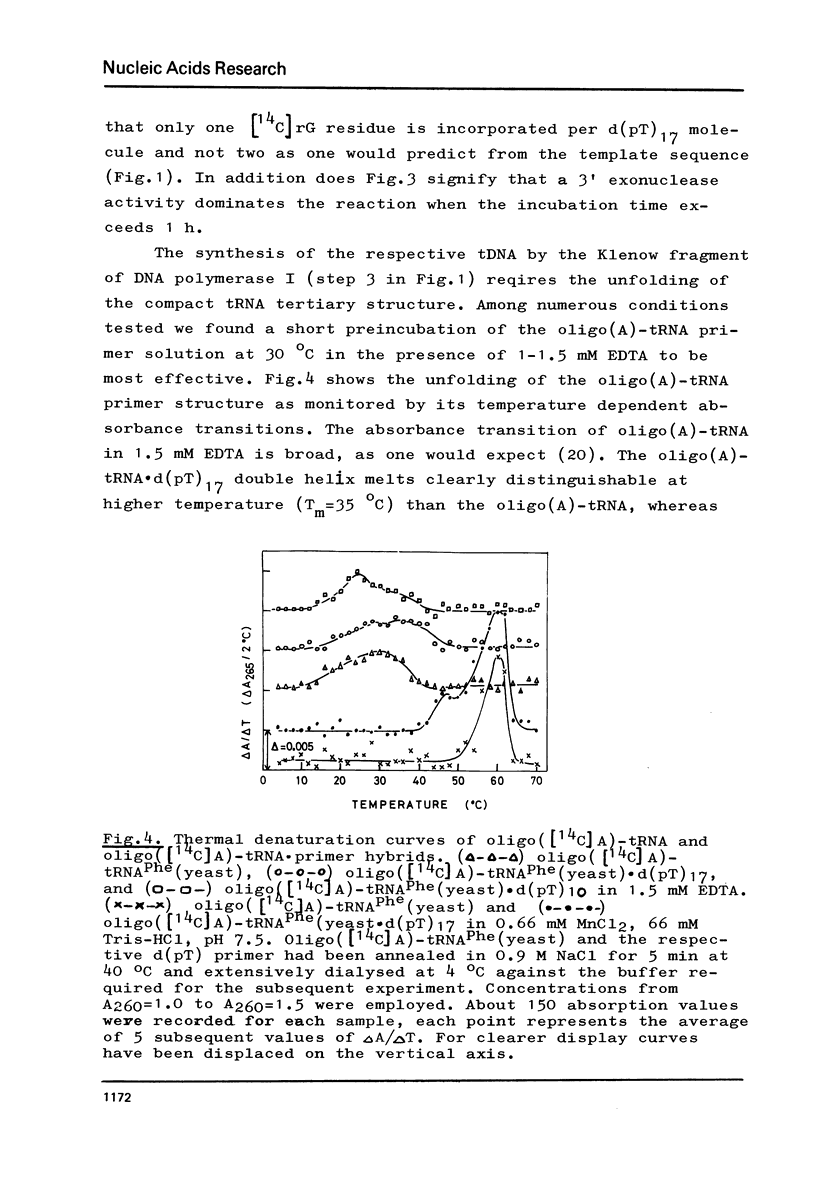
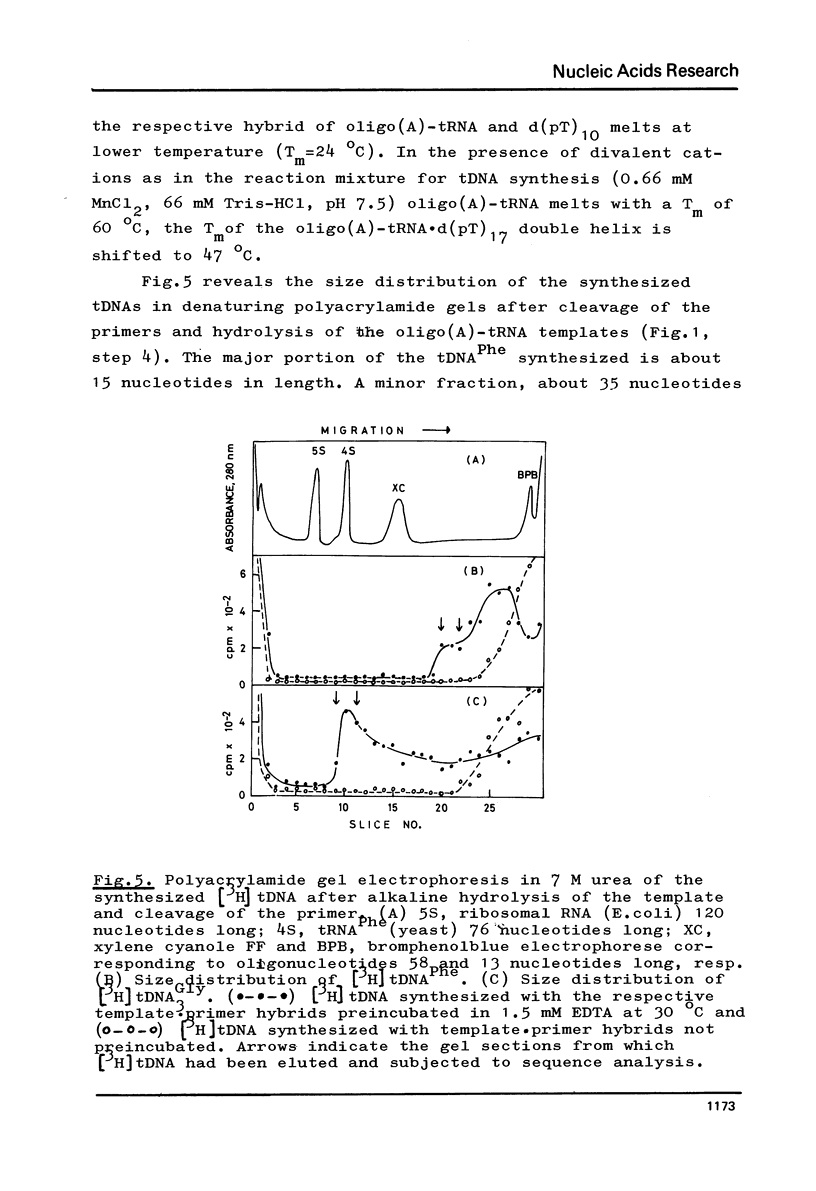
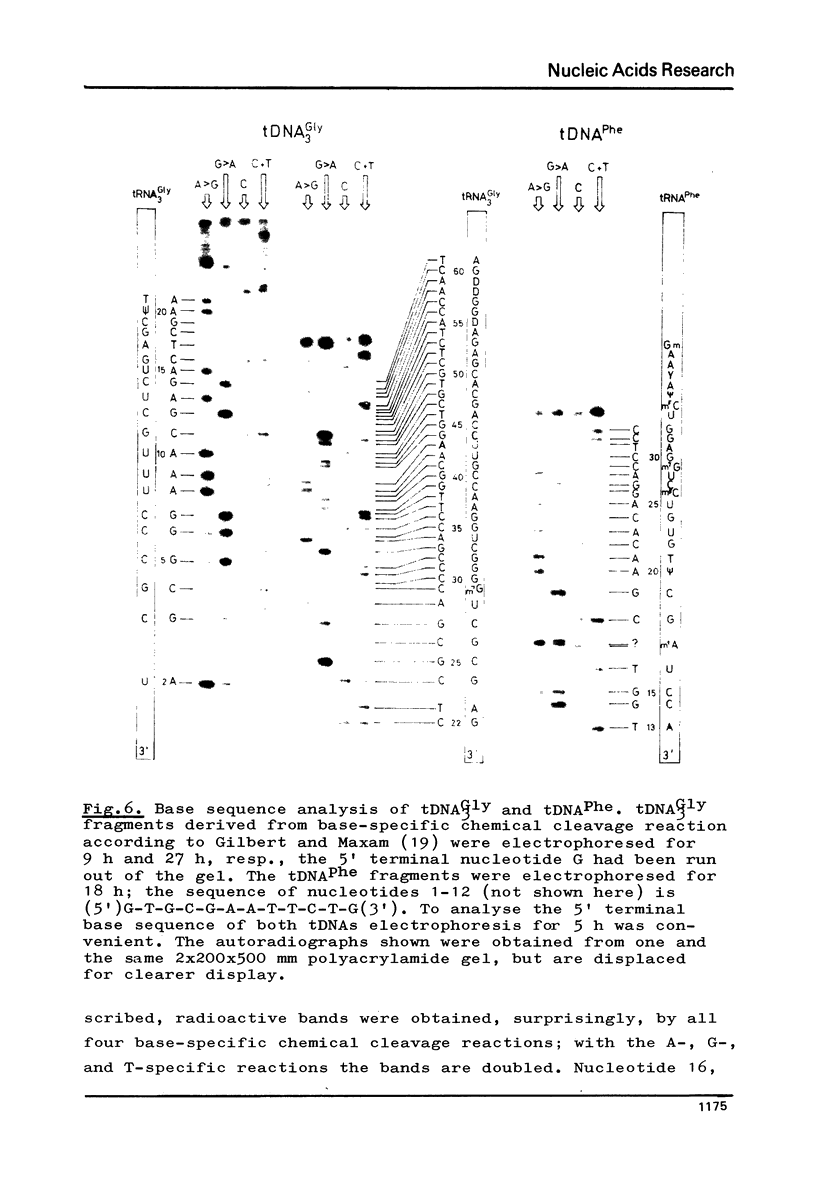
Abstract
The 3' terminus of tRNA was enzymatically elongated by an oligo(A) tail. A fragment of DNA polymerase I (E. coli) was used in the presence of manganese to phase and synthesize a cleavable primer at the oligo(A)-tRNA template. When the threedimensional structure of oligo(A)-tRNA is being unfolded under conditions where the primer is still hybridized at the oligo(A) tail, the DNA polymerase I fragment transcribes oligo(A)-tRNA into DNA. Reverse transcription is slowed down and its fidelity suspended by the 1-methyladenine in oligo(A)-tRNAPhe(yeast). The reaction is stopped by the highly modified Y-base present in this template. Approximately full length transcripts can be obtained from oligo(A)-tRNA3Gly(E.coli). The transcription products were characterized by sequence analysis.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T., Fresco J. R. Conformational differences between the biologically active and inactive forms of a transfer ribonucleic acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1684–1691. doi: 10.1073/pnas.57.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. N., Schimke R. T. Partial purification of the ovalbumin gene. Cell. 1976 Mar;7(3):331–338. doi: 10.1016/0092-8674(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M. Rapid gel sequencing of RNA by primed synthesis with reverse transcriptase. J Mol Biol. 1977 Jul;114(1):93–117. doi: 10.1016/0022-2836(77)90285-6. [DOI] [PubMed] [Google Scholar]
