Abstract
Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. Its major projections are to the septal area and prefrontal cortex, mediating emotional responses to sensory stimuli. The temporal lobe contains much subcortical white matter, with such named bundles as the anterior commissure, arcuate fasciculus, inferior longitudinal fasciculus and uncinate fasciculus, and Meyer's loop of the geniculocalcarine tract. This article also reviews arterial supply, venous drainage, and anatomical relations of the temporal lobe to adjacent intracranial and tympanic structures.
1. Introduction
In this paper, I attempt to explain the positions of the parts of the normal human temporal lobe in relation to one another and to nearby structures. Some physiological and pathological correlates are mentioned, but this is primarily an anatomical account. References are provided for further reading, especially in areas where there is clinical interest or controversy or where anatomical details are not easily found in ordinary textbooks of neuroanatomy. A few historical references are included to remind readers that many of the “discoveries” made with modern imaging techniques simply confirm what has been known about the temporal lobe for many years. No attempt is made to provide references for the original descriptions of gross anatomical structures, but synonyms are mentioned to accommodate the differences in terminology used by anatomists, pathologists, and radiologists in textbooks and other literature.
Bulges on the lateral sides of the forebrain in insectivores (considered ancestral to primates) are anatomically related to the temporal bone and contain cortical areas and other structures such as the hippocampus and amygdala that are medially located in the temporal lobes of the human brain. A true temporal lobe, delineated above (dorsally) by a lateral sulcus (sylvian fissure), and containing an extension of the lateral ventricle, occurs only in primates and is largest in man [1–3].
Approximately 17% of the volume of the human cerebral cortex, 16% in the right and 17% in the left hemisphere, forms the surfaces of the temporal lobes [4]. Temporal cortex includes areas involved with the auditory, olfactory, vestibular, and visual senses, and in the perception of spoken and written language. In addition to cortex, the temporal lobe contains white matter, part of the lateral ventricle, the tail of the caudate nucleus, the stria terminalis, the hippocampal formation, and the amygdala. The medial side of the temporal lobe includes regions concerned with olfaction (the uncus and nearby cortex) and semantic memory (the hippocampal formation). The nearby amygdala generates responses to perceived sensory stimuli that have been partly analyzed elsewhere in the brain. Such responses include largely involuntary ones, mediated by the autonomic and somatic motor systems, and mental functions, especially those called feelings or emotions, that motivate decision and voluntary actions [5–12].
The temporal lobe can be damaged by infection, trauma, ischaemia, and neoplasia. Lesions in the temporal lobe can stimulate or inhibit the functions mentioned in the preceding paragraph. The syndrome of Kluver and Bucy [13, 14] provided an extreme example of changed behaviour following bilateral temporal lobectomy in monkeys. The animals became unnaturally docile, exhibited excessive and abnormal sexual behaviour, lost the ability to be trained, and had a condition that the authors termed “psychic blindness,” in which tactile exploration of objects with the mouth replaced their visual recognition. The equivalent human syndrome is rare and usually associated with pathology extending beyond the temporal lobes [15–17]. Fragments of the classical syndrome, such as visual field defects, visual agnosia, and inability to consolidate new memories, occur more frequently, with destructive lesions in parts of one or both temporal lobes.
2. Surface Features and Delimitation
Like the other lobes of the cerebral hemisphere, the temporal lobe is delineated by cortical landmarks. On the lateral surface, the stem and posterior ramus of the lateral sulcus mark the separation of the temporal lobe from the frontal and parietal lobes. The lateral sulcus or sylvian fissure is a deep cleft, but in anatomical terms it is not a fissure, because its extensive internal surfaces are all bounded by cerebral cortex. Latin sulcus means a furrow or trench, whereas fissus and related words translate to the English slit or split. A fissure separates different structures, such as the two cerebral hemispheres (longitudinal fissure), the cerebrum from the cerebellum (transverse fissure), or the fornix from the thalamus (choroid fissure). The lateral sulcus, in addition to defining the superior border of the temporal lobe, accommodates a cistern of the subarachnoid space, the middle cerebral artery, and the superficial and deep middle cerebral veins. The term perisylvian is often used when referring to cortex on both sides of the lateral sulcus, especially in neuroimaging studies of patients with aphasias [18, 19].
The lateral surface of the temporal lobe is indented by the superior and inferior temporal sulci, thus delineating superior, middle, and inferior temporal gyri. The last of these curves around onto the inferior surface of the brain and extends posteriorly into the occipital lobe; it is also called the lateral occipitotemporal gyrus. The sulci of the inferior surface of the temporal lobe are variable. Typically, the occipitotemporal sulcus separates the medial border of the inferior temporal gyrus from the lateral border of the fusiform or medial occipitotemporal gyrus. Medial to the fusiform gyrus is the collateral sulcus, and medial to the collateral sulcus, the parahippocampal gyrus forms the medial border of the inferior surface of the lobe. The anterior end of the collateral sulcus, which curves anteromedially below the temporal pole, is called the rhinal sulcus. The uncus is a small projection of the medial surface of the anterior end of the parahippocampal gyrus, a region that will be discussed in more detail in connection with the hippocampal formation.
The superior surface of the temporal lobe, which forms the floor of the lateral sulcus, is continuous with the superior temporal gyrus. It is marked by two obliquely oriented ridges, the transverse temporal gyri, which constitute the primary auditory cortex, posterior to which is the planum temporale, a cortical area that is usually larger on the left than on the right side in men, but not in women [20]. The superior surface of the temporal lobe is bounded medially by the circular sulcus, which surrounds the insula, a lobe of the cortex that forms the expanded floor of the lateral sulcus. The anterior end of the insula, the limen insulae, is continuous, in the stem of the lateral sulcus, with cortices of the anteromedial part of the parahippocampal gyrus, the anterior perforated substance, and the medial frontal cortex (subcallosal or paraterminal gyrus) below the rostrum of the corpus callosum [21].
