Abstract
The uridine-5-0-derivatives, 5-methoxyuridine (mo5U) and uridine-5-oxyacetic acid (cmo5U) occupy the first position of anticodons in certain tRNA species of B. subtilis and E. coli, respectively. Here we present experimental evidence showing that both modifications are derived from a common precursor, 5-hydroxyuridine. Incompletely modified tRNAAla, tRNAThr and tRNAVal were purified from B. subtilis, and submodified tRNASer and tRNAVal from E. coli met− rel−. All five tRNAs accepted methyl groups from S-adenosylmethionine with B. subtilis extracts in vitro and mo5U was formed. In B. subtilis tRNAs the mo5U was proved to be at the specific site; in. E. coli tRNAVal the mo5U was demonstrated to be present in the oligonucleotide that comprises the anticodon. In submethylated E. coli tRNAVal, 5-hydroxyuridine was detected whereas considerable amounts of cmo5U were lacking.
Full text
PDF



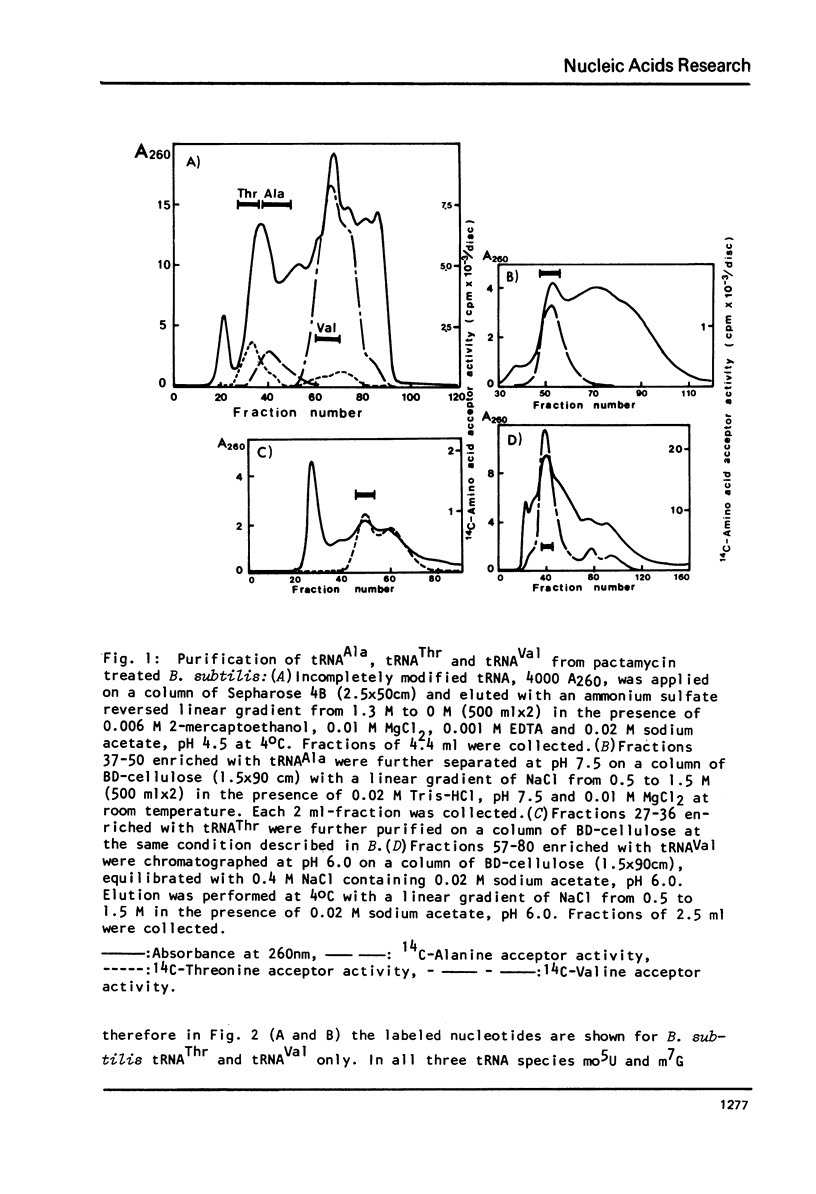
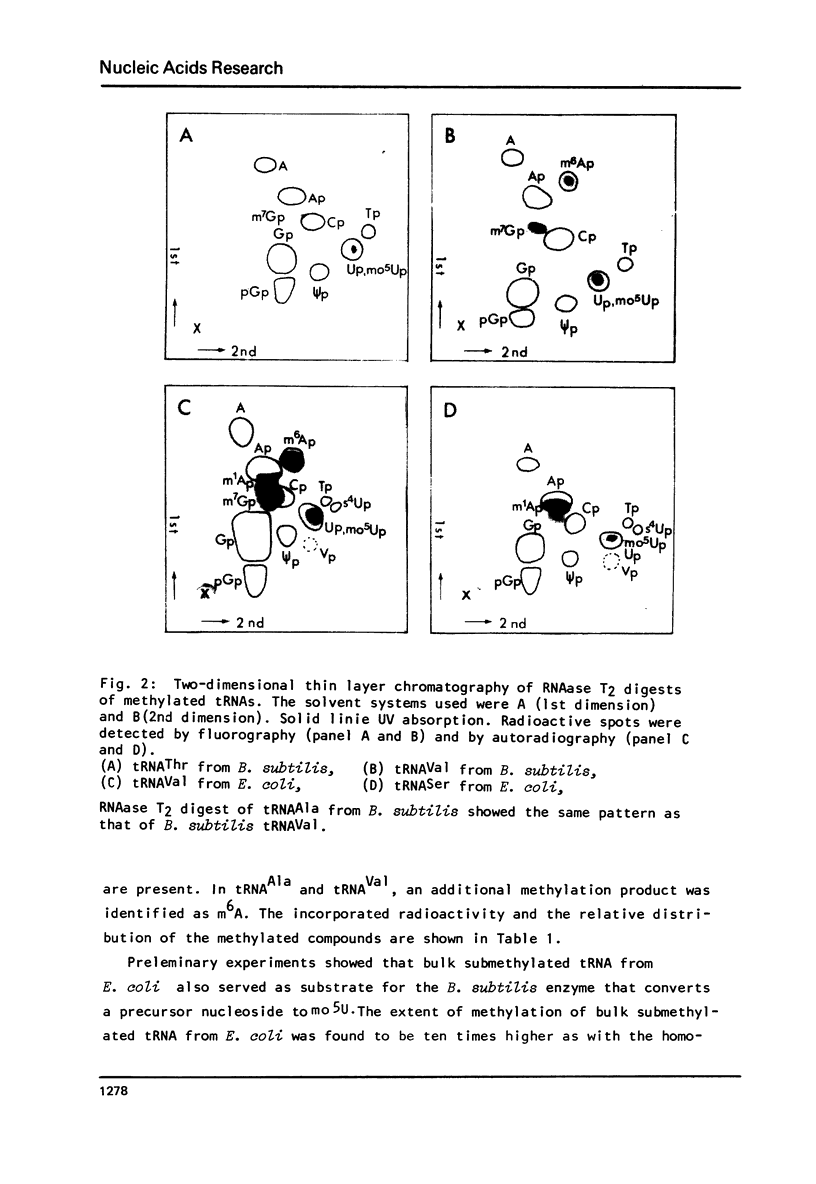
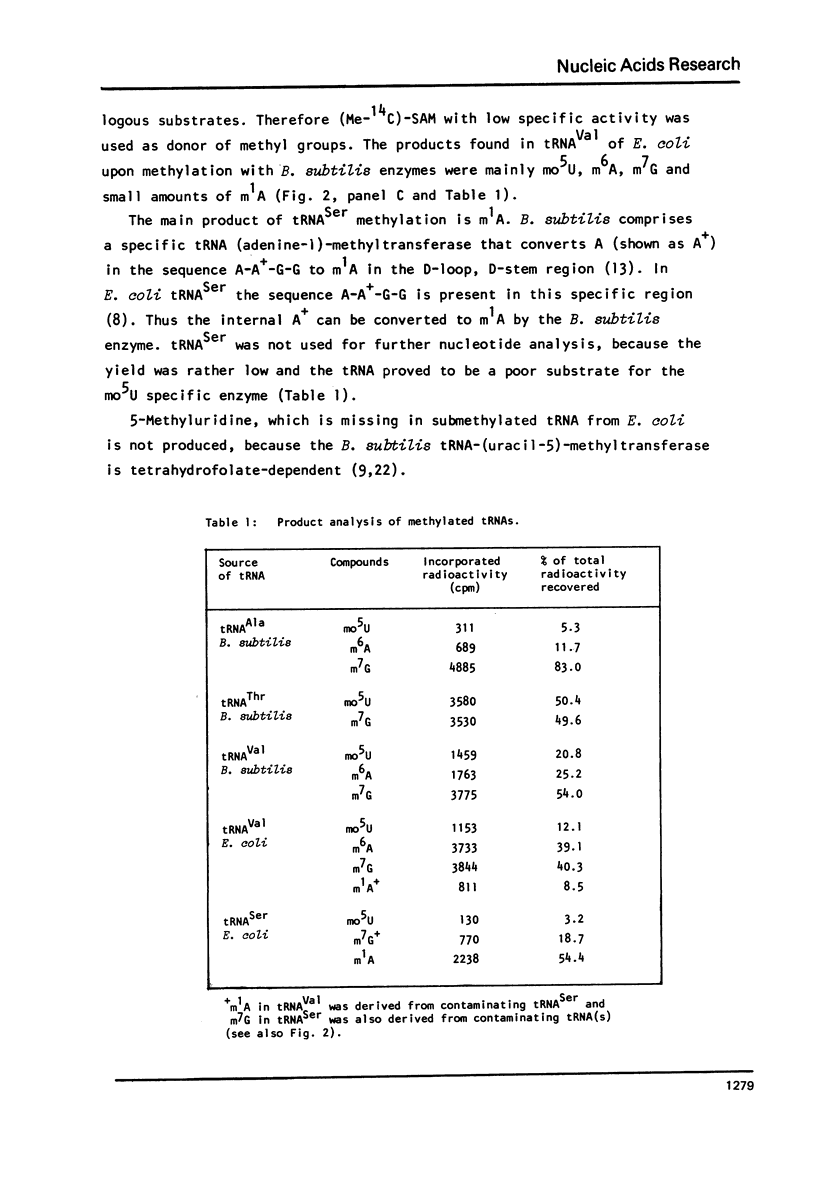
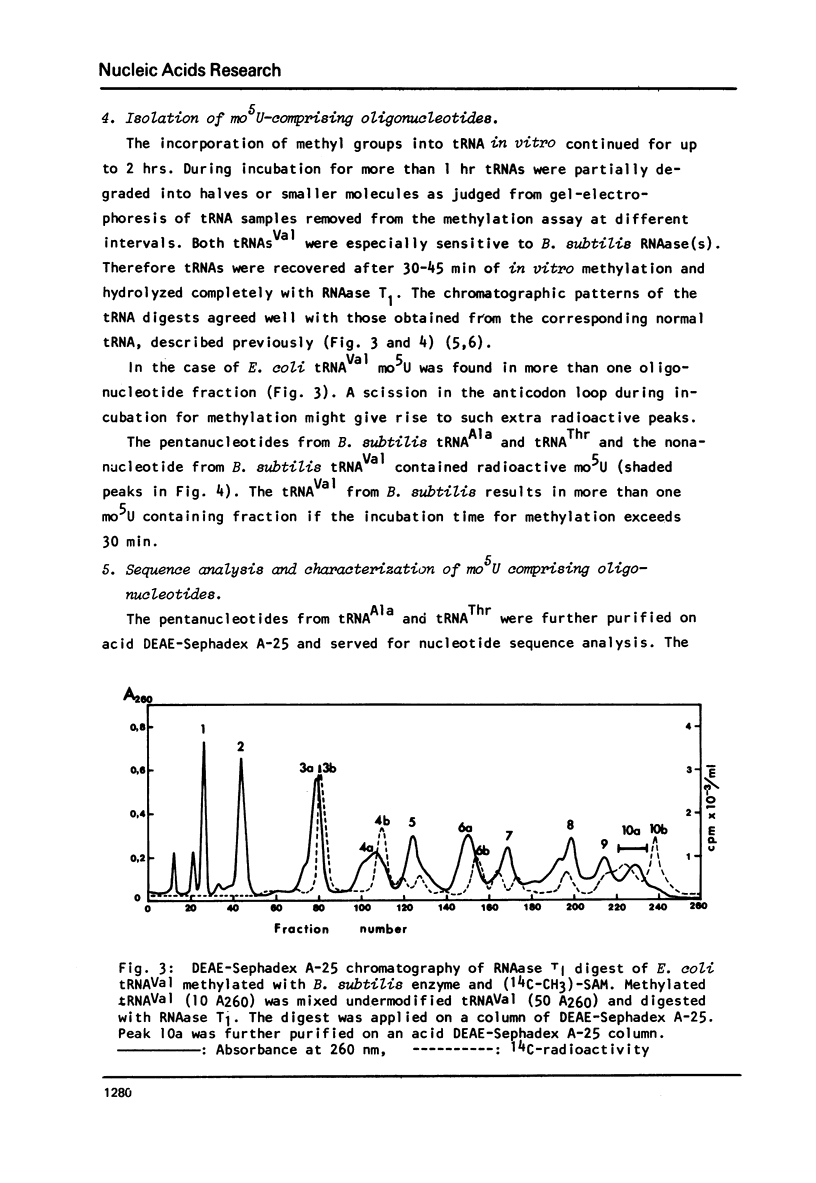
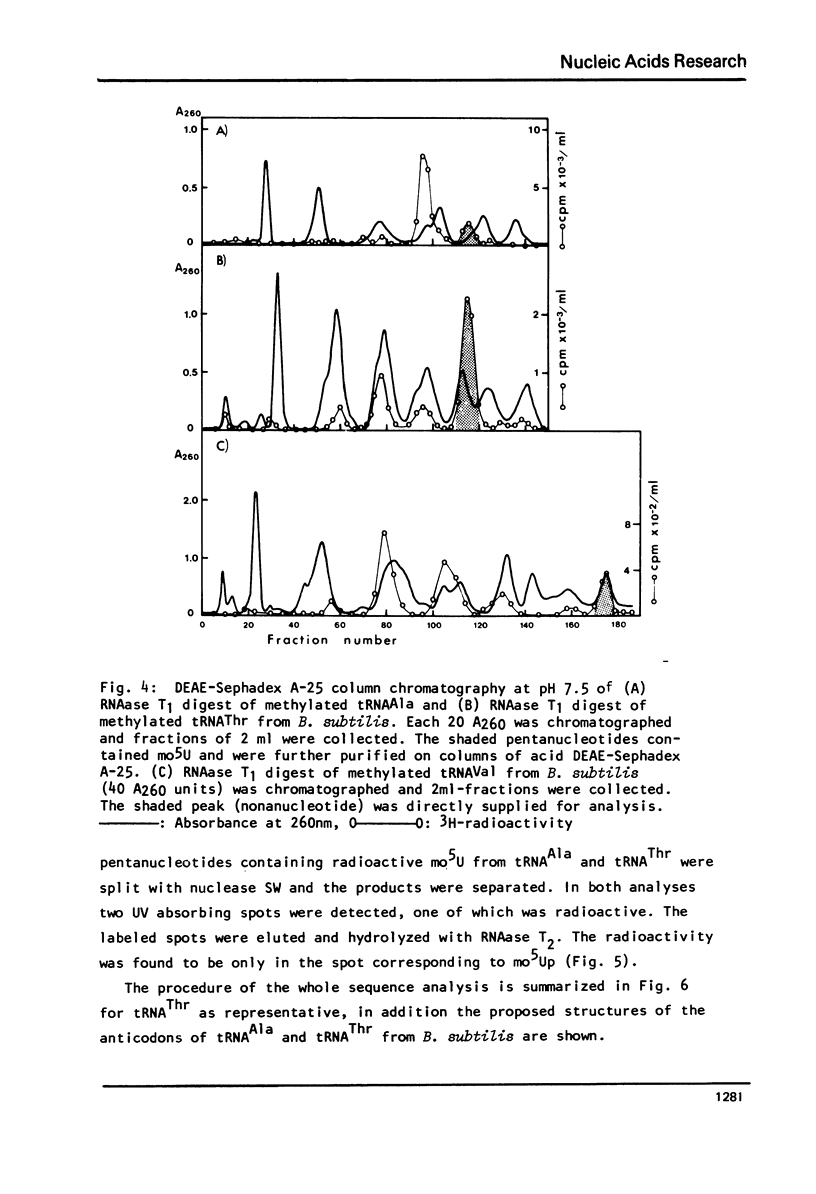
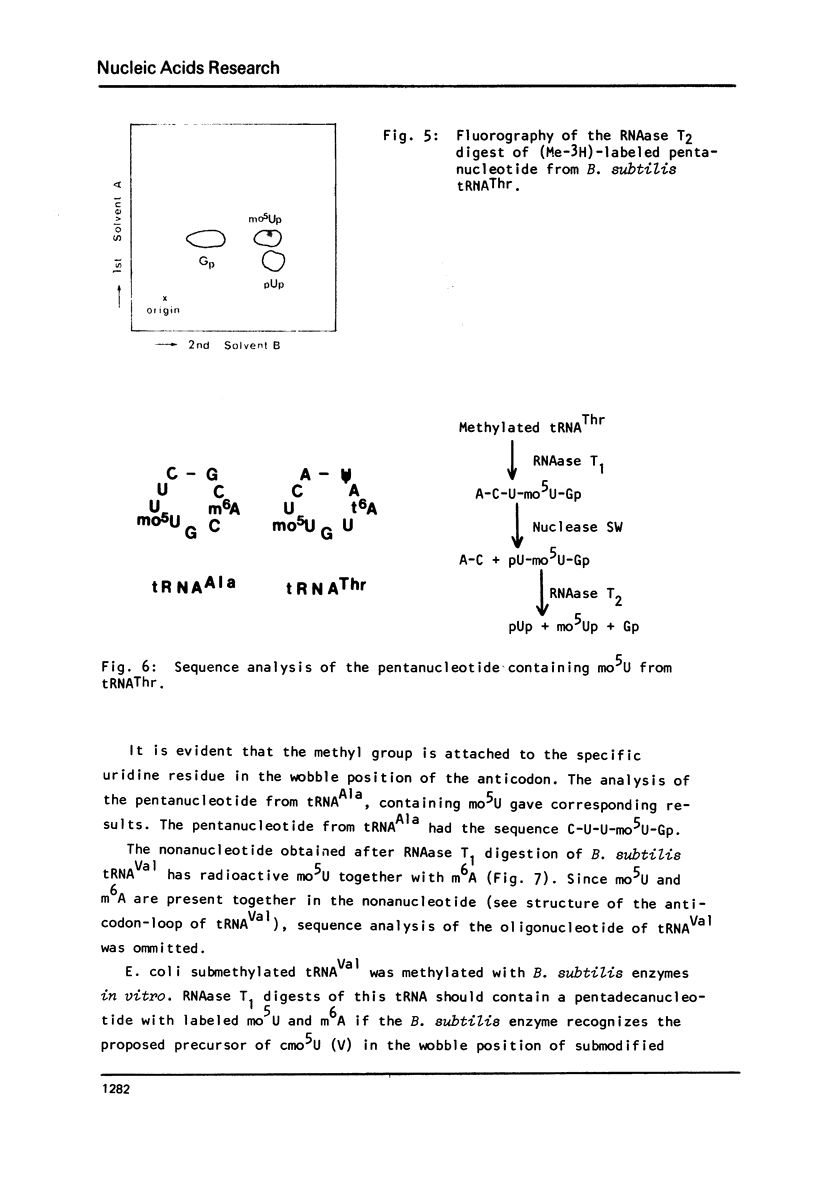
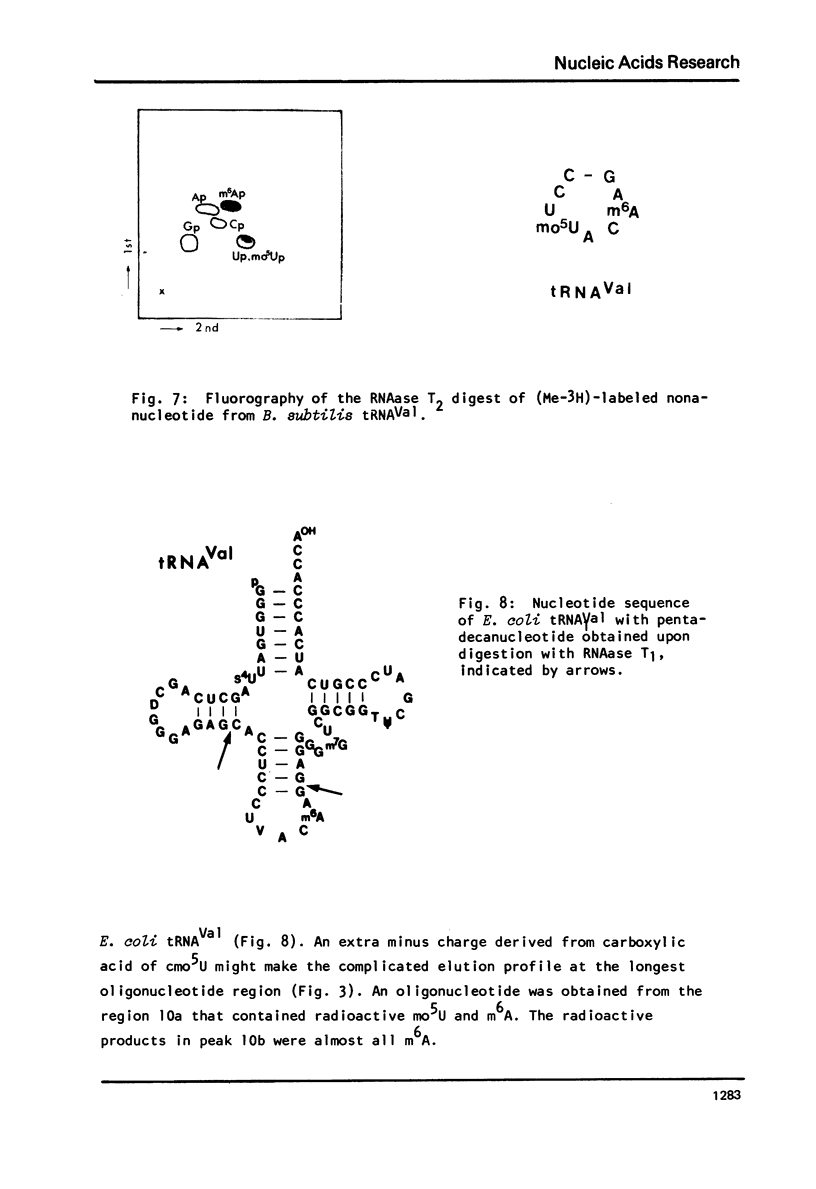
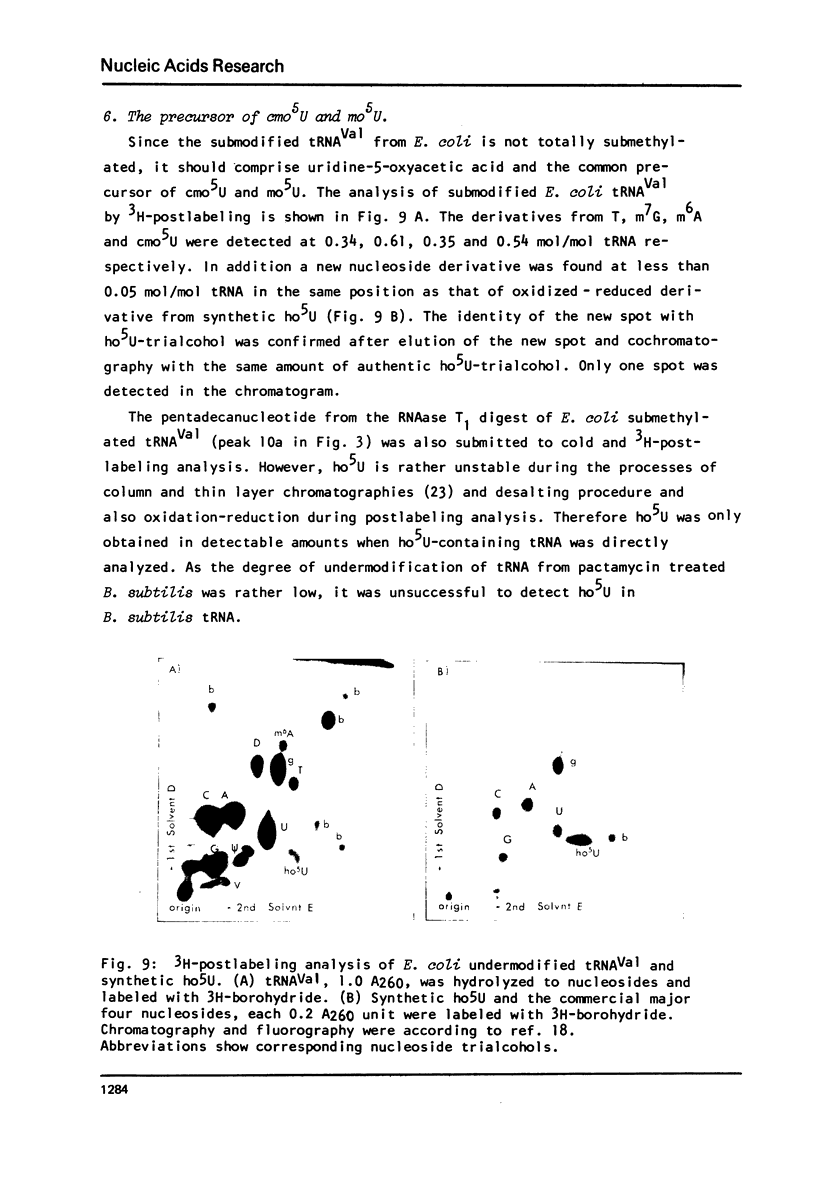



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albani M., Schmidt W., Kersten H., Geibel K., Lüderwald I. 5-Methoxyuridine, a new modified constituent in tRNAs of Bacillaceae. FEBS Lett. 1976 Nov;70(1):37–42. doi: 10.1016/0014-5793(76)80721-1. [DOI] [PubMed] [Google Scholar]
