Abstract
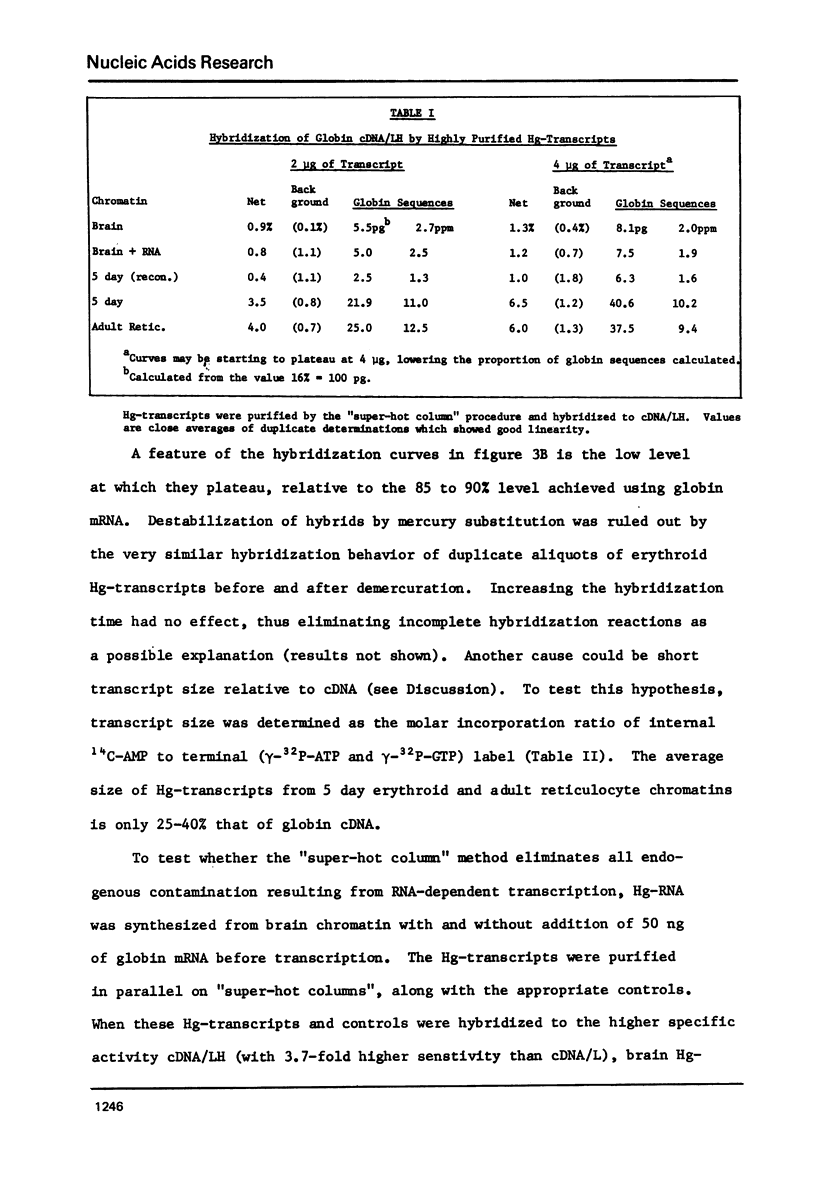
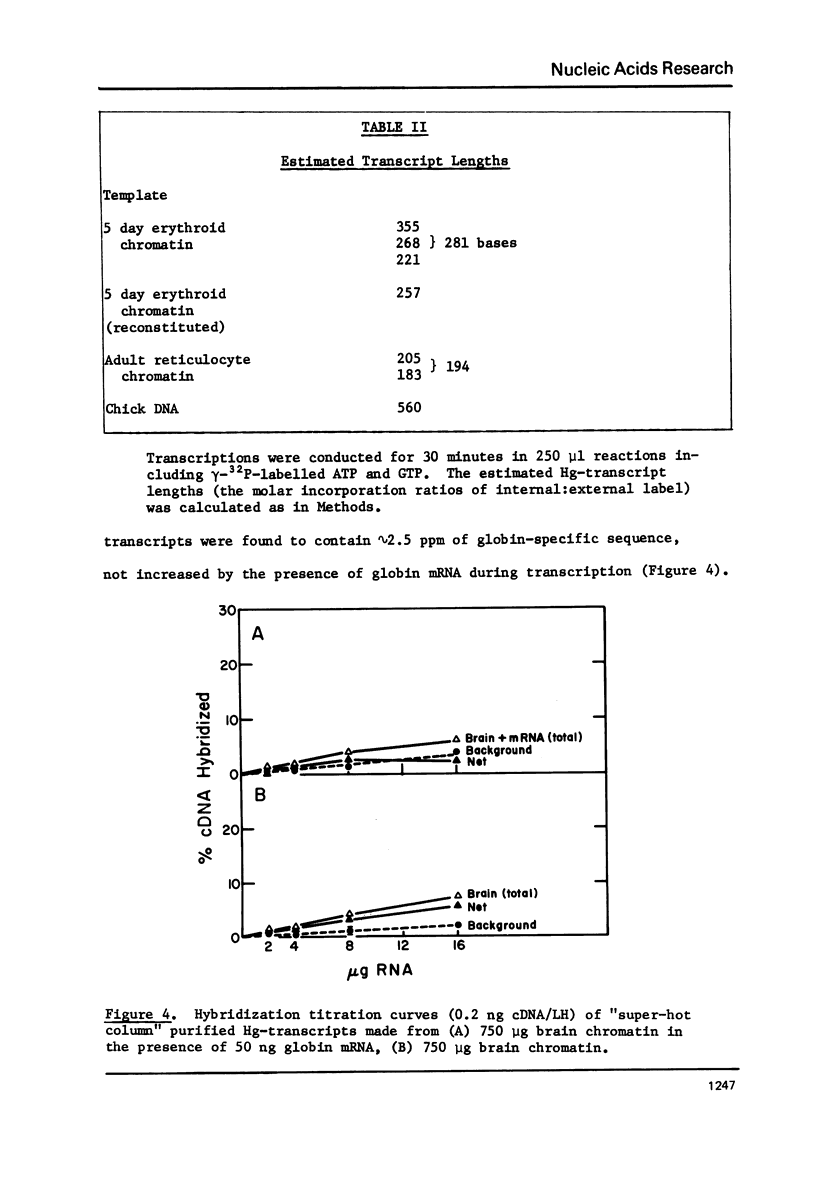
Hg-UMP-containing transcripts made from chick erythroid chromatins with E. coli RNA polymerase hybridize to chick globin cDNA. Contamination with endogenous globin RNA has been largely removed by purification on SH-agarose columns at 55 degrees C. Some endogenous globin mRNA sequences remain, probably as hybrids with "anti-sense" Hg-transcripts produced by RNA-dependent RNA synthesis. Heating to 115 degrees C before SH-agarose chromatography eliminates these contaminants. Hg-transcripts from adult and embryonic erythroid chromatins purified by this method are hybridized to globin cDNA; they contain a 4- to 6-fold higher proportion of globin-specific sequences (10-13 PPM) than do transcripts from brain chromatin. Dissociation of erythroid chromatins in salt and urea, followed by reconstitution using standard methods, destroys even this low degree of specificity.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfield G. The structure of the globin genes in chromatin. Biochemistry. 1975 Jun 3;14(11):2489–2495. doi: 10.1021/bi00682a031. [DOI] [PubMed] [Google Scholar]
- Barrett T., Maryanka D., Hamlyn P. H., Gould H. J. Nonhistone proteins control gene expression in reconstituted chromatin. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5057–5061. doi: 10.1073/pnas.71.12.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Gjerset R. A., Levy B., McCarthy B. J. Fidelity of chromatin transcription in vitro. Biochemistry. 1976 Oct 5;15(20):4356–4363. doi: 10.1021/bi00665a002. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Ingram V. M. Structural studies on chick embryonic hemoglobins. J Biol Chem. 1974 Jun 25;249(12):3960–3972. [PubMed] [Google Scholar]
- Chan L. N., Wiedmann M., Ingram V. M. Regulation of specific gene expression during embryonic development: Synthesis of globin messenger RNA during red cell formation in chick embryos. Dev Biol. 1974 Sep;40(1):174–185. doi: 10.1016/0012-1606(74)90117-1. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Tsai Y. H., Sakuma K., Hnilica L. S. Regulation of in vitro mRNA transcription by a fraction of chromosomal proteins. J Biol Chem. 1975 Dec 25;250(24):9431–9433. [PubMed] [Google Scholar]