The posterior part of the temporal lobe blends into the parietal lobe above and the occipital lobe behind. The limits of the lobes are arbitrary straight lines connecting anatomical landmarks. The preoccipital notch is an indentation in the inferior temporal gyrus, about 3 cm anterior to the occipital pole, formed by the petrous part of the temporal bone. A straight line drawn from the parietooccipital sulcus to the preoccipital notch defines the anterior border of the occipital lobe on the lateral aspect of the hemisphere. From the midpoint of this line, a horizontal line passing forward to the lateral sulcus separates the temporal from the parietal lobe. On the inferior surface, a line connecting the preoccipital notch with the cortex immediately behind the splenium of the corpus callosum separates temporal from occipital cortex. The sulci gyri and boundaries of the temporal lobe are illustrated in Figures 1 and 2.
Figure 1.
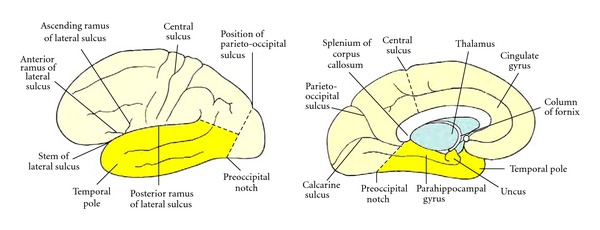
Boundaries of the temporal lobe and positions of major sulci and gyri and other anatomical landmarks of the lateral and medial surfaces of the left cerebral hemisphere.
Figure 2.
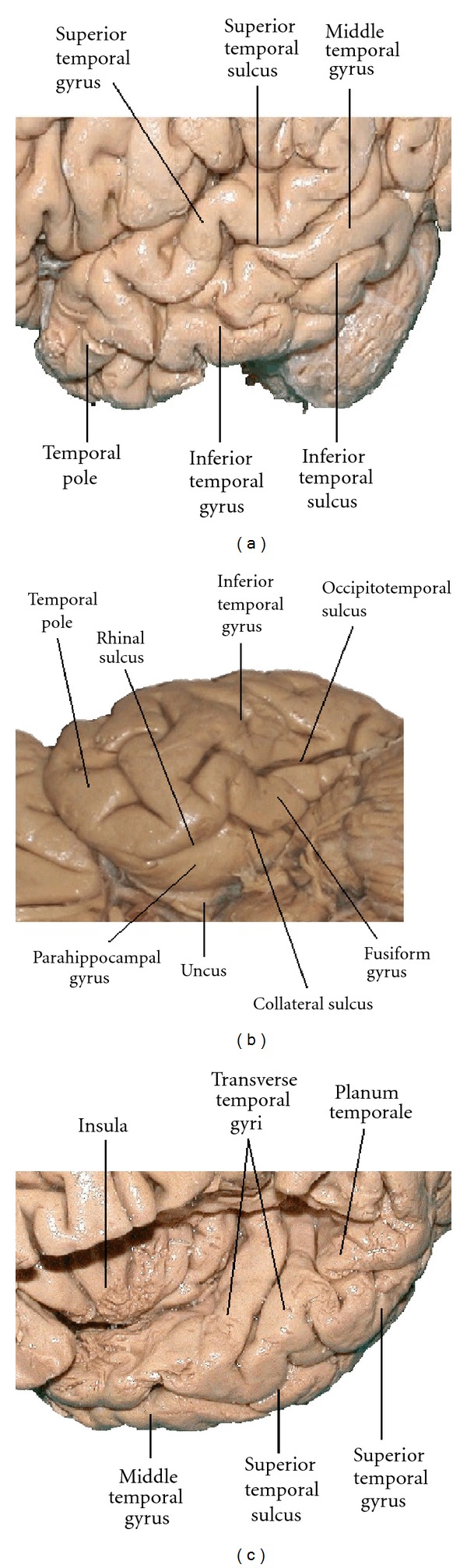
Anatomical landmarks of the cortex of the left temporal lobe. Photographs are of the lateral (a), inferior (b), and superior (c) surfaces. The superior surface, along with the insula, was exposed by removal of parts of the frontal and parietal lobes above the lateral sulcus.
3. Hippocampal Formation
In comparative anatomy, the word pallium (Latin for cloak) is often applied to the cerebral cortex of mammals and homologous parts of the forebrain in submammalian vertebrates. A cloak has a continuous edge. The edge of the cerebral cortex around the base of each cerebral hemisphere forms a ring, often called the limbic lobe (from Latin limbus, a hem or fringe), a name bestowed by Broca in 1877 [22]. In the human brain the most conspicuous components of this ring of cortex are the parahippocampal gyrus and the cingulate gyrus. These gyri are continuous behind the splenium of the corpus callosum as the isthmus or retrosplenial cortex. Anteriorly, the continuity includes cortical areas that extend from the uncus and cortex overlying the amygdala (periamygdaloid cortex) across the stem of the lateral sulcus (the limen insulae) and the anterior perforated substance to the medial surface of the frontal lobe, below the rostrum of the corpus callosum (the subcallosal gyrus), which is continuous around the genu with the anterior end of the cingulate gyrus. The components of the hippocampal formation are the hippocampus, an enrolled gyrus adjacent to the parahippocampal gyrus, the dentate gyrus, which represents the free edge of the pallium, and the associated white matter, the alveus, fimbria, and fornix. The cortex adjacent to the hippocampus is known as the entorhinal area; it is present along the whole length of the parahippocampal gyrus [21]. The subiculum is a transitional zone between the entorhinal and hippocampal cortices. The hippocampal formation has indirect afferent connections from the whole of the cerebral cortex, funneled through the adjacent temporal cortex and the subiculum. The best understood function of the hippocampus is the consolidation of memory.
Other components of the limbic lobe, very small in the human brain, are the fasciolar gyrus, and the indusium griseum. These are continuations of the hippocampal formation, forming an inconspicuous but thin continuous ring of grey matter at the edge of the pallium. The limbic lobe includes some grossly visible bodies of white matter. Of these, the alveus and fimbria of the hippocampal formation and the crus of the fornix are within the temporal lobe. The body and column of the fornix are outside the territory of the temporal lobe, as are the longitudinal striae of Lancisi, beneath the indusium griseum. The components of the limbic lobe are also parts of the limbic system, a term that includes also the amygdala and several functionally connected nuclei in the cerebral hemisphere, diencephalon, and brain stem.
