Abstract
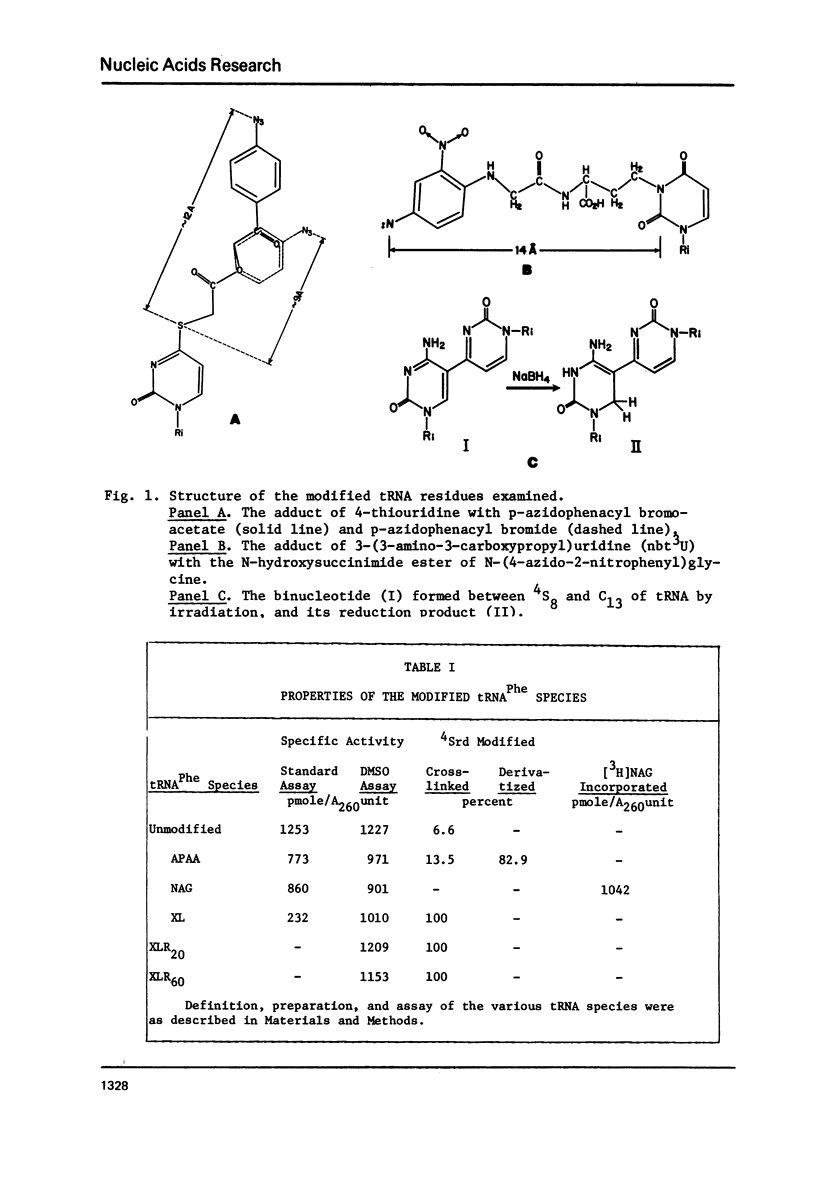
