Abstract
Traditionally monoclonal antibody (MAb) titer is determined by indirect enzyme-linked immunosorbent assay (ELISA), which is primarily used to evaluate the quality of MAbs. In this study, the titer and affinity of a group of MAbs against ovalbumin (OVA) were tested by indirect ELISA and the ELISA method reported previously. Data showed that there may be great differences between the indirect ELISA antibody titer and affinity value of MAbs. For the first time, a simple and effective reverse direct ELISA (RD-ELISA) was established for the detection of high-affinity MAbs. Among the group of MAbs to OVA, a certain proportion of antibodies with high affinity but low indirect ELISA titer do exist and can be clearly and efficiently detected by RD-ELISA. This study demonstrates that RD-ELISA is an effective method for high-affinity MAb screening.
Introduction
MAbs play very important roles in many scientific disciplines such as immunology, histology, and tumor pathology.(1) It is therefore crucial for a researcher to screen for a readily available antibody of superior quality. Conventionally an antibody of superior quality was selected by the antibody titer, which, in spite of a high degree of dilution, yielded a positive result.(2)
In this study, our results indicate some discrepancy between the titer and affinity of MAbs; some MAbs existed with a lower titer but higher affinity. This was easily missed by antibody titer determination by the traditional method. Therefore, it is necessary to improve the efficacy of screening high-affinity MAbs by ELISA. For this purpose, a new format of ELISA was developed by employing a horseradish peroxidase (HRP)-conjugated antigen reactor with a coating of MAbs at constant dilutions. The results of the RD-ELISA correlated well with antibody affinity.
Materials and Methods
Reagents
Freund's incomplete adjuvant, Freund's complete adjuvant, Tween-20, 3,3’,5,5’-tetramethyl-benzidine dihydrochloride hydrate (TMB), and horseradish peroxidase (HRP) were purchased from Sigma (St. Louis, MO). PEG 4000 was purchased from Merck (MW 4000; Darmstadt, Germany). SBA Clonotyping System was purchased from Southern Biotech (Birmingham, AL). Ovalbumin (OVA, Grade VII) was purchased from Sigma. Fetal bovine serum (FBS) was purchased from Gibco (Invitrogen, Grand Island, NY). 0.05% Tween-20 (v/v) in phosphate-buffered saline (PBS) was used as the washing buffer. PBS containing 10% FBS (v/v) and 0.05% Tween-20 (v/v) were applied as blocking buffer. PBS containing 10% FBS (v/v) and 0.05% Tween-20 (v/v) were used as dilution buffer. TMB solution containing 2.5 mg TMB, 10 μL of 3% H2O2, and 10 mL substrate buffer was applied as ELISA color development substrate. RPMI 1640 (HyClone, Logan, UT) was also used in this study.
Apparatus
The ELISA plate was purchased from Corning-Costar (Corning, NY). A microplate reader (Bio-Rad, Hercules, CA) and Fast Protein Liquid Chromatography (FPLC) system (Amersham, Buckinghamshire, United Kingdom) were used in this study.
Production of MAbs
OVA MAbs were generated using a conventional protocol in our laboratory.(3–6) Briefly, female BALB/c mice (8 weeks old) were treated in accordance with the Guide for Care and Use of Experimental Animals approved by the Animal Care Committee of The Fourth Military Medical University, and immunized with 20 μg OVA antigen in complete Freund's adjuvant by subcutaneous (s.c.) injection. Subsequent immunizations were carried out twice with 20 μg OVA antigen in incomplete Freund's adjuvant by s.c. and intraperitoneal (i.p.) injection at 3-week intervals, respectively. Ten days after the third immunization, blood sera titers were determined by indirect ELISA. The mouse with the highest serum titers was boosted with 20 μg OVA. At 72 h, splenocytes were isolated from the boosted mice and were fused with Sp2/0 murine myeloma cells in the presence of PEG 4000. The positive hybrids were selected by indirect ELISA with OVA as coating antigen and then subcloned three times using limiting dilution method. MAbs were produced from ascites of BALB/c mice and purified by Q Sepharose Fast Flow ion-exchange chromatography column connected to a Fast Protein Liquid Chromatography (FPLC) system. The immunoglobulin class and subclass of each MAb was determined by the isotype kit following the manufacturer's recommendations.