- Crouse G. F., Fodor J. B., Doty P. In vitro transcription of chromatin in the presence of a mercurated nucleotide. Proc Natl Acad Sci U S A. 1976 May;73(5):1564–1567. doi: 10.1073/pnas.73.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Curtis P. J., Weissmann C. Purification of globin messenger RNA from dimethylsulfoxide-induced Friend cells and detection of a putative globin messenger RNA precursor. J Mol Biol. 1976 Oct 5;106(4):1067–1075. doi: 10.1016/0022-2836(76)90353-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Windass J. D., Affara N., Paul J. Control of transcription of the globin gene. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):449–458. doi: 10.1002/jcp.1040850411. [DOI] [PubMed] [Google Scholar]
- Humphries S., Windass J., Williamson R. Mouse globin gene expression in erythroid and non-erythroid tissues. Cell. 1976 Feb;7(2):267–277. doi: 10.1016/0092-8674(76)90026-x. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Ingram V. M. RNA aggregation during sulfhydryl-agarose chromatography of mercurated RNA. Nucleic Acids Res. 1977 Jun;4(6):1979–1988. doi: 10.1093/nar/4.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre S. S., Rutter W. J. Isolation of chicken hemoglobin mRNA and synthesis of complementary DNA. J Biol Chem. 1977 Apr 25;252(8):2742–2752. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. In vitro synthesis of a DNA probe for antisense globin sequences. J Biol Chem. 1977 Aug 25;252(16):5606–5608. [PubMed] [Google Scholar]
- Orkin S. H., Swerdlow P. S. Globin RNA synthesis in vitro by isolated erythroleukemic cell nuclei: direct evidence for increased transcription during erythroid differentiation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2475–2479. doi: 10.1073/pnas.74.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Young H. A., Parks W. P., Scolnick E. M. In vitro transcription of Moloney leukemia virus genes in infected cell nuclei and chromatin: elongation of chromatin associated ribonucleic acid by Escherichia coli ribonucleic acid polymerase. Biochemistry. 1977 May 3;16(9):1795–1801. doi: 10.1021/bi00628a005. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Huang R. C. Transcription in vitro of immunoglobulin kappa light chain genes in isolated mouse myeloma nuclei and chromatin. Proc Natl Acad Sci U S A. 1976 Mar;73(3):775–779. doi: 10.1073/pnas.73.