- Altman S. A modified uridine in the anticodon of E. coli tRNA I Tyr su + oc. Nucleic Acids Res. 1976 Feb;3(2):441–448. doi: 10.1093/nar/3.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold H. H., Schmidt W., Raettig R., Sandig L., Domdey H., Kersten H. S-Adenosylmethionine and tetrahydrofolate-dependent methylation of tRNA in Bacillus subtilis. Incomplete methylations caused by trimethoprim, pactamycin, or chloramphenicol. Arch Biochem Biophys. 1976 Sep;176(1):12–20. doi: 10.1016/0003-9861(76)90135-1. [DOI] [PubMed] [Google Scholar]
- Biezunski N., Giveon D., Littauer U. Z. Purification and properties of Escherichia coli methyl-deficient phenylalanine tRNA. Biochim Biophys Acta. 1970 Feb 18;199(2):382–393. doi: 10.1016/0005-2787(70)90081-x. [DOI] [PubMed] [Google Scholar]
- Harada F., Kimura F., Nishimura S. Primary sequence of tRNA val from Escherichia coli B. I. Oligonucleotide sequences of digests of Escherichia coli tRNA val with RNase T and pancreatic RNase. Biochemistry. 1971 Aug 17;10(17):3269–3277. doi: 10.1021/bi00793a017. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. The nucleotide sequence of a serine tRNA from Escherichia coli. FEBS Lett. 1971 Jul 15;16(1):68–70. doi: 10.1016/0014-5793(71)80688-9. [DOI] [PubMed] [Google Scholar]
- Kersten H., Chandra P., Tanck W., Wiedemhöver W., Kersten W. Effect of pactamycin on methylation of RNA and protein synthesis. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):659–663. doi: 10.1515/bchm2.1968.349.1.659. [DOI] [PubMed] [Google Scholar]