Anti-OVA MAbs (FMU-OVA 1∼9) had been prepared previously by our group.(4)
MAb titer detection
The titer of OVA MAbs was determined by indirect ELISA employing the conventional protocol.(7) The wells of the ELISA plate were coated with 2 μg/mL OVA in coating buffer and incubated overnight at 4°C. After three washings, each MAb (2 mg/mL) was serially diluted from 1:1×103 to 1:1×1010 with dilution buffer, added to the wells (100 μL/well), and incubated for 1 h at 37°C. After washing, 100 μL/well of 1:2500 diluted HRP-conjugated goat anti-mouse IgG was added into the plates and incubated for 45 min at 37°C. After a final washing, color development was performed by adding 100 μL of TMB solution. The plates were incubated for 15 min at 37°C. The reaction was subsequently quenched with 2 M sulfuric acid (50 μL/well), and the absorbance at 450 nm was measured with a microplate reader.
Measurement of MAb affinity
The affinity of MAbs was measured by the method described previously.(8) Briefly, the ELISA plate was coated with 2 μg/mL of OVA. After incubation for a 15 h period at 4°C, the plate was washed three times, then blocked by adding dilution buffer, and incubated for 1 h at room temperature. At the same time, OVA antigen at various concentrations (from 3×10−9 M to 1×10−7 M) was incubated with anti-OVA antibodies at constant concentration (deduced from preliminary ELISA calibration for each MAb) for 15 h at room temperature and 100 μL of each mixture was transferred into the wells of the ELISA plate previously coated with OVA and incubated for 1 h at 37°C. After washing, 1:2500 diluted HRP-conjugated goat anti-mouse IgG was added and the plate was incubated for 45 min at 37°C. Finally, the plate was washed five times and then observed with TMB. The dissociation constant (KD) was calculated according to the following formula:
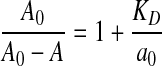 |
(Eq 1) |
RD-ELISA
The microplate was coated with anti-OVA (10 μg/mL) overnight at 4°C. Next, the microplate was washed three times with washing buffer, and the HRP-conjugated OVA (serially diluted from 1:100 to 1:12,800 in dilution buffer) was added to the wells (100 μL/well). After incubation for 1 h at 37°C and extensive washing, 100 μL/well of substrate solution containing TMB was added in the plate and incubated for 15 min at 37°C. The reaction was subsequently quenched with 2 M sulfuric acid (50 μL/well), and its optical density (OD) was measured at a wavelength of 450 nm using a microplate reader.
Statistical analysis
Statistical analysis was done using SPSS 17.0 (SPSS Inc., Chicago, IL). For the analysis of correlation between indirect ELISA antibody titer and affinity and RD-ELISA titer and affinity, Pearson correlation analysis was used. All data were representative of at least two separate experiments.
Results
Determination of anti-OVA MAb titer and affinity
The titers of 30 MAbs were tested by indirect ELISA (Table 1). Ten clones with a titer of ≤1×10−3 were discarded after indirect ELISA titer detection. Depending on the value of indirect ELISA titers, the prepared 20 MAbs were divided into three subgroups. Group 1 has a relative lower antibody titer, ranging from 5.0×10−7 to 1.0×10−7; group 2 has a middle level antibody titer between 6.3×10−8 to 2.5×10−8; and group 3 has an antibody titer higher than 5.0×10−9.
Table 1.