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggles A. W., Wilson G. N., Kantor J. A., Picciano D. J., Falvey A. K., Anderson W. F. Cell-free transcription of mammalian chromatin: transcription of globin messenger RNA sequences from bone-marrow chromatin with mammalian RNA polymerase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1219–1223. doi: 10.1073/pnas.71.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G., Park W., Thrall C., Mans R., Stein J. Regulation of cell cycle stage-specific transcription of histone genes from chromatin by non-histone chromosomal proteins. Nature. 1975 Oct 30;257(5529):764–767. doi: 10.1038/257764a0. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Tsai M. J., Tsai S. Y., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. J Biol Chem. 1977 Apr 10;252(7):2396–2404. [PubMed] [Google Scholar]
- Weiss G. B., Wilson G. N., Steggles A. W., Anderson W. F. Importance of full size complementary DNA in nucleic acid hybridization. J Biol Chem. 1976 Jun 10;251(11):3425–3431. [PubMed] [Google Scholar]
- Wilson G. N., Steggles A. W., Kantor J. A., Nienhuis A. W., Anderson W. F. Cell-free transcription of mammalian chromatin. Quantitative measurement of newly synthesized globin messenger RNA sequences. J Biol Chem. 1975 Nov 25;250(22):8604–8613. [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Analysis of in vitro transcription of duck reticulocyte chromatin using mercury-substituted ribonucleoside triphosphates. Biochemistry. 1977 Nov 15;16(23):5135–5145. doi: 10.1021/bi00642a029. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Use of mercury-substituted ribonucleoside triphosphates can lead to artefacts in the analysis of in vitro chromatin transcrits. Biochem Biophys Res Commun. 1977 Apr 11;75(3):598–603. doi: 10.1016/0006-291x(77)91514-5. [DOI] [PubMed] [Google Scholar]
- de Pomerai D. I., Chesterton C. J., Butterworth P. H. Preparation of chromatin. Variation in the template properties of chromatin dependent on the method of perparation. Eur J Biochem. 1974 Aug 1;46(3):461–471. doi: 10.1111/j.1432-1033.1974.tb03639.x. [DOI] [PubMed] [Google Scholar]