- Kimura F., Harada F., Nishimura S. Primary sequence of tRNA-Val-1 from Escherichia coli B. II. Isolation of large fragments by limited digestion with RNases, and overlapping of fragments to reduce the total primary sequence. Biochemistry. 1971 Aug 17;10(17):3277–3283. doi: 10.1021/bi00793a018. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. An evolutionary study of the methylation of transfer and ribosomal ribonucleic acid in prokaryote and eukaryote organisms. J Biol Chem. 1973 Apr 10;248(7):2612–2620. [PubMed] [Google Scholar]
- Lis A. W., Passarge W. E. Isolation of 5-hydroxyuridine (iso-barbituridine) from yeast ribonucleic acids. Arch Biochem Biophys. 1966 Jun;114(3):593–595. doi: 10.1016/0003-9861(66)90384-5. [DOI] [PubMed] [Google Scholar]
- Mukai J. I., Soeta E. I. Action of silkworm endonuclease on oligoribonucleotides terminating in 3'-phosphate. Biochim Biophys Acta. 1967 Mar 29;138(1):1–9. doi: 10.1016/0005-2787(67)90580-1. [DOI] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. 5-methoxyuridine: a new minor constituent located in the first position of the anticodon of tRNAAla, tRNAThr, and tRNAVal from Bacillus subtilis. Nucleic Acids Res. 1976 Oct;3(10):2851–2860. doi: 10.1093/nar/3.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K., Saneyoshi M., Harada F., Nishimura S. Uridin-5-oxy acetic acid: a new minor constituent from E. coli valine transfer RNA I. Biochem Biophys Res Commun. 