Characteristics of Anti-OVA MAbs: Indirect ELISA Titer, Affinity, and RD-ELISA Titer
| Clone | Subclass | Titer detected by indirect ELISA | Affinity detected by ELISA | Titer detected by RD-ELISA |
|---|---|---|---|---|
| 25 | IgG1(κ) | 5.0×10−7 | 1.9×10−9 | 7.8×10−5 |
| 4 | IgG1(κ) | 3.1×10−7 | 2.8×10−9 | 3.1×10−5 |
| 20 | IgG1(κ) | 2.5×10−7 | 2.6×10−8 | 6.3×10−4 |
| 1 | IgG1(κ) | 2.5×10−7 | 9.5×10−9 | 3.1×10−4 |
| 3 | IgG1(κ) | 2.5×10−7 | 3.5×10−9 | 3.9×10−5 |
| 22 | IgG1(κ) | 2.0×10−7 | 2.1×10−8 | 6.3×10−4 |
| 24 | IgG1(κ) | 2.0×10−7 | 3.3×10−9 | 3.9×10−5 |
| 27 | IgG1(κ) | 1.6×10−7 | 2.7×10−8 | 6.3×10−4 |
| 23 | IgG1(κ) | 1.0×10−7 | 2.7×10−9 | 3.9×10−5 |
| 19 | IgG1(κ) | 6.3×10−8 | 5.6×10−9 | 3.1×10−4 |
| 21 | IgG1(κ) | 6.3×10−8 | 4.8×10−9 | 3.1×10−4 |
| 9 | IgG1(κ) | 5.0×10−8 | 8.9×10−9 | 6.3×10−4 |
| 12 | IgG1(κ) | 5.0×10−8 | 8.1×10−9 | 3.1×10−4 |
| 13 | IgG1(κ) | 5.0×10−8 | 1.1×10−9 | 7.8×10−5 |
| 7 | IgG1(κ) | 2.5×10−8 | 7.8×10−10 | 3.9×10−5 |
| 17 | IgG1(κ) | 5.0×10−9 | 2.8×10−8 | 6.3×10−4 |
| 14 | IgG1(κ) | 5.0×10−9 | 6.2×10−10 | 3.9×10−5 |
| 6 | IgG1(κ) | 3.1×10−9 | 8.7×10−10 | 3.9×10−5 |
| 5 | IgG2b(κ) | 2.5×10−9 | 3.6×10−8 | 6.3×10−4 |
| 10 | IgG1(κ) | 1.6×10−9 | 6.2×10−10 | 3.9×10−5 |
| 2 | IgG2b(κ) | 1.0×10−3 | ND | ND |
| 8 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 11 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 15 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 16 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 18 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 26 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 28 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 29 | IgG1(κ) | 1.0×10−3 | ND | ND |
| 30 | IgG1(κ) | 1.0×10−3 | ND | ND |
Titers of 30 MAbs were tested by indirect ELISA, and 20 MAbs with a titer higher than 1×10−3 were selected for the subsequent affinity and RD-ELISA titer detection.
ND, not detected.
The affinity of antibody is the attraction force of the reaction between an antigenic determinant and a combining site on the antibody.(9) For the affinity detection, antigen at various concentrations was incubated with a constant amount of antibody in dilution buffer until equilibrium was reached. Then KD can be evaluated by measuring the slope of the linear dependence of A0/(A0 – A) upon 1/a0.
