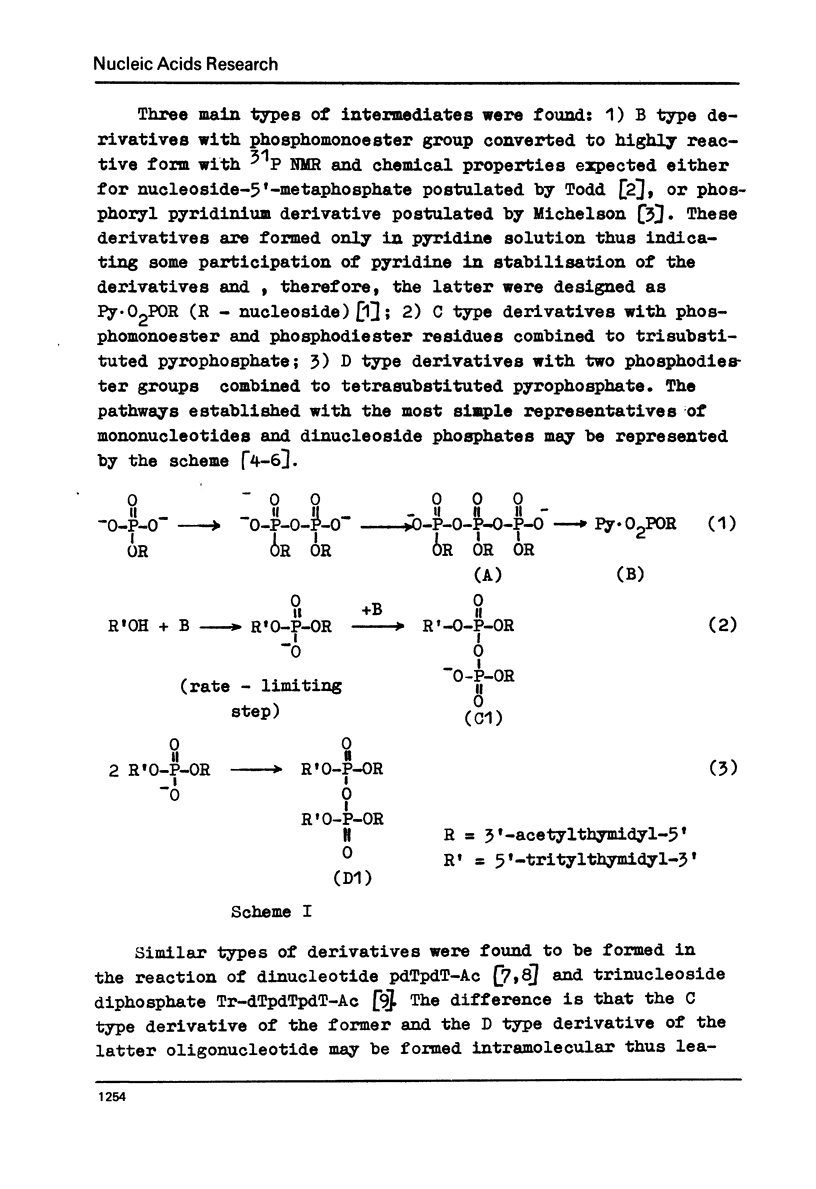
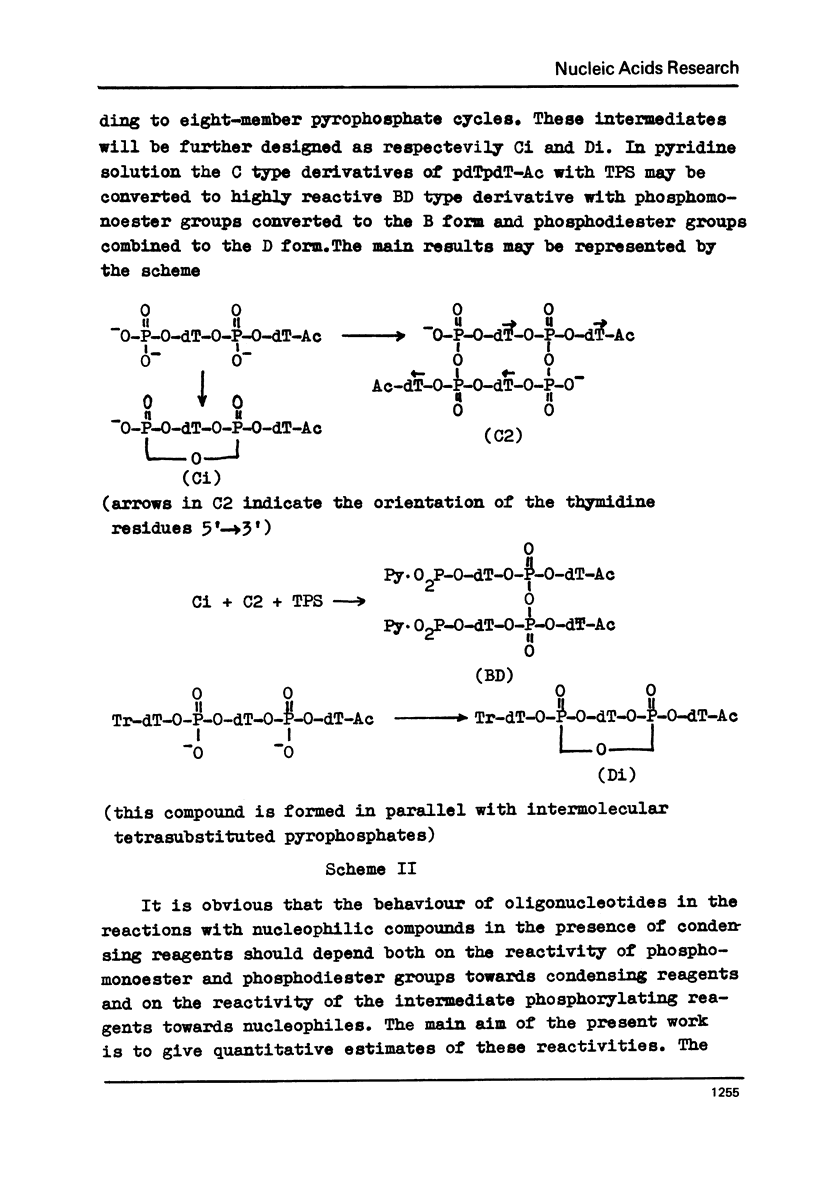
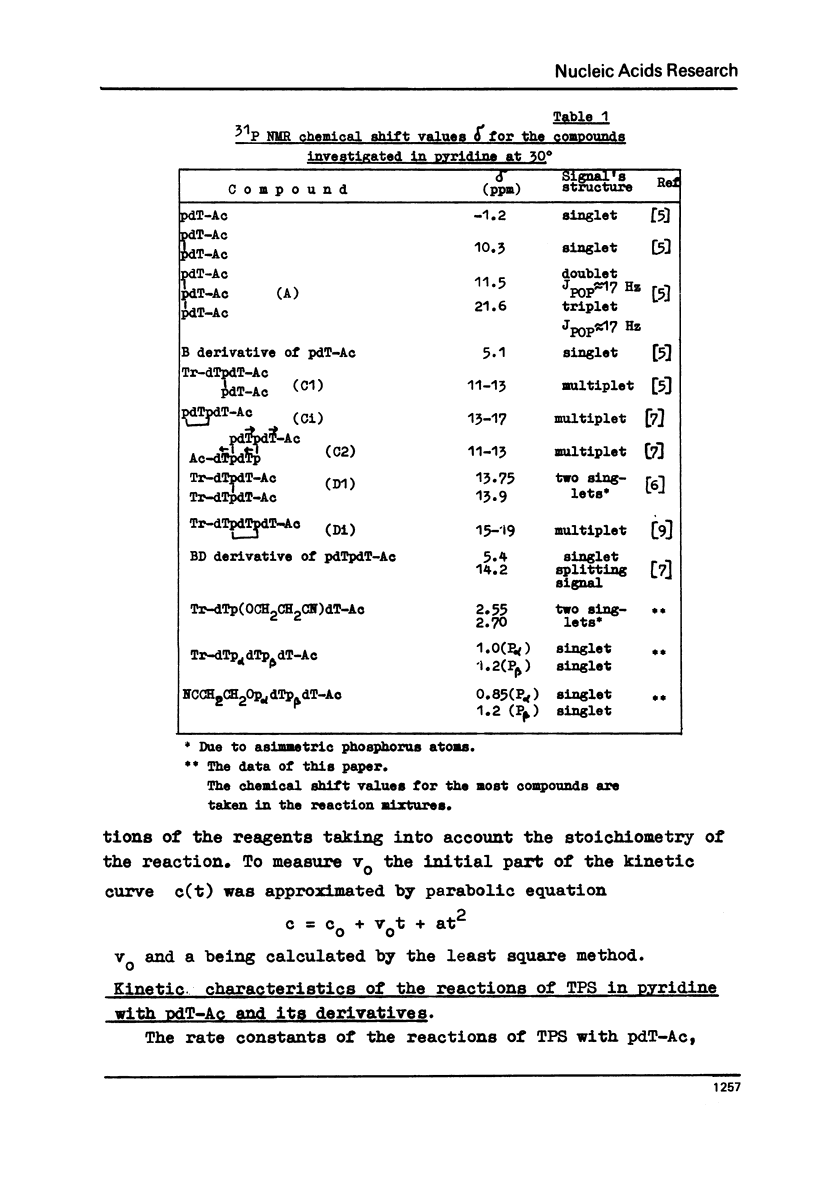
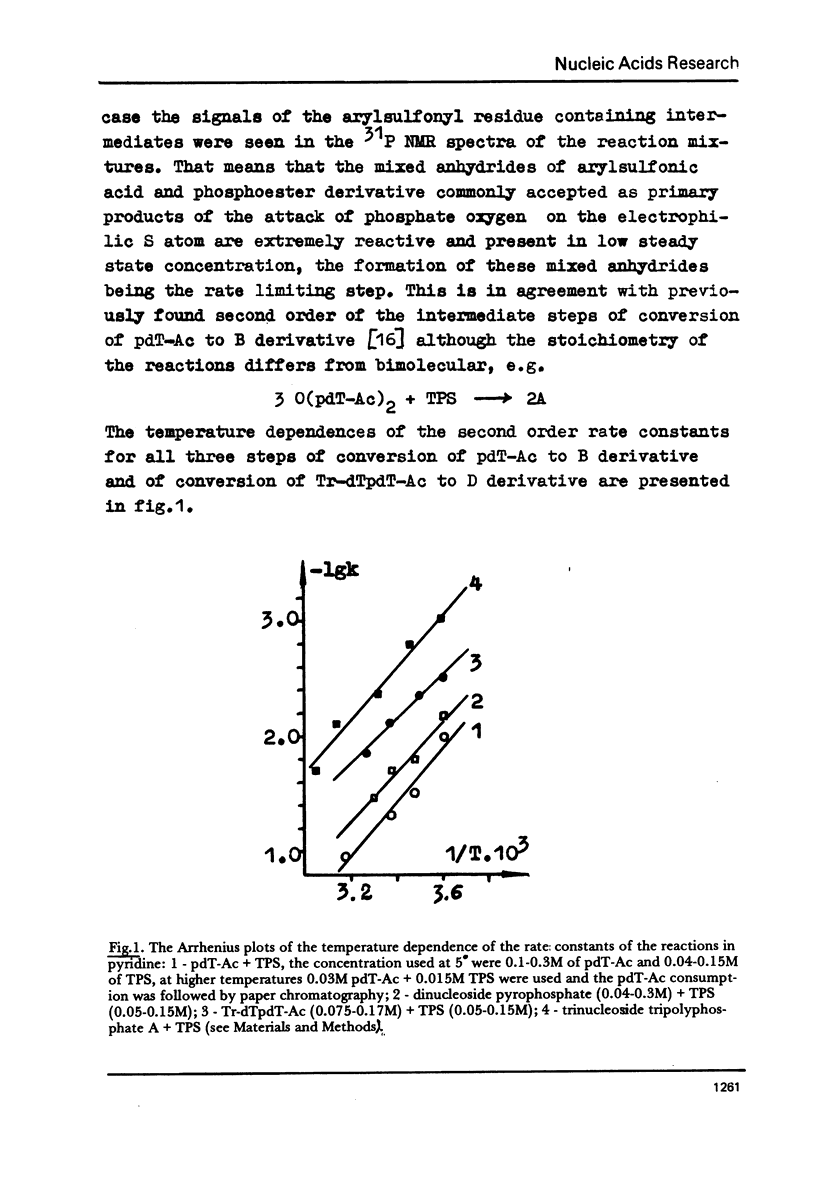
Abstract
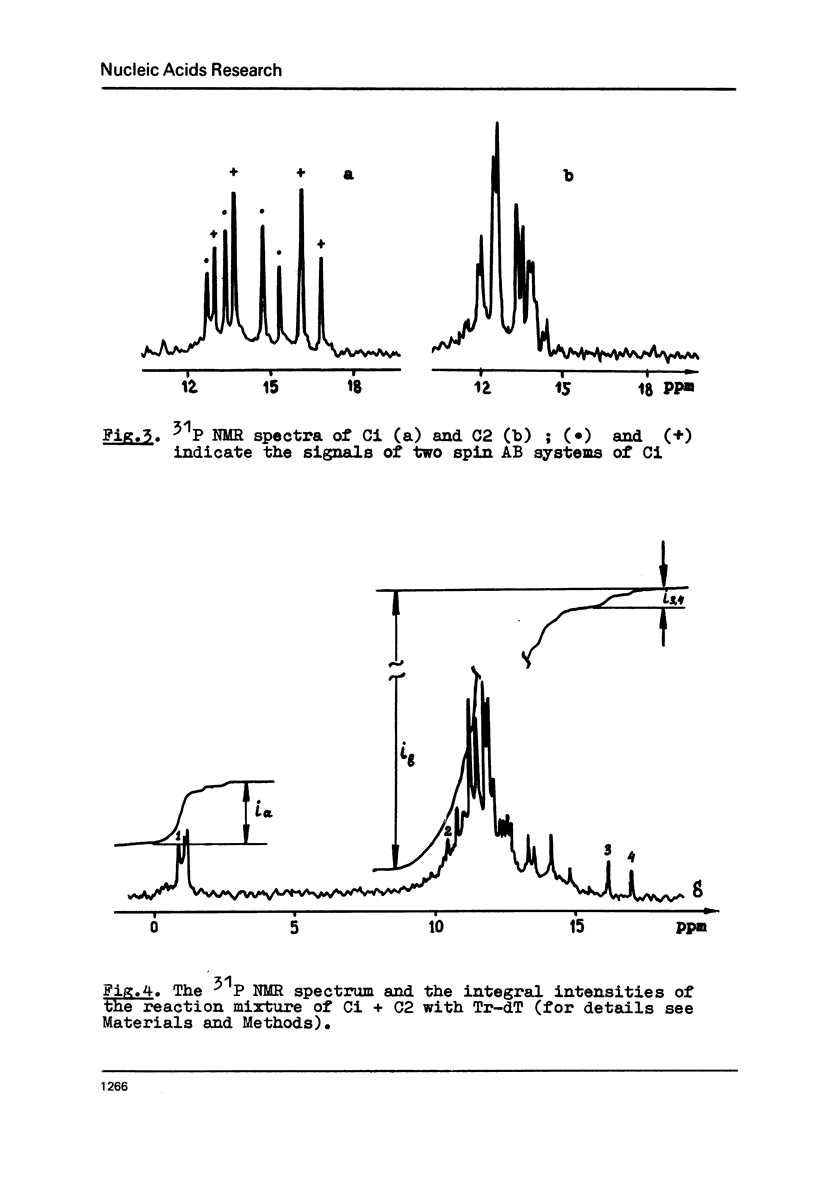
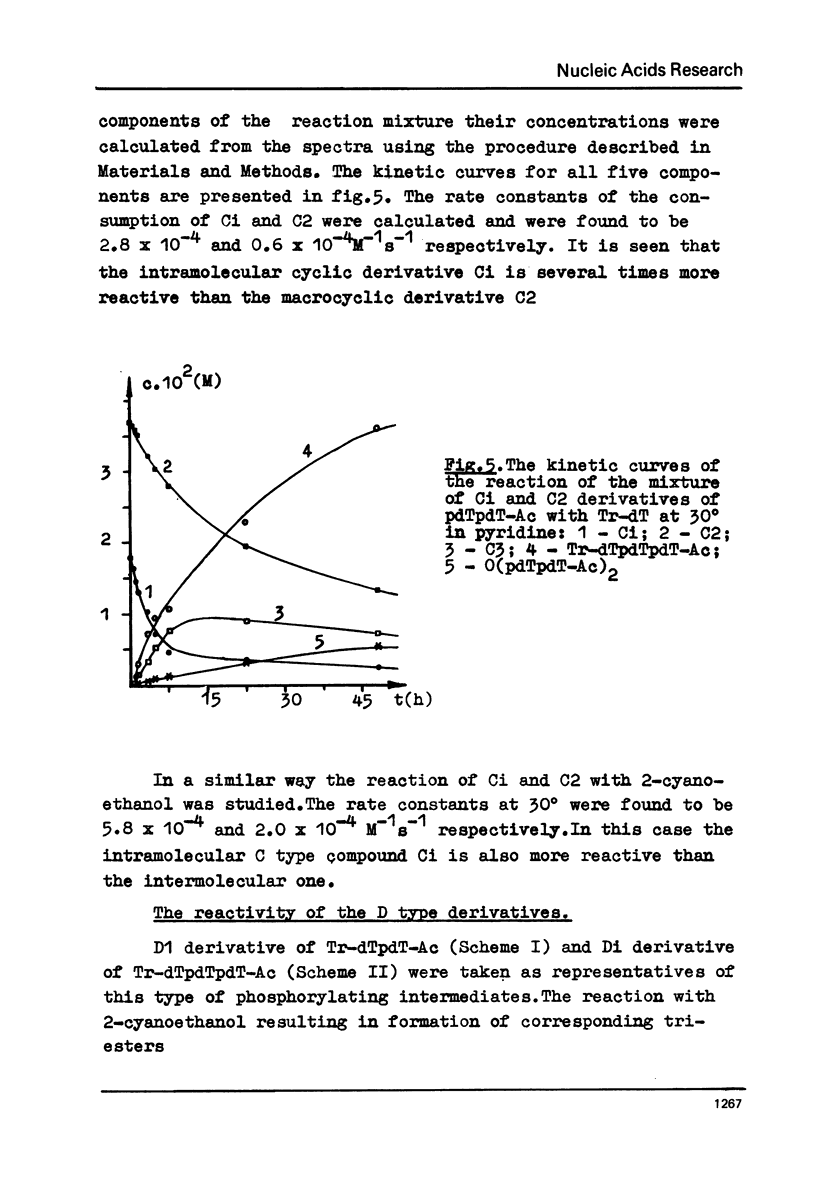
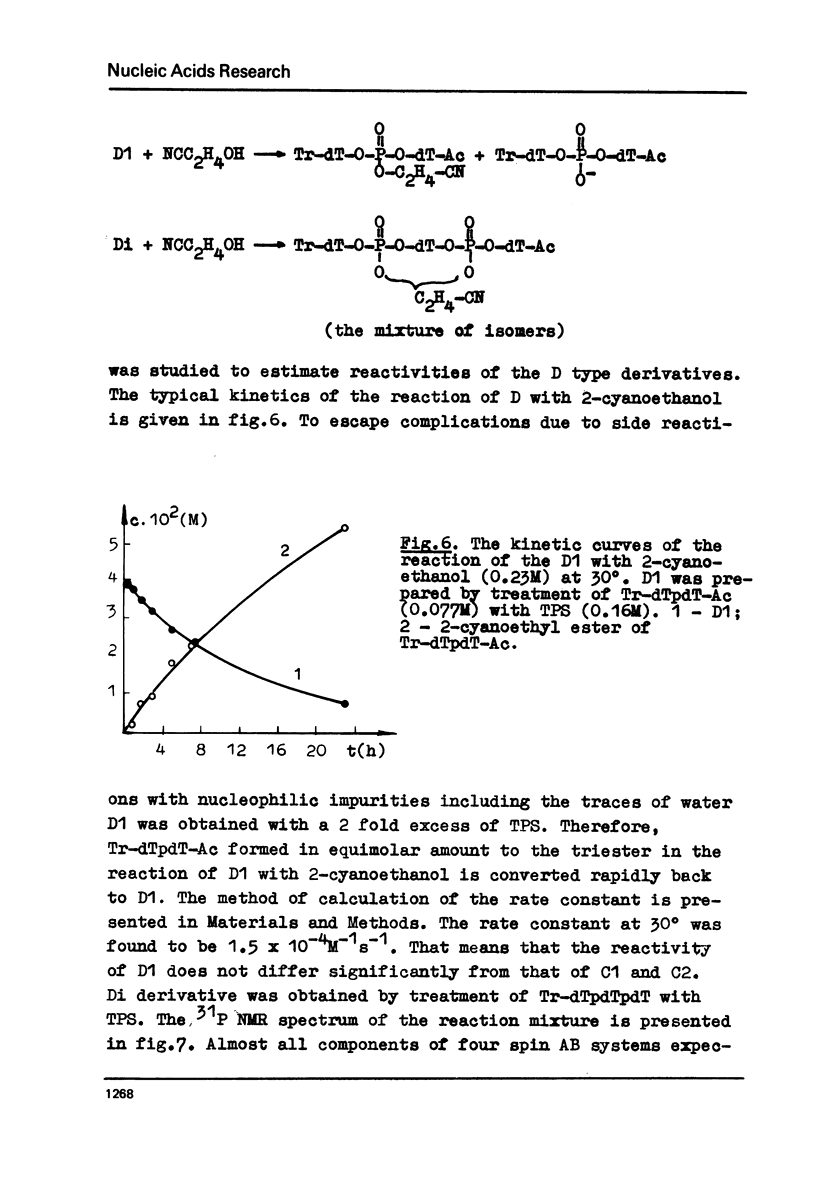