1970 Feb 20;38(4):657–662. doi: 10.1016/0006-291x(70)90631-5. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Raettig R., Kersten H., Weissenbach J., Dirheimer G. Methylation of an adenosine in the D-loop of specific transfer RNAs from yeast by a procaryotic tRNA (adenine-1) methyltransferase. Nucleic Acids Res. 1977 Jun;4(6):1769–1782. doi: 10.1093/nar/4.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raettig R., Schmidt W., Mahal G., Kersten H., Arnold H. H. Purification and characterization of tRNAMet-f, tRNAPhe and tRNATyr2 from Baccillus subtilis. Biochim Biophys Acta. 1976 Jun 18;435(2):109–118. doi: 10.1016/0005-2787(76)90241-0. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Arnold H. H., Kersten H. Biosynthetic pathway of ribothymidine in B. subtilis and M. lysodeikticus involving different coenzymes for transfer RNA and ribosomal RNA. Nucleic Acids Res. 1975 Jul;2(7):1043–1051. doi: 10.1093/nar/2.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Comer M. M., McClain W. H. Nucleotide alterations in the bacteriophage T4 glutamine transfer RNA that affect ochre suppressor activity. J Mol Biol. 1974 Dec 25;90(4):677–689. doi: 10.1016/0022-2836(74)90532-4. [DOI] [PubMed] [Google Scholar]
- Singhal R. P., Vold B. Changes in transfer ribonucleic acids of Bacillus subtilis during different growth phases. Nucleic Acids Res. 1976 May;3(5):1249–1262. doi: 10.1093/nar/3.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]