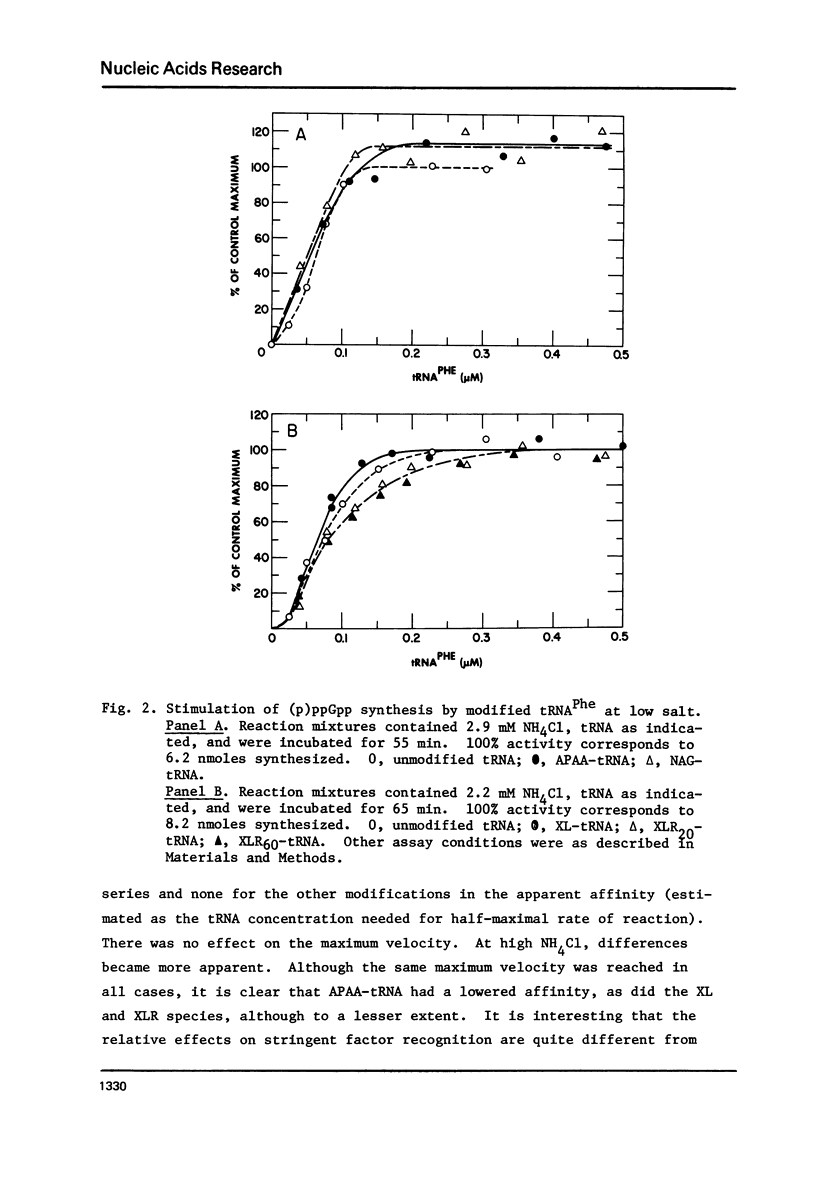
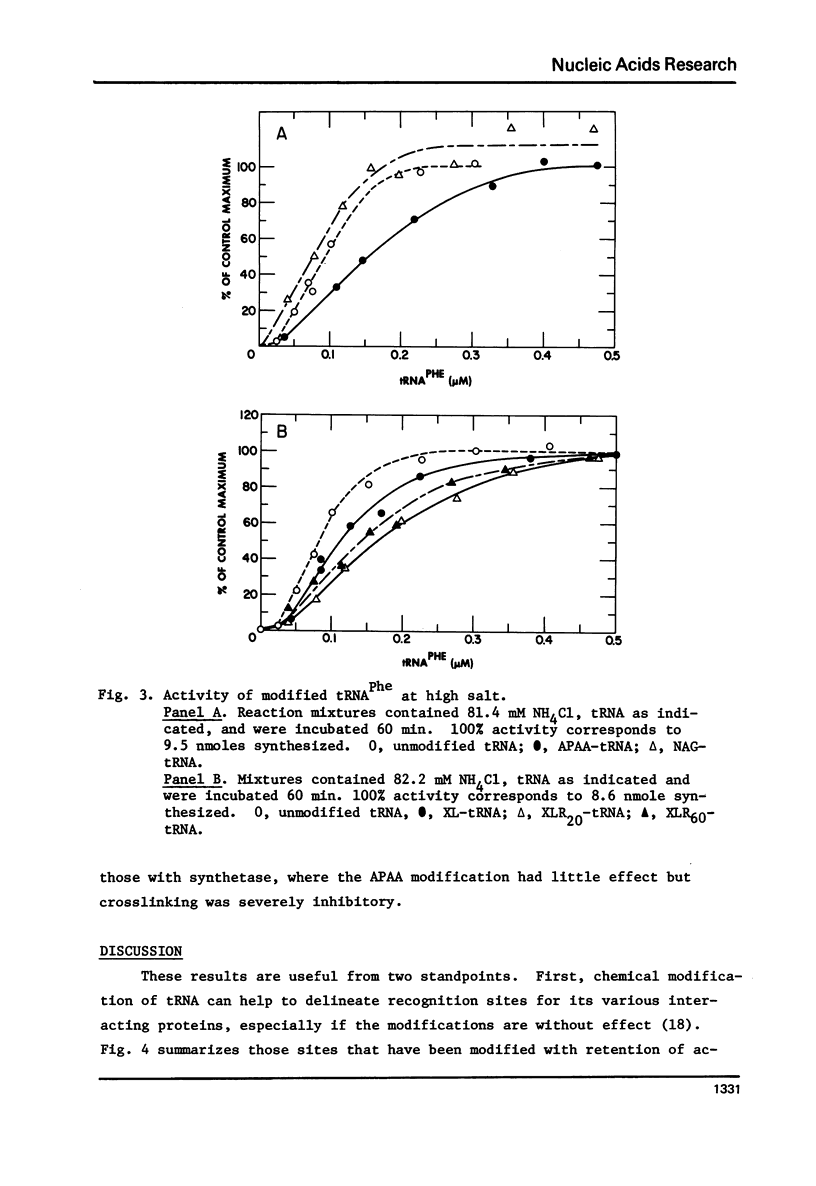