The adult anatomy of the human hippocampal formation is best understood in the context of its development. The hippocampus and dentate gyrus become recognizable late in the embryonic period of development [23], in the edge of the pallium. Growth of the cerebral cortex and associated subcortical white matter, especially the radiations of the corpus callosum, pushes the relatively small hippocampus downward and forward to become the medial surface of the developing temporal lobe. The folding that characterizes the adult hippocampal formation occurs during fetal development, from 13 to 20 weeks after fertilization [24, 25]. A shallow indentation, the hippocampal sulcus, forms on the medial surface of the temporal lobe, indenting the temporal horn of the lateral ventricle. With continued growth, the hippocampal sulcus becomes deeper and narrower, and by 18 weeks it is largely obliterated, so that the ventral surface of the dentate gyrus is fused with the surface of the subiculum, which is the cortical area along the medial edge of the parahippocampal gyrus, adjacent to the hippocampus. The resulting bulge into the temporal horn is the hippocampus. Its surface, the alveus, is its subcortical white matter, the fibres of which converge along the edge of the hippocampus as the fimbria (from Latin fimbriae, fringe), which becomes progressively larger posteriorly, eventually becoming the crus of the fornix.
The hippocampus is widest at its head or anterior end, which includes a narrow reflexed prolongation into the anterior part of the uncus. The inferior surface of the uncus is crossed by an obscure transverse marking the band of Giacomini, which represents the rostral end of the dentate gyrus. Posteriorly the structure has a narrow horizontal body and a slender upwardly curved tail (Figure 3). The imaginative alternative name cornu ammonis (from Amun, an early Egyptian deity with a ram's head), dates back to the 18th century. In modern usage it excludes the dentate gyrus and is memorialized in terminology for sectors seen in transverse (coronal) sections of the hippocampus, with CA1 next to the subiculum and CA4 in the concavity (hilum) of the dentate gyrus [21, 26].
Figure 3.
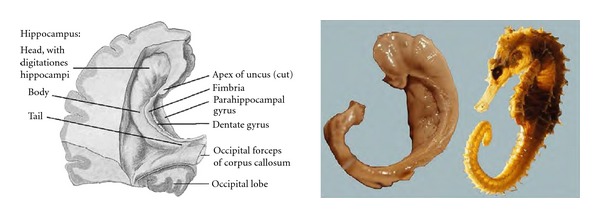
The hippocampus, dentate gyrus and fimbria as they appear after removal of the roof of the temporal horn of the lateral ventricle and of the choroid plexus (modified from Piersol, 1928 [27]). The photograph on the right (courtesy of Dr. Laszlo Seriss, University of Pecs, Hungary) shows a dissected hippocampal formation, including the reflexed intrauncal component, with a sea-horse alongside.
The choroid fissure is formed by invagination of the ependyma of the medial wall of the lateral ventricle and ingrowth of vascular tissue that will form the choroid plexus. In the temporal lobe, the fissure is alongside the hippocampus and fimbria, which form its inferior wall. Subcortical grey matter, the tail of the caudate nucleus, forms the opposing wall of the fissure, along with an associated fibre bundle, the stria terminalis, which contains axons from the amygdala. In the adult human brain, the amygdala is near the temporal pole and indents tip of the temporal horn of the lateral ventricle, overlapping slightly with the more conspicuous indentation due to the hippocampus. In a dissected adult brain with the lateral ventricle opened from above, the hippocampus is seen as the white floor (inferomedial surface) of the inferior horn. The ventricular surface of the human hippocampus is shallowly grooved (the digitationes hippocampi), with the hippocampal head giving an impression of the dorsal surface of an animal's paw (Figure 3). The name pes hippocampi is still occasionally encountered for the anterior end of the hippocampus [28, 29].
The name hippocampus (Latin for sea-horse) dates from the Renaissance and a fancied resemblance to the fish (Figure 3), with its thin snout, flexed neck, plump body, and curved tail [30]. The curved shape of the hippocampus has been likened also to the horn of a ram (cornu ammonis), a shape that relates also to the appearance of a transverse section [31].
The architecture of the hippocampal formation is largely uniform throughout its length, as seen in transverse sections (Figure 4). Most of the human cerebral cortex (the isocortex) has 6 layers, two of which contain principal cells: neurons with axons that enter the subcortical white matter. The cortex of the hippocampal formation, however, has the principal cells in just one layer (allocortex). The molecular layer, which is continuous with the most superficial layer of the isocortex of the parahippocampal gyrus, consists largely of axodendritic synapses and is adjacent to the vestigial hippocampal sulcus. In the dentate gyrus this is quite a thin layer, but in the hippocampus it is thick, with 4 sublayers, the stratum molecular, stratum lacunosum, stratum radiatus, and stratum lucidum, which contain synapses from different groups of afferent fibres. The layer of principal cells in the hippocampus is called the stratum pyralidae; it contains neurons whose sectioned somata have triangular outlines and large apical dendrites that extend into the molecular layer. In the dentate gyrus the principal cells are small and very numerous, constituting the stratum granulosum. The axons of hippocampal pyramidal cells pass through the stratum oriens and then enter the alveus. The axons of dentate gyrus granule cells pass through the polymorphic layer into the hilus of the gyrus (sector CA4) and then into the strata lucidum and pyralidae of sector CA3 of the hippocampus [32].
Figure 4.
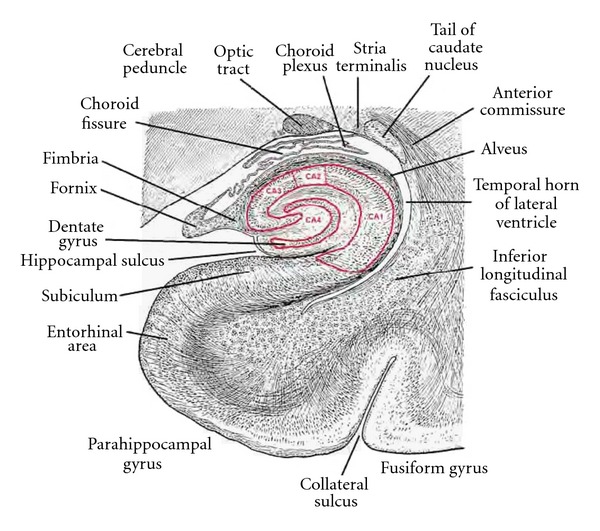
A transverse section through the body of the hippocampus and dentate gyrus, choroid fissure, and inferior horn of the lateral ventricle. The dentate gyrus and CA sectors of the hippocampus are outlined in red (modified from Edinger, 1899 [33]).