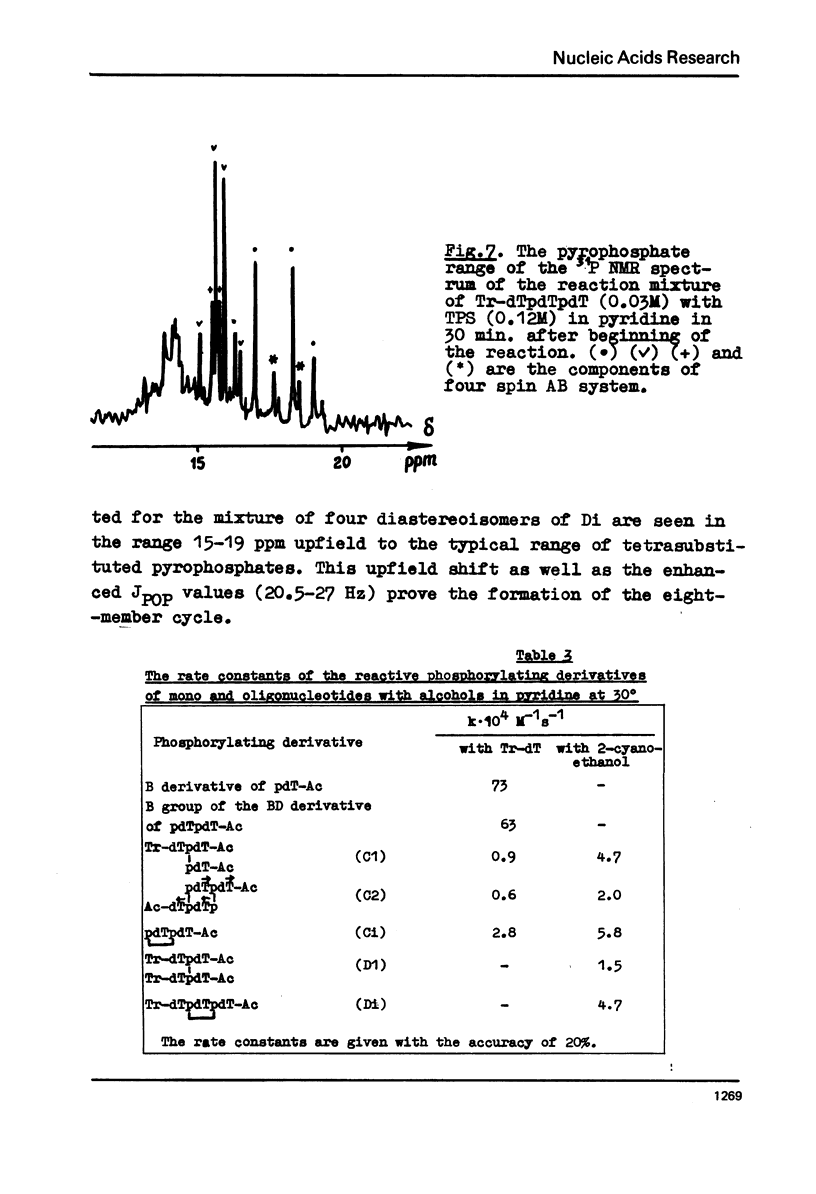
The rate constants were estimated by phosphorus NMR spectroscopy for the reactions of alcohols (Tr-dT, 2-cyanoethanol) in pyridine with the main types of the reactive phosphorylating intermediates formed by treatment of pdT-Ac, pdTpdT-Ac, Tr-dTpdT-Ac, Tr-dTpdTpdT-Ac