Abstract
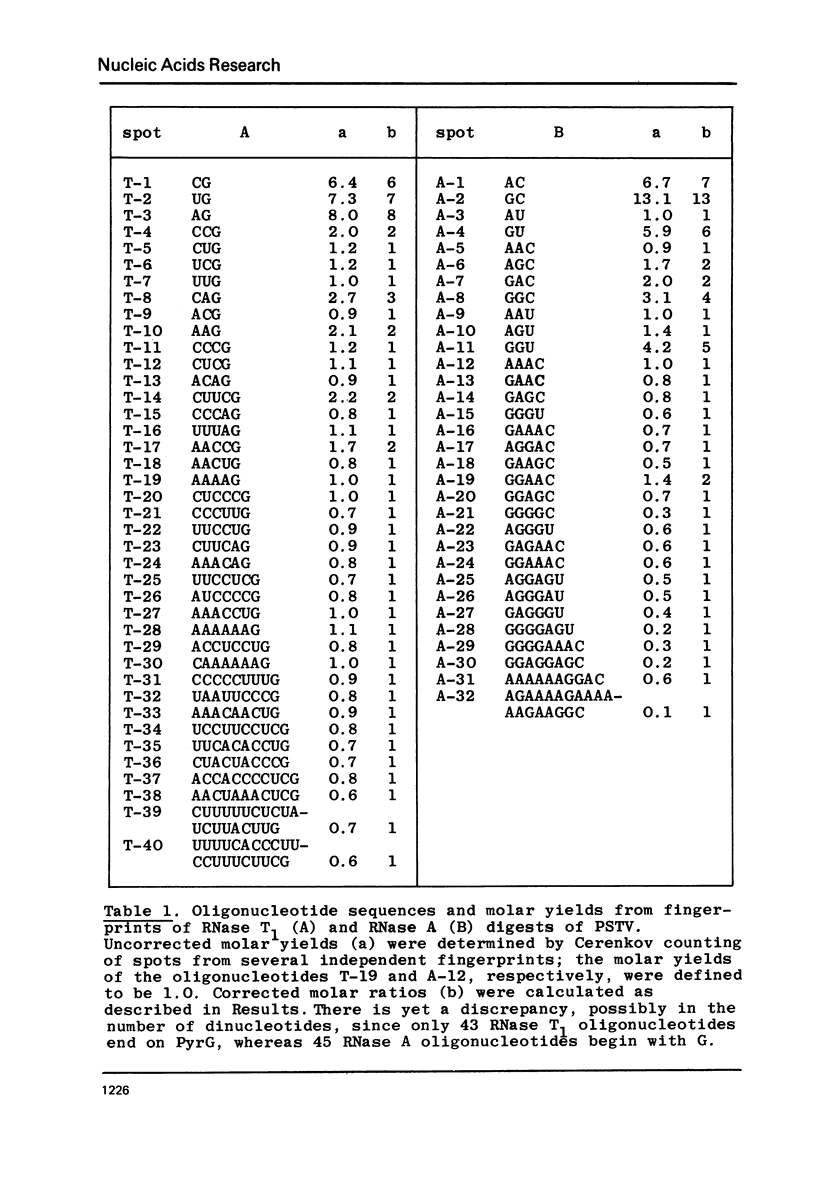
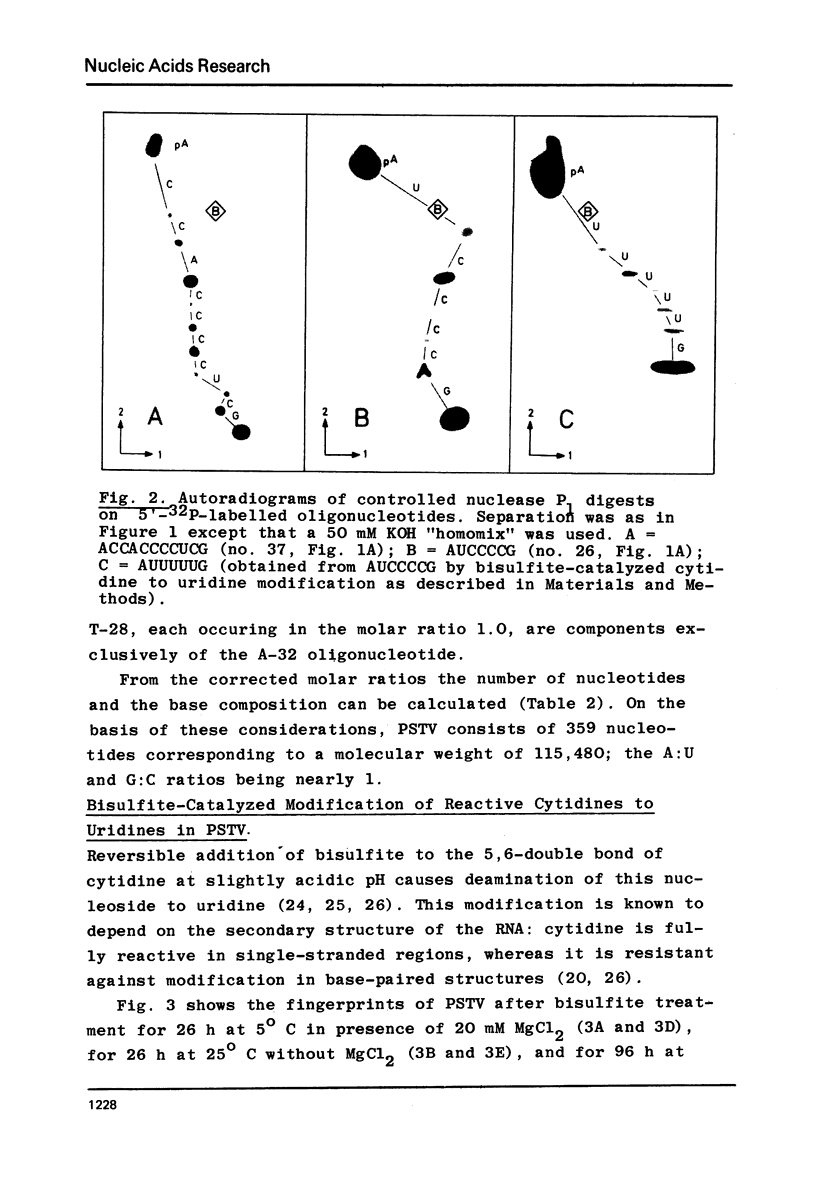
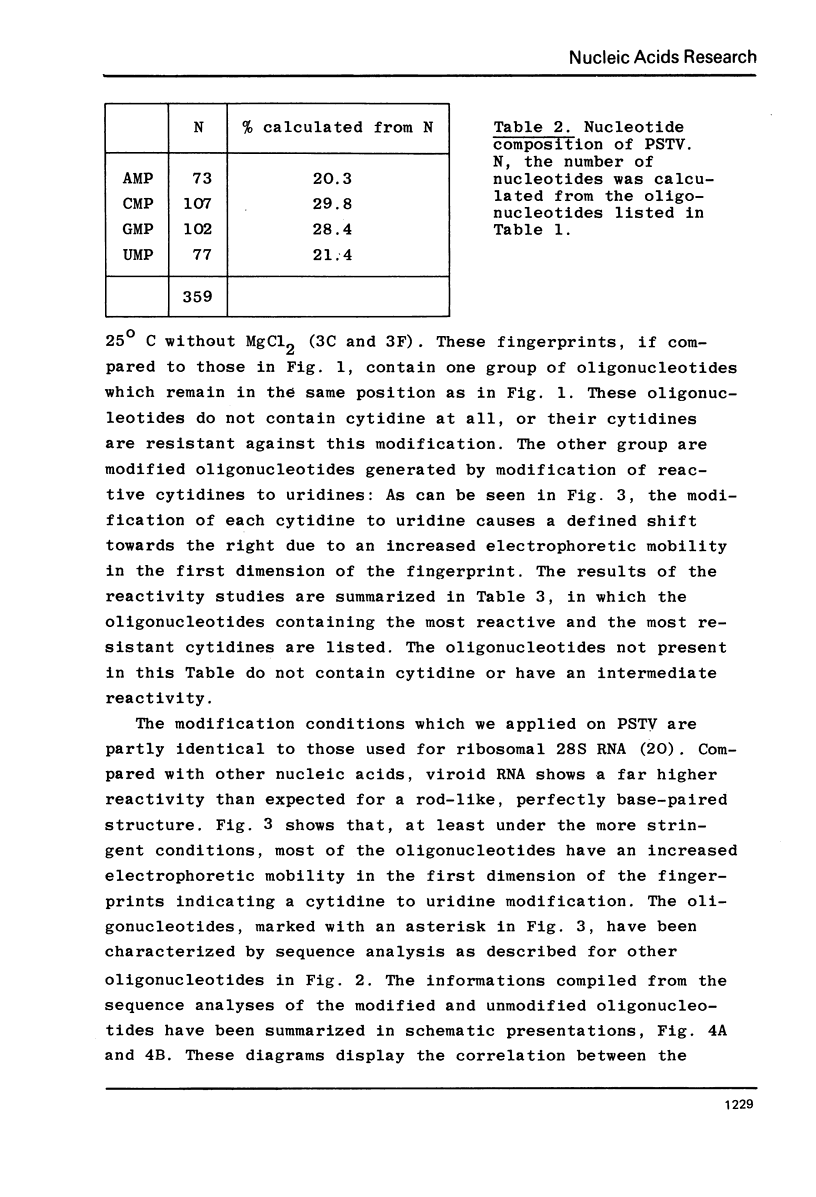
Potato spindle tuber viroid (PSTV), a small infectios RNA, has been completely digested with RNase T1 and RNase A, and the resulting oligonucleotides have been sequenced using 5'-terminal 32p-labelling with gamma-32p ATP and T4 polynucleotide kinase, fingerprinting and controlled nuclease P1 digestion. Modified nucleotides have not been detected in 5'-positions of these oligonucleotides. PSTV consists of about 359 nucleotides and contains a remarkable stretch of 18 purines, mainly adenosines; there is no AUG initiation triplet present. The established oligonucleotide sequences preclude a perfect intramolecular base complementarity within the covalently closed viroid circle. Therefore, the rigid, rod-like native secondary structure of PSTV, as seen in the electron microscope, must be based on a defective rather than on a homogeneous RNA helix. The detailed analysis of the bisulfite-catalized modification of cytidine to uridine in PSTV revealed a higher reactivity for the majority of the cytidines than would be expected for a perfect helix. Since only cytidines in single-stranded regions are knonw to be fully reactive, this finding provides additional evidence for defects in the helical secondary structure of PSTV.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty H., Raba M., Gross H. J. Isolation from rat liver and sequence of a RNA fragment containing 32 nucleotides from position 5 to 36 from the 3' end of ribosomal 18S RNA. Nucleic Acids Res. 1978 Feb;5(2):425–434. doi: 10.1093/nar/5.