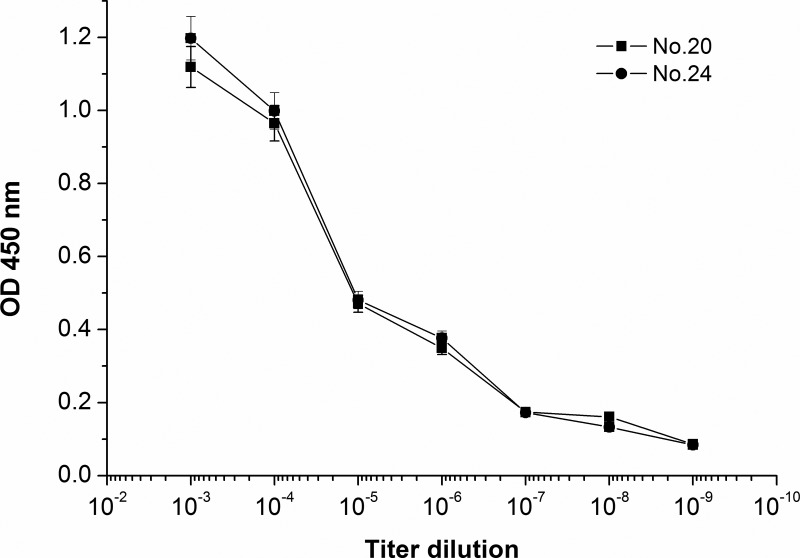
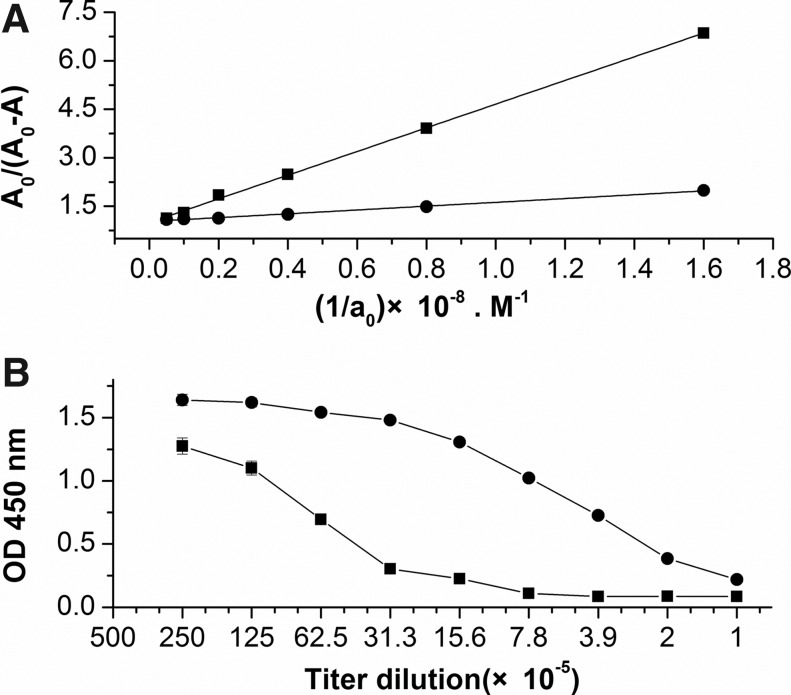
Despite the fact that MAbs of groups 1–3 have different indirect ELISA titer, affinity detection results have no significant correlation with MAb titer (Table 1). On the other hand, a part of MAbs like FMU-OVA 20 and FMU-OVA 24 have the same indirect ELISA titer but different affinity (Figs. 1, 2A). Statistical analysis of the 20 MAbs listed in Table 1 indicate that the correlation coefficient (R2) between indirect ELISA antibody titers and affinity was 0.206 (p=0.2).
FIG. 1.
The antibody titer of purified anti-OVA MAbs determined by indirect ELISA. Antibody titer was defined as the highest dilution with absorbance value greater than 2-fold of mean negative control value. Representative titer curves (FMU-OVA 20 and FMU-OVA 24) are shown. The points are given as mean±SD values of triplicate determinations.
FIG. 2.
The KD values and titers of anti-OVA MAbs determined by ELISA and RD-ELISA. (A) KD values of (•)[solid circle] no. 20 and (▪)[solid square] no. 24 were 2.6×10−8 M and 3.3×10−9 M, respectively, calculated by Eq 1, in which A is the absorbance measured for the antibody reacting with different concentrations of antigen, A0 is the absorbance measured for the antibody in the absence of antigen, and a0 is the total antigen concentration. (B) The titers of (•)[solid circle] no. 20 and (▪)[solid square] no. 24 were 6.3×10−4 and 3.9×10−5. The points are given as mean±SD values of triplicate determinations.