with 2, 4, 6-triisopropylbenzene-sulfonyl chloride (TPS): 1) B type derivatives with phosphomono ester (PME) group converted to a phosphoryl pyridinium residue; 2) C type derivatives with PME and phosphodiester (PDE) groups converted to trisubstituted pyrophosphate; 3) D type derivatives with PDE groups converted to tetrasubstituted pyrophosphate. The two latter types are partially present as cyclic intramolecular pyrophosphates Ci and Di. The reactivity of the intermediates decrease in the series B greater than Ci approximately Di greater than C approximately D. The Ci derivative of pdTpdT-Ac when obtained in dimethylformamide was found to be rather stable to hydrolysis and could be separated from the other dinucleotide derivatives by ion-exchange chromatography. The Arrhenius parameters of all steps of the conversion of PME group of pdT-Ac to B derivative and of the reaction of TPS with PDE group of dinucleoside phosphate Tr-dTpdT-Ac were measured.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Knorre D. G., Zarytova V. F. The mechanism of the chemical synthesis of oligonucleotides and its synthetic consequences. Nucleic Acids Res. 1976 Oct;3(10):2709–2729. doi: 10.1093/nar/3.10.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S. A. SOME ASPECTS OF PHOSPHATE CHEMISTRY. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1389–1397. doi: 10.1073/pnas.45.9.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]


