on T cell proliferation and cytokine production in vitro was performed by assays of CCK8 and ELISA. Interestingly, we found that MAb 18F9 could obviously promote the proliferation of T cells in a dose-dependent manner (Fig. 4A). The secretion of cytokines (IL-2 and IFN-γ) reached maximum levels at 24 h stimulated by MAb 18F9 (Fig. 4B)
FIG. 4.
T cell proliferation and cytokine production stimulated by MAb 18F9. Purified T cells were activated with plate-bound agonistic anti-CD3 MAb. MAb 18F9 was applied and cell proliferation (A) and cytokines production (B) were measured. Rat IgG2b was used as isotype control (*p<0.05). The data shown are representative of four independent experiments with similar results.
Discussion
B7-H3, a sixth member of the B7 ligand family, was first identified as a positive co-stimulatory regulator of T cell responses.(6) However, after further investigation, the regulatory roles of B7-H3 are controversial. Both positive and negative effects of B7-H3 during immune response have been reported.(6–8) Mouse B7-H3 mRNA was expressed in multiple tissues while the expression profile of B7-H3 protein is not clear. B7-H3 has a soluble form and its level was increased in the sera of lung cancer patients as well as other diseases.(16–18) But the physiologic and pathologic roles of B7-H3 are little understood.
In order to explore the functions of B7-H3 molecule, one specific rat anti-mouse MAb 18F9 was prepared. Titration studies showed that 18F9 could recognize a different epitope of B7-H3 from M3.2D7 (data not shown). Our further studies found that this MAb could be used in Western blot analysis, immunocytostaining, and immunohistochemical staining. We also analyzed the expression of the MAb by immune cells. The results showed that mouse B7-H3 molecule was expressed on B cells, monocytes, immature and mature dendritic cells, but not on NK cells, resting cells, and activated T cells. We also found the MAb we generated could promote T cell proliferation and the secretion of IL-2 and IFN-γ. Murine B7-H3 protein was expressed only on the cytoplasma and membrane of bladder epithelial cells rather than on any other tissues we investigated. In conclusion, our studies provide a novel and useful tool to investigate the expression and functions of mouse B7-H3 for both basic and applied purposes.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (30972718 and 31100626).
Author Disclosure Statement
The authors have no financial conflict of interest to disclose. The antibody we generated was distributed solely to nonprofit research organizations for research purposes only.
References
- 1.Sun M. Richards S. Prasad DV. Mai XM. Rudensky A. Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 2.Ling V. Wu PW. Spaulding V. Kieleczawa J. Luxenberg D. Carreno BM. Collins M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–377. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 3.Steinberger P. Majdic O. Derdak SV. Pfistershammer K. Kirchberger S. Klauser C. Zlabinger G. Pickl WF. Stöckl J. Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of B7 family with four Ig-like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 4.Hashiguchi M. Kobori H. Ritprajak P. Kamimura Y. Kozono H. Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitner J. Klauser C. Pickl WF. Stockl J. Majdic O. Bardet AF. Kreil DP. Dong C. Yamazaki T. Zlabinger G. Pfistershammer K. Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapoval AI. Ni J. Lau JS. Wilcox RA. Flies DB. Liu D. Dong H. Sica GL. Zhu G. Tamada K. Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 7.Prasad DV. Nguyen T. Li Z. Yang Y. Duong J. Wang Y. Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 8.Suh WK. Gajewska BU. Okada H. Gronski MA. Bertram EM. Dawicki W. Duncan GS. Bukczynski J. Plyte S. Elia A. Wakeham A. Itie A. Chung S. Da Costa J. Arya S. Horan T. Campbell P. Gaida K. Ohashi PS. Watts TH. Yoshinaga SK. Bray MR. Jordana M. Mak TW. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 9.Luo L. Chapoval AI. Files DB. Zhu G. Hirano F. Wang S. Lau JS. Dong H. Tamada K. Files AS. Liu Y. Chen L. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific cd8+ cytolytic T cells. J Immunol. 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 10.Crispen PL. Sheinin Y. Roth TJ. Lohse CM. Kuntz SM. Frigola X. Thompson RH. Boorjian SA. Dong H. Leibovich BC. Blute ML. Kwon ED. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth TJ. Sheinin Y. Lohse CM. Kuntz SM. Frigola X. Inman BA. Krambeck AE. McKenney ME. Karnes RJ. Blute ML. Cheville JC. Sebo TJ. Kwon ED. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 12.Zang X. Thompson RH. Al-Ahmadie HA. Serio AM. Reuter VE. Eastham JA. Scardino PT. Sharma P. Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CP. Jiang JT. Tan M. Zhu YB. Ji M. Xu KF. Zhao JM. Zhang GB. Zhang XG. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis: World. J Gastroenterol. 2006;12:457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang G. Xu Y. Lu X. Huang H. Zhou Y. Lu B. Zhang X. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer. 2009;66:245–249. doi: 10.1016/j.lungcan.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.de StGroth SF. Scheidegger D. Production of monoclonal antibodies: strategy tactics. J Immunol Methods. 1980;35:1–12. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen X. Zhang G. Li Y. Feng X. Wan F. Zhang L. Wang J. Zhang X. Circulating B7-H3 (CD276) elevations in cerebrospinal fluid and plasma of children with bacterial meningitis. J Mol Neurosci. 2009;37:86–94. doi: 10.1007/s12031-008-9133-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhang G. Hou J. Shi J. Yu G. Lu B. Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells, and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G. Wang J. Kelly J. Gu G. Hou J. Zhou Y. Redmond HP. Wang JH. Zhang X. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185:3677–3684. doi: 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]