4. Amygdala
In embryonic development, the origin of the amygdaloid body or complex, usually more simply called the amygdala, has been traced to populations of diencephalic and telencephalic cells that form the floor of the lateral ventricle about 3 weeks after conception [34], long before the first appearance of the developing temporal lobe at about 7 weeks [35]. The adult amygdala is a group of several nuclei located in the medial part of the temporal pole, anterior to and partly overlapping the hippocampal head. The medial part of the complex, present in the anterior part of the uncus, receives fibres of the olfactory tract. Two named gyri of the anterior end of the uncus, the ambient and semilunar gyri consist of periamygdaloid cortex that receives fibres from the olfactory tract [28, 32]. The larger lateral part of the amygdala, like the hippocampal formation, receives direct and indirect input from most of the cerebral cortex. Functional imaging confirms experimental studies involving stimulation or destructive lesions, indicating that the amygdala extracts affective content from multiple sensory inputs [36, 37].
The posterior pole of the amygdala is prolonged for a short distance around the stria terminalis, which is one of the two major efferent tracts of this nuclear group. The stria terminalis runs in the roof of the temporal horn of the lateral ventricle alongside the tail of the caudate nucleus, which ends close to but not in contiguity with the amygdala [4, 37, 38]. At the junction of the temporal horn with the central part (“body”) of the lateral ventricle, the stria terminalis turns upward and forward into the frontal horn, in the sulcus between the head of the caudate nucleus and the superior surface of the thalamus. At the anterior tubercle of the thalamus the stria terminalis converges with a minority of the fibres of the fornix that descend anterior to the anterior commissure into the septal area. Grey matter surrounding the stria terminalis in this region constitutes the bed nucleus of the stria terminalis, which, in the human brain, has 7 named divisions [4]. The bed nucleus of the stria terminalis is currently described as part of the “extended amygdala”, located in the frontal rather than the temporal lobe [37, 39]. Dorsomedially the posterior part of the amygdala is bounded by the substantia innominata, a region contiguous with the lateral hypothalamus that includes several cell groups, notably the large cholinergic neurons of the basal nucleus of Meynert, whose much branched axons innervate the whole cerebral cortex. The second major group of efferent amygdalar fibres, the ventral amygdalofugal pathway, passes through the substantia innominata before distributing axons to extensive areas of the cerebral cortex, the mediodorsal nucleus of the thalamus, the hypothalamus, and all levels of the brain stem. Laterally the amygdala is bounded by white matter of the temporal lobe, through which afferent fibres are received principally from sensory association areas of the cerebral cortex.
On the basis of cytoarchitectonics and comparison with experiments tracing neuronal connectivity in animals, 24 nuclei are recognized in the human amygdala [4], though some authorities [40] recognize only 12. The nuclei are organized in three groups: corticomedial nuclei in the anterior part of the uncus, basolateral nuclei comprising the inferolateral two-thirds of the amygdala, and a central group, which receives afferent fibers from the other two groups of nuclei. The corticomedial nuclei blend into the thin overlying cortical layer (periamygdaloid cortex), and like that cortex they receive afferent fibres from the olfactory tract. The basolateral nuclei receive indirect olfactory input from the primary olfactory areas [37, 41], but most of the afferents to the basolateral nuclei are from association cortex of the visual, auditory, and somatosensory systems. There are also reciprocal connections with the prefrontal and anterior cingulate cortex but few or no connections with the parietal lobe [37, 39–42]. Fibres leaving the amygdala originate mainly in the basolateral and central nuclei. The position and relations of the amygdala are shown in Figure 5.
Figure 5.
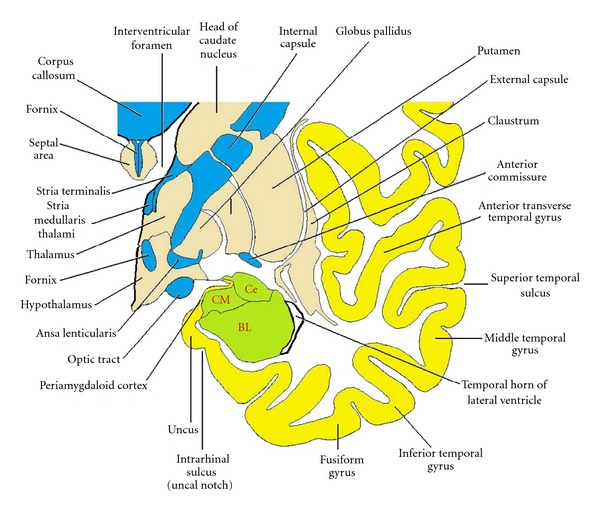
Drawing of a coronal section through the temporal lobe and adjacent structures, at a level anterior to the hippocampal head. The amygdala is coloured green, with the positions of its three nuclear groups indicated: corticomedial (CM), basolateral (BL), and central (Ce). Selected bodies of white matter are coloured blue.
Electrical stimulation of the human amygdala causes feelings of fear. In laboratory animals, such stimulation leads to autonomic and other behavioural responses associated with fear [43, 44]. The extensive connections with sensory association cortex support a more general role of the amygdala in mediating emotional responses to sensations [45, 46]. Electrical stimulation and recording in patients prior to surgery for temporal lobe epilepsy show that a dreamy state or déjà vu is associated with activity in the amygdala as well as with the hippocampus and both medially and laterally located isocortex of the temporal lobe [47], indicating that the amygdala is part of a system of memory recall [48]. In fMRI studies, activity in the amygdala, bilaterally, has been associated with emotionally significant word pairs [49] and with feared aversive sensory stimuli [50].
5. White Matter
Subcortical white matter comprises three populations of axons. Association fibres connect cortical areas within the same cerebral hemisphere. Commissural fibres connect mainly but not exclusively [51] symmetrical cortical areas. Projection fibres connect cortical areas with subcortical nuclei of grey matter. The three types of fibre intersect extensively, but certain bundles can be demonstrated by dissection. The same bundles also can be imaged in the living brain by diffusion tensor (DT) imaging, a nuclear magnetic resonance technique. The production of DT images of fibre tracts is, however, heavily dependent on knowledge of axonal orientation gained by traditional dissection [52], and the method has not yet contributed new information about the white matter of the temporal lobe.