Developed RD-ELISA for selection of MAbs
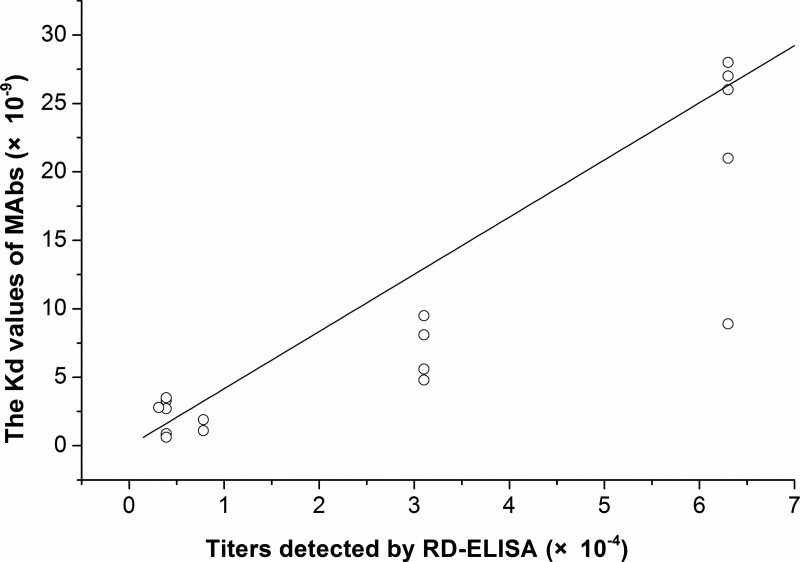
Several methods can be used to measure the affinity of MAbs, such as immunoprecipitation, surface plasmon resonance, and ELISA. These methods are complicated, time-consuming, or expensive compared with antibody titer detection by indirect ELISA. In order to acquire high-affinity MAbs conveniently and quickly, we established a simple method, known as RD-ELISA (Fig. 2B). The titers determined by RD-ELISA were consistent with the affinity of MAbs (Table 1). Correlation coefficient (R2) between RD-ELISA titer and affinity was 0.869 (p<0.01; Fig. 3).
FIG. 3.
Correlation of KD values with titers detected by RD-ELISA. R2=0.869; p<0.01.
Discussion
In this study, a group of anti-OVA MAbs were selected by indirect ELISA during preparation process; however, a large proportion (9/20) of MAbs have a relatively lower antibody titer (group 1). We determined the affinity of all MAbs by ELISA, and the results indicated that part of the MAbs that had lower antibody titer showed discrepancy between antibody titer and affinity. As reported previously, most antigens adsorbed on polystyrene were partially or largely denatured.(10) Therefore, the process of OVA coating may affect its binding to anti-OVA MAbs. To avoid the impact of the coating process to antigen and MAb binding, we tested a RD-ELISA method. Data showed that 6 MAbs (FMU-OVA 3, 4, 23, 24, 25) had a relatively higher affinity but a lower indirect ELISA titer, indicating the efficacy of RD-ELISA for anti-OVA MAb screening. Furthermore, affinity and RD-ELISA titer of the whole 20 MAbs correlate well. In the process of hybridoma cell cloning, each round of limit dilution is followed by one round of antibody screening, and indirect ELISA is a popular and convenient screening assay by MAb detection in cell culture supernatant. Coating induced antigen denaturation influenced supernatant screening in the case of MAbs with lower antibody titer or lower antigen coating concentration. In our experiment, when the OVA coating concentration was changed to 0.2 μg/mL, MAbs with lower titer but higher affinity (FMU-OVA 3, 4, 23, 24, 25) cannot be detected in the supernatant, whereas all the MAbs with higher titer (group 3) had positive results (data not shown). In conclusion, our study demonstrates for the first time that RD-ELISA can yield quicker results as well as be a powerful tool in the screening of high-affinity MAbs, and further suggests that some low titer but high-affinity MAbs may be missed by indirect ELISA.