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O., Lawson R. H. Chrysanthemum stunt: a viroid disease. Virology. 1973 Jan;51(1):94–101. doi: 10.1016/0042-6822(73)90369-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Frisby D. Oligonucleotide mapping of non-radioactive virus and messenger RNAs. Nucleic Acids Res. 1977 Sep;4(9):2975–2996. doi: 10.1093/nar/4.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. P., Maden B. E. Reaction of HeLa cell methyl-labelled 28S ribosomal RNA with sodium bisulphite: a conformational probe for methylated sequences. Nucleic Acids Res. 1976 Feb;3(2):431–440. doi: 10.1093/nar/3.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. P., Schulman L. H. Conversion of exposed cytidine residues to uridine residues in Escherichia coli formylmethionine transfer ribonucleic acid. J Biol Chem. 1972 Jun 25;247(12):3864–3867. [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Sänger H. L. Comparative oligonucleotide fingerprints of three plant viroids. Nucleic Acids Res. 1977 Jun;4(6):2021–2028. doi: 10.1093/nar/4.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kai K., Iida S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry. 1970 Jul 7;9(14):2858–2865. doi: 10.1021/bi00816a016. [DOI] [PubMed] [Google Scholar]
- Henco K., Riesner D., Sanger H. L. Conformation of viroids. Nucleic Acids Res. 1977 Jan;4(1):177–194. doi: 10.1093/nar/4.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Lockard R. E., Alzner-deWeerd B., RajBhandary U. L., Bol J. F. Nucleotide sequence of 5' terminus of alfalfa mosaic virus RNA 4 leading into coat protein cistron. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5504–5508. doi: 10.1073/pnas.74.