The temporal cortex is connected by association fibres with all the other lobes of the forebrain. The largest named bundle is the arcuate fasciculus, whose anterior end is in the frontal lobe. The arcuate fasciculus passes above the insula and lentiform nucleus, where it is also named the superior longitudinal fasciculus and follows a curved course into the temporal lobe, thus providing two-way communication between frontal cortex, including Broca's expressive speech area, and Wernicke's receptive language area in the posterior part of the superior temporal gyrus. The condition of conduction aphasia is traditionally attributed to a destructive lesion that interrupts the arcuate fasciculus [18, 19, 53]. Another frontotemporal association bundle is the uncinate fasciculus, named for its hooklike shape, which passes around the stem of the lateral sulcus and connects the cortex of the temporal pole with the prefrontal cortex. The ventral amygdalofugal pathway is more dorsally and posteriorly located, above the anterior perforated substance. Visual association cortex extends from the occipital lobe to the middle and inferior temporal and fusiform gyri. The inferior longitudinal fasciculus, which is in the white matter inferolateral to the temporal horn (Figure 4) connects these visual areas with one another and with the temporal polar cortex, an important source of fibres afferent to the amygdala. The fornix and stria terminalis, already discussed in connection with the hippocampal formation and amygdala, respectively, can also be considered association fasciculi.
The largest group of commissural fibres is the corpus callosum. Degeneration studies indicate that axons from the middle and posterior parts of the temporal cortex cross the midline in the central part of the body of the corpus callosum [54]. The temporal poles, transverse temporal gyri, and amygdalae may be interconnected mainly by fibers of the anterior commissure [55].
Projection fibres afferent to the temporal cortex include those from the medial geniculate body to the primary auditory area of the transverse temporal gyri. These travel in the sublenticular limb of the internal capsule, where they probably are accompanied by fibres from the medioventral thalamic nucleus, which is connected with the amygdala, hypothalamus, hippocampal formation, and parahippocampal gyrus. For much of the temporal cortex, the sources of thalamic afferents have not been determined [40, 42]. All thalamocortical projections are accompanied by reciprocal corticothalamic fibres. An important thalamocortical pathway that passes through the temporal lobe is Meyer's loop of the geniculocalcarine tract, which is drawn into the anterior temporal white matter with the growth of the nearby temporal horn of the lateral ventricle. This loop carries signals derived from the upper quadrants of the contralateral visual fields to the corresponding primary visual cortex of the anterior half of the inferior bank of the calcarine sulcus. Some efferent temporal cortical projection fibres go to the amygdala and hippocampus and are thus confined to the temporal lobe. Corticothalamic fibres have already been mentioned. Most textbooks of neuroanatomy show a large temporopontine or temporoparietopontine tract occupying the lateral quarter of the basis pedunculi in the midbrain. Degeneration studies following temporal lobe lesions in monkeys, however, show only a small temporopontine projection, originating in the superior temporal gyrus and ending in the most lateral of the pontine nuclei [56]. In the absence of comparable information for the human brain, we must guess that the projection is similar to that of the monkey, and that the temporal cortex does not have a large, direct influence on the workings of the cerebellum.
6. Anatomical Relations of the Temporal Lobe
The locations of structures close to the temporal lobe are summarized in Figures 6, 7, 8, and 9. The temporal lobe occupies the middle cranial fossa, which is bounded anteriorly by the greater wing of the sphenoid bone, inferiorly by the superior surface of the petrous part of the temporal bone, and laterally by the squamous part of the temporal bone and the adjoining parietal bone. The dura mater adheres closely to these bones and is separated from the surface of the brain by the arachnoid, subarachnoid space and pia mater. The inner surfaces of the sphenoid, parietal, and squamous temporal bones are grooved by branches of the middle meningeal artery, which enters the cranial cavity by way of the foramen spinosum, beneath the fusiform gyrus. The tentorium cerebelli lies beneath the posterior and medial parts of the temporal lobe, with the free edge of the tentorial incisura being beneath the rostral end of the parahippocampal gyrus, lateral to the uncus and posterior to the rhinal sulcus, causing a shallow indentation, the intrarhinal sulcus [21] or uncal notch (Figure 5). This is a normal feature, not to be confused with the much deeper indentations that result from transtentorial herniation of the temporal lobe. The attachment of the tentorium splits to include the transverse sinus medially and the sphenoparietal sinus anterolaterally.
Figure 6.
Figure 7.
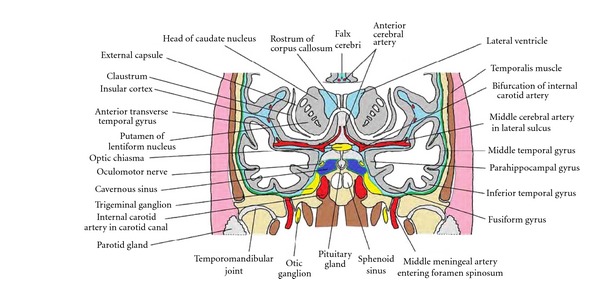
Anatomical relations of the temporal lobe, as seen in a schematic coronal section passing through the temporal pole, anterior to the amygdala, hippocampus, and temporal horn.
Figure 8.
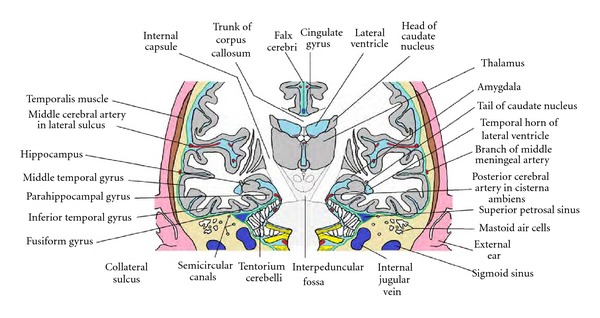
Anatomical relations of the temporal lobe, as seen in a schematic coronal section passing through the amygdala and the head of the hippocampus. This section is in a plane anterior to that shown in Figure 4.