Recently, a sandwich-ELISA with very high sensitivity was successfully established using FMU-OVA 24 as a coating antibody for the detection of OVA (unpublished data). In our opinion, RD-ELISA is more effective than traditional indirect ELISA for the selection of MAbs for use as coating antibodies in the development of sandwich-ELISA since the reaction conditions of RD-ELISA and sandwich-ELISA are similar when used as coating antibodies.
Acknowledgment
This work was supported by grants from the National Key Technology R&D Program (2009BAK61B04).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Song C. Wang F. Jin L. Chen L. Qin W. Yang A. Yang K. Jin B. Ultrahigh spacing tissue arrays for screening hybridoma by immunohistochemistry. J Immunol Methods. 2009;343:130–133. doi: 10.1016/j.jim.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Bai Y. Wu C. Zhao J. Liu YH. Ding W. Ling WL. Role of iron and sodium citrate in animal protein-free CHO cell culture medium on cell growth and monoclonal antibody production. Biotechnol Prog. 2011;27:209–219. doi: 10.1002/btpr.513. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang W. Xue J. Liu J. Jia W. Li Z. Xie X. Liu X. Jian J. Li Q. Zhu Y. Yang A. Jin B. Establishment of an ELISA system for determining soluble LAIR-1 levels in sera of patients with HFRS and kidney transplant. J Immunol Methods. 2004;292:109–117. doi: 10.1016/j.jim.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Li Y. Song C. Zhang K. Wang M. Yang K. Yang A. Jin B. Establishment of a highly sensitive sandwich enzyme-linked immunosorbent assay specific for ovomucoid from hen's egg white. J Agri Food Chem. 2008;56:337–342. doi: 10.1021/jf0724522. [DOI] [PubMed] [Google Scholar]
- 5.Jia W. Liu XS. Zhu Y. Li Q. Han WN. Zhang Y. Zhang JS. Yang K. Zhang XH. Jin BQ. Preparation and characterization of mabs against different epitopes of CD226 (PTA1) Hybridoma. 2000;19:489–494. doi: 10.1089/027245700750053986. [DOI] [PubMed] [Google Scholar]
- 6.Liu XS. Zhu Y. Han WN. Li YN. Chen LH. Jia W. Song CJ. Liu F. Yang K. Li Q. Jin BQ. Preparation and characterization of a set of monoclonal antibodies to TRAIL and TRAIL receptors DR4, DR5, DcR1, and DcR2. Hybridoma Hybridomics. 2003;22:121–125. doi: 10.1089/153685903321948058. [DOI] [PubMed] [Google Scholar]
- 7.Liu F. Li Y. Song C. Dong B. Liu Z. Zhang K. Li H. Sun Y. Wei Y. Yang A. Yang K. Jin B. Highly sensitive microplate chemiluminescence enzyme immunoassay for the determination of staphylococcal enterotoxin B based on a pair of specific monoclonal antibodies and its application to various matrices. Anal Chem. 2010;82:7758–7765. doi: 10.1021/ac101666y. [DOI] [PubMed] [Google Scholar]
- 8.Friguet B. Chaffotte AF. Djavadi-Ohaniance L. Goldberg ME. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985;77:305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 9.Chalquest RR. Calculation of antibody affinity in homogeneous and heterogeneous systems. J Clin Microbiol. 1988;26:2561–2563. doi: 10.1128/jcm.26.12.2561-2563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang M. Klakamp SL. Funelas C. Lu H. Lam B. Herl C. Umble A. Drake AW. Pak M. Ageyeva N. Pasumarthi R. Roskos LK. Detection of high- and low-affinity antibodies against a human monoclonal antibody using various technology platforms. Assay Drug Devel Technol. 2007;5:655–662. doi: 10.1089/adt.2007.089. [DOI] [PubMed] [Google Scholar]