- Cheng C. C., Brownlee G. G., Carey N. H., Doel M. T., Gillam S., Smith M. The 3' terminal sequence of chicken ovalbumin messenger RNA and its comparison with other messenger RNA molecules. J Mol Biol. 1976 Nov 15;107(4):527–547. doi: 10.1016/s0022-2836(76)80081-2. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Frolova L. Y., Metelyev V. G., Ratmanova K. I., Smirnov V. D., Shabarova Z. A., Prokofyev M. A., Berzin V. M., Jansone I. V., Gren E. J., Kisselev L. L. Reverse transcription of phage RNA and its fragment directed by synthetic heteropolymeric primers. Nucleic Acids Res. 1977 Jul;4(7):2145–2159. doi: 10.1093/nar/4.7.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Birnie G. D., Paul J. Transcription of in vitro polyadenylated ribonucleic acid with reverse transcriptase. Biochemistry. 1974 May 7;13(10):2235–2240. doi: 10.1021/bi00707a033. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Khorana H. G., Agarwal K. L., Besmer P., Büchi H., Caruthers M. H., Cashion P. J., Fridkin M., Jay E., Kleppe K., Kleppe R. Total synthesis of the structural gene for the precursor of a tyrosine suppressor transfer RNA from Escherichia coli. 1. General introduction. J Biol Chem. 1976 Feb 10;251(3):565–570. [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K. Proteolytic cleavage of DNA polymerase from Escherichia Coli B into an exonuclease unit and a polymerase unit. FEBS Lett. 1970 Jan 15;6(1):25–27. doi: 10.1016/0014-5793(70)80032-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mans R. J., Huff N. J. Utilization of ribonucleic acid and deoxyoligomer primers for polyadenylic acid synthesis by adenosine triphosphate: polynucleotidylexotransferase from maize. J Biol Chem. 1975 May 25;250(10):3672–3678. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L., Filimonov V. V., Venkstern T. V., Bayev A. A. A calorimetric investigation of tRNAVal1 melting. J Mol Biol. 1975 Sep 25;97(3):279–288. doi: 10.1016/s0022-2836(75)80041-6. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Sequence analysis of the 3' non-coding regions of rabbit alpha- and beta-globin messenger RNAs. J Mol Biol. 1976 Nov 15;107(4):491–525. doi: 10.1016/s0022-2836(76)80080-0. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Scheit K. H. The stereochemical basis of template function. Eur J Biochem. 1977 Jan 3;72(1):191–200. doi: 10.1111/j.1432-1033.1977.tb11239.x. [DOI] [PubMed] [Google Scholar]
- Riesner D., Maass G., Thiebe R., Philippsen P., Zachau H. G. The conformational transitions in yeast tRNAPhe as studied with tRNAPhe fragments. Eur J Biochem. 1973 Jul 2;36(1):76–88. doi: 10.1111/j.1432-1033.1973.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Venetianer P., Leder P. Enzymatic synthesis of solid phase-bound DNA sequences corresponding to specific mammalian genes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3892–3895. doi: 10.1073/pnas.71.10.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Reuter S., Gottschling H. Studies on phenylalanine-specific transfer ribonucleic acid from chick embryos. Eur J Biochem. 1974 Dec 2;49(3):521–529. doi: 10.1111/j.1432-1033.1974.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Wittig B., Wittig S. Nucleosome mono, di, tri-, and tetramers from chicken embryo chromatin. Nucleic Acids Res. 1977 Nov;4(11):3901–3917. doi: 10.1093/nar/4.11.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]