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer H. A calorimetric investigation of the heats of binding of Mg ++ to poly A, to poly U, and to their complexes. Biopolymers. 1972;11(4):811–828. doi: 10.1002/bip.1972.360110406. [DOI] [PubMed] [Google Scholar]
- McClements W. L., Kaesberg P. Size and secondary structure of potato spindle tuber viroid. Virology. 1977 Feb;76(2):477–484. doi: 10.1016/0042-6822(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Erbe E., Hadidi A., Steere R. L., Diener T. O. Separation and infectivity of circular and linear forms of potato spindle tuber viroid. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3859–3863. doi: 10.1073/pnas.74.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randles J. W., Rillo E. P., Diener T. O. The viroidlike structure and cellular location of anomalous RNA associated with the cadang-cadang disease. Virology. 1976 Oct 1;74(1):128–139. doi: 10.1016/0042-6822(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Romaine C. P., Horst R. K. Suggested viroid etiology for chrysanthemum chlorotic mottle disease. Virology. 1975 Mar;64(1):86–95. doi: 10.1016/0042-6822(75)90081-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis disease: evidence for a new species of "infectious" low molecular weight RNA in plants. Nat New Biol. 1972 Jun 21;237(77):242–244. doi: 10.1038/newbio237242a0. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely M., Sanger F. Use of polynucleotide kinase in fingerprinting non-radioactive nucleic acids. J Mol Biol. 1969 Aug 14;43(3):607–617. doi: 10.1016/0022-2836(69)90362-3. [DOI] [PubMed] [Google Scholar]