Figure 9.
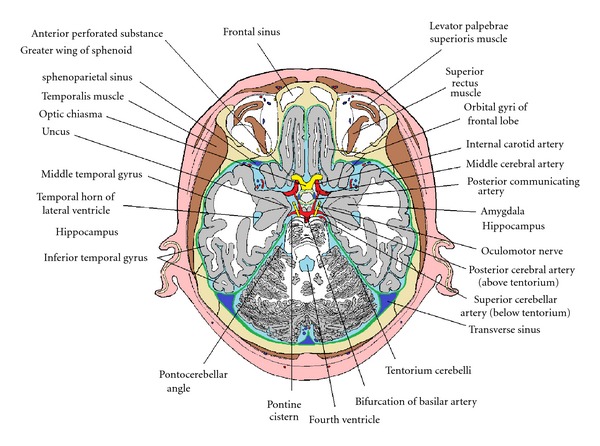
Anatomical relations of the temporal lobe, shown in a schematic horizontal section in the plane of the pituitary gland.
The petrous part of the temporal bone contains the structures of the inner ear and also the mastoid air cells, which are in continuity with the middle ear and the nasopharynx. Although the name “petrous” indicates that this bone looks like a rock, the layer of bone between the mastoid air cells and the dura of the middle cranial fossa (the tegmen tympani) is quite thin and is susceptible to erosion by bacterial infections arising in the middle ear. Septic lesions can extend through the tegmen tympani and the dura mater and arachnoid. Meningitis can follow such an invasion, but the defensive formation of fibrous connective tissue around the lesion often protects the subarachnoid space from the bacteria, which move upward into the temporal lobe, evoking the formation of an abscess that typically involves the inferior temporal gyrus and the white matter around the temporal horn of the lateral ventricle [57, 58].
The internal carotid artery enters the cranium below and medial to the anterior end of the parahippocampal gyrus, passing through the cavernous sinus and then coming between the optic chiasma and the anterior part of the temporal lobe before dividing into the anterior and middle cerebral arteries. The middle cerebral artery (MCA) enters the lateral sulcus from below, giving off frontal, parietal, and temporal branches that supply the cortex of the lateral surfaces of the lobes for which they are named. The MCA also gives rise to the anterolateral group of central arteries (lateral striate arteries), which enter the brain through the anterior perforated substance and supply much of the corpus striatum and external and internal capsules. The posterior cerebral artery, as it ascends through the tentorial notch, and its first branch, the posterior communicating artery, are also close to the medial surface of the temporal lobe, lying between the uncus and the oculomotor nerve.
The oculomotor nerve, which arises from the medial surface of the cerebral peduncle, passes through the subarachnoid space, just below the uncus, on its way to the cavernous sinus and superior orbital fissure. If the temporal lobe is displaced medially and downward into the tentorial notch by a space-occupying lesion, especially an extradural or subdural haemorrhage, the uncus is pushed medially against the oculomotor nerve, compressing first that nerve's preganglionic parasympathetic fibres, which are located superficially in the upper sector of the nerve [59], cause ipsilateral pupillary dilation [60].
7. Arterial Blood Supply and Venous Drainage
The temporal lobe receives blood from both the carotid and the vertebrobasilar systems. The anterior choroidal artery, which is the preterminal branch of the internal carotid, runs alongside the optic tract and then along the choroid fissure, which at this level indents the medial surface of the temporal lobe, separating the fimbria of the hippocampus from the stria terminalis and tail of the caudate nucleus (Figure 4). Structures in the temporal lobe supplied by the anterior choroidal artery are the anterior end of the parahippocampal gyrus, the uncus, the amygdala, and the choroid plexus in the temporal horn of the lateral ventricle.
The middle cerebral artery, a terminal branch of the internal carotid, crosses the insula in the floor of the lateral sulcus, giving off branches that supply the cortex of the superior and middle temporal gyri and the temporal pole. The posterior cerebral artery gives off two to four temporal branches, before it divides into the calcarine and parieto-occipital arteries, which supply the occipital lobe. The temporal branches of the posterior cerebral artery supply the inferior surface of most of the temporal lobe, but not the temporal pole.
The arteries supplying the temporal lobe are illustrated in Figure 10. Detailed anatomical accounts with photographs of serial slices of injected specimens are available [4, 61], and neuroradiology textbooks and atlases identify the vessels as they appear in angiograms.
Figure 10.
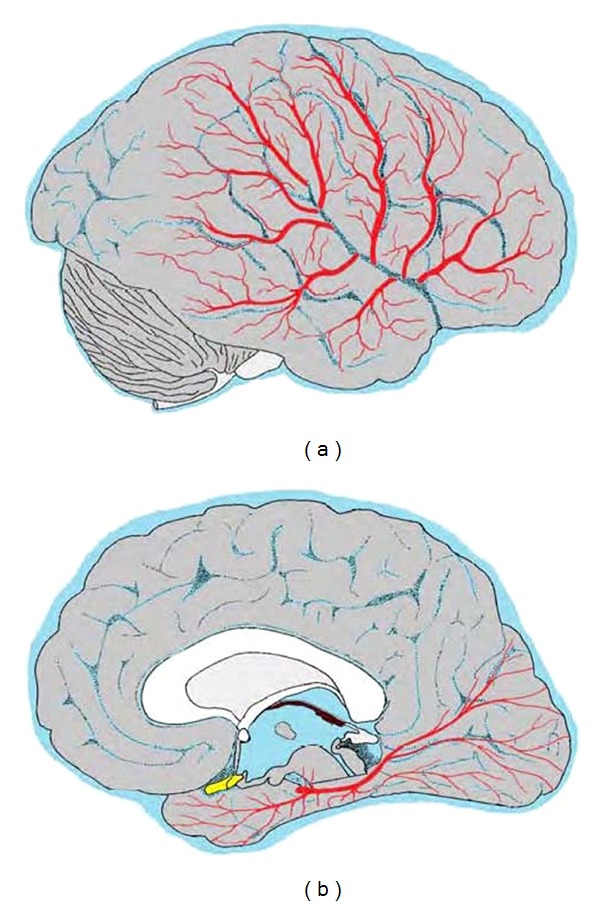
Arteries supplying the temporal lobe. The colour scheme is explained in Figure 6. (a) Three frontal, two parietal, and three temporal branches of the middle cerebral artery emerging from the lateral sulcus. (b) Hemisected brain with the pons, medulla, and cerebellum removed. The posterior cerebral artery is shown, with anterior and posterior temporal branches to the inferior temporal gyrus, and calcarine and other branches supplying the occipital lobe. Central branches to the midbrain and thalamus are represented by two small upwardly directed vessels. The medial temporal structures, supplied by the anterior choroidal artery, are hidden from view by the optic chiasma, hypothalamus and midbrain.
The venous drainage of the temporal cortex and underlying white matter goes anteriorly into the superficial middle cerebral vein, in the cistern of the lateral sulcus and also into the inferior anastomotic vein (vein of Labbé), which connects the superficial middle cerebral vein with the transverse sinus (Figure 11). Blood from interior of the lobe, including the amygdala, hippocampus, and fornix, flows into the posterior choroidal vein. This vessel lies alongside the choroid plexus of the lateral ventricle, passing upward and then forward, joining the thalamostriate vein immediately behind the interventricular foramen to form the internal cerebral vein. The left and right internal cerebral veins run posteriorly in the transverse fissure between the crura of the fornix and below the splenium of the corpus callosum, where they are joined by the basal veins and unite to form the great cerebral vein, a midline structure that continues into the straight sinus. The basal vein (vein of Rosenthal), which carries blood from the cortex and the interior of the frontal lobe, traverses the subarachnoid space in the cisterna ambiens, medial to the temporal lobe.
Figure 11.
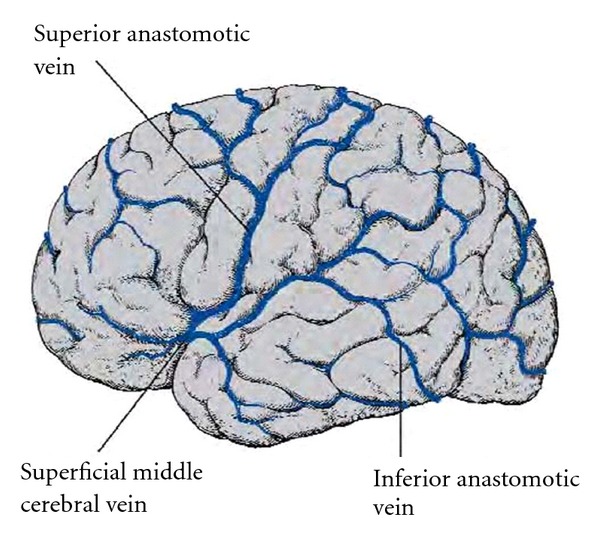
The larger superficial cerebral veins, including those draining the cortex of the temporal lobe.
References
- 1.Young JZ. The Life of Vertebrates. Oxford, UK: Clarendon Press; 1950. [Google Scholar]
- 2.Pirlot P. Morphologie Evolutive des Chordes. Montreal, Canada: Presses de l'Universite de Montreal; 1969. [Google Scholar]
- 3.Sarnat HB, Netsky MB. Evolution of the Nervous System. 2nd edition. New York, NY, USA: Oxford University Press; 1981. [Google Scholar]
- 4.Mai JG, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edition. Amsterdam, The Netherlands: Elsevier; 2008. [Google Scholar]
- 5.Penfield W, Rasmussen T. The Cerebral Cortex of Man. A Clinical Study of Localization of Function. New York, NY, USA: Macmillan; 1957. [Google Scholar]
- 6.Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 7.Heath RG. Correlation of brain activity with emotion: a basis for developing treatment of violent-aggressive behavior. Journal of the American Academy of Psychoanalysis. 1992;20(3):335–346. doi: 10.1521/jaap.1.1992.20.3.335. [DOI] [PubMed] [Google Scholar]
- 8.Jones-Gotman M, Zatorre RJ, Cendes F, et al. Contribution of medial versus lateral temporal-lobe structures to human odour identification. Brain. 1997;120(10):1845–1856. doi: 10.1093/brain/120.10.1845. [DOI] [PubMed] [Google Scholar]
- 9.Rolls ET. Memory systems in the brain. Annual Review of Psychology. 2000;51:599–630. doi: 10.1146/annurev.psych.51.1.599. [DOI] [PubMed] [Google Scholar]
- 10.Nader K. Memory traces unbound. Trends in Neurosciences. 2003;26(2):65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 11.Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 12.Baloh RW, Kerber KA. Neurophysiology of the Vestibular System. 4th edition. New York, NY, USA: Oxford University Press; 2011. [Google Scholar]
- 13.Kluver H, Bucy PC. ‘Psychic blindness’ and other symptoms following bilateral temporal lobectomy in rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- 14.Kluver H, Bucy PC. Preliminary analysis of functions of temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. [Google Scholar]
- 15.Lilly R, Cummings JL, Benson F, Frankel M. The human Kluver-Bucy syndrome. Neurology. 1983;33(9):1141–1145. doi: 10.1212/wnl.33.9.1141. [DOI] [PubMed] [Google Scholar]
- 16.Ozawa H, Sasaki M, Sugai K, et al. Single-photon emission CT and MR findings in Kluver-Bucy syndrome after Reye syndrome. American Journal of Neuroradiology. 1997;18(3):540–542. [PMC free article] [PubMed] [Google Scholar]
- 17.Jha S, Patel R. Kluver-Bucy syndrome—an experience with six cases. Neurology India. 2004;52(3):369–371. [PubMed] [Google Scholar]
- 18.Kertesz A. Aphasia and Associated Disorders: Taxonomy, Localization and Recovery. New York, NY, USA: Grune & Stratton; 1979. [Google Scholar]
- 19.Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 20.Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cerebral Cortex. 1994;4(2):107–118. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- 21.Insausti R, Amaral DG. Hippocampal formation. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2nd edition. Amsterdam, The Netherlands: Elsevier; 2004. pp. 871–914. [Google Scholar]
- 22.Broca P. Sur la circonvolution limbique et la scissure limbique. Bulletins de la Société D'anthropologie de Paris. 1877;12(12):646–657. [Google Scholar]
- 23.O’Rahilly R, Müller F. Significant features in the early prenatal development of the human brain. Annals of Anatomy. 2008;190(2):105–118. doi: 10.1016/j.aanat.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lemire RJ, Loeser JD, Leech RW, Alvord EC. Normal and Abnormal Development of the Human Nervous System. Hagerstown, Md, USA: Harper & Row; 1975. [Google Scholar]
- 25.Kier EL, Kim JH, Fulbright RK, Bronen RA. Embryology of the human fetal hippocampus: mr imaging, anatomy, and histology. American Journal of Neuroradiology. 1997;18(3):525–532. [PMC free article] [PubMed] [Google Scholar]
- 26.Lorente de No R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. Journal of Psychologie and Neurologie. 1934;46:113–177. [Google Scholar]
- 27.Piersol GA, editor. Human Anatomy Including Structure and Development and Practical Considerations. 8th edition. Philadelphia, Pa, USA: Lippincott; 1923. [Google Scholar]
- 28.Williams PL, editor. Gray's Anatomy. The Anatomical Basis of Medicine and Surgery. New York, NY, USA: Edinburgh etc: Churchill Livingstone; 1995. [Google Scholar]
- 29.Bruni JE. Human Neuroanatomy: A Text, Brain Atlas and Laboratory Dissection Guide. 3rd edition. New York, NY, USA: Oxford University Press; 2009. [Google Scholar]
- 30.Lewis FT. The significance of the term hippocampus. The Journal of Comparative Neurology. 1923;35:213–230. [Google Scholar]
- 31.Field EJ, Harrison RJ. Anatomical Terms: Their Origin and Derivation. 3rd edition. Cambridge, UK: Heffer; 1968. [Google Scholar]
- 32.Duvernoy H. The Human Hippocampus. Functional Anatomy, Vascularization and Serial Sections with MRI. 3rd edition. Berlin, Germany: Springer; 2005. [Google Scholar]
- 33.Edinger L. The Anatomy of the Central Nervous System of Man and of Vertebrates in General. 5th edition. Philadelphia, Pa, USA: F.A. Davis; 1899. Edited by W. S. Hall assisted by P. L. Holland and E. P. Carlton. [Google Scholar]
- 34.Müller F, O’Rahilly R. The amygdaloid complex and the medial and lateral ventricular eminences in staged human embryos. Journal of Anatomy. 2006;208(5):547–564. doi: 10.1111/j.1469-7580.2006.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streeter GL. The development of the nervous system. In: Keibel F, Mall FP, editors. Manual of Human Embryology. Vol. 2. Philadelphia, Pa, USA: Lippincott; 1912. pp. 1–156. [Google Scholar]
- 36.Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. NeuroReport. 1996;7(11):1765–1769. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- 37.de Olmos JS. Amygdala. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2nd edition. Amsterdam, The Netherlands: Elsevier; 2004. pp. 739–868. [Google Scholar]
- 38.Crosby EC, Humphrey T, Lauer EW. Correlative Anatomy of the Nervous System. New York, NY, USA: Macmillan; 1962. [Google Scholar]
- 39.Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. Journal of Comparative Neurology. 1991;309(4):445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- 40.Parent A. Carpenter's Human Neuroanatomy. 9th edition. Baltimore, Md, USA: Williams & Wilkins; 1996. [Google Scholar]
- 41.Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. Journal of Comparative Neurology. 1994;346(3):403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- 42.Kiernan JA. Barr's The Human Nervous System: An Anatomical Viewpoint. 9th edition. Philadelphia, Pa, USA: Lippincott-Raven; 2009. [Google Scholar]
- 43.Maquet P, Peters JM, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 44.LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 45.Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends in Neurosciences. 1993;16(8):328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- 46.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 47.Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of deja vu and vivid ’memories’ in human temporal lobe epilepsy. Brain. 1994;117(1):71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- 48.Buchanan TW. Retrieval of emotional memories. Psychological Bulletin. 2007;133(5):761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramponi C, Barnard PJ, Kherif F, Henson RN. Voluntary explicit versus involuntary conceptual memory are associated with dissociable fMRI responses in hippocampus, amygdala, and parietal cortex for emotional and neutral word pairs. Journal of Cognitive Neuroscience. 2011;23:1935–1951. doi: 10.1162/jocn.2010.21565. [DOI] [PubMed] [Google Scholar]
- 50.Bach DR, Weiskopf N, Dolan RJ. A stable sparse fear memory trace in human amygdala. Journal of Neuroscience. 2011;31(25):9383–9389. doi: 10.1523/JNEUROSCI.1524-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Research. 1971;26(2):217–233. [PubMed] [Google Scholar]
- 52.Oishi K, Faria A, van Zijl PCM, Mori S. MRI Atlas of Human White Matter. 2nd edition. Amsterdam, The Netherlands: Elsevier; 2011. [Google Scholar]
- 53.de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. Journal of Neuropathology and Experimental Neurology. 1985;44(6):578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Damasio AR. Medical progress: aphasia. The New England Journal of Medicine. 1992;326(8):531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- 55.Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. The Journal of Comparative Neurology. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- 56.Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101(2):251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- 57.Tutton GK. Cerebral abscess—the present position. Annals of the Royal College of Surgeons of England. 1953;13(5):281–311. [PMC free article] [PubMed] [Google Scholar]
- 58.Sennaroglu L, Sozeri B. Otogenic brain abscess: review of 41 cases. Otolaryngology. 2000;123(6):751–755. doi: 10.1067/mhn.2000.107887. [DOI] [PubMed] [Google Scholar]
- 59.Sunderland S, Bradley KC. Disturbances of oculomotor function accompanying extradural haemorrhage. Journal of Neurology, Neurosurgery, and Psychiatry. 1953;16(1):35–46. doi: 10.1136/jnnp.16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sunderland S, Hughes ESR. The pupillo-constrictor pathway and the nerves to the ocular muscles in man. Brain. 1946;69(4):301–309. doi: 10.1093/brain/69.4.301. [DOI] [PubMed] [Google Scholar]
- 61.Salamon G. Atlas de la Vascularization Arterielle du Cerveau chez l'Homme. 2nd edition. Paris, Farnce: Sandoz Editions; 1973. [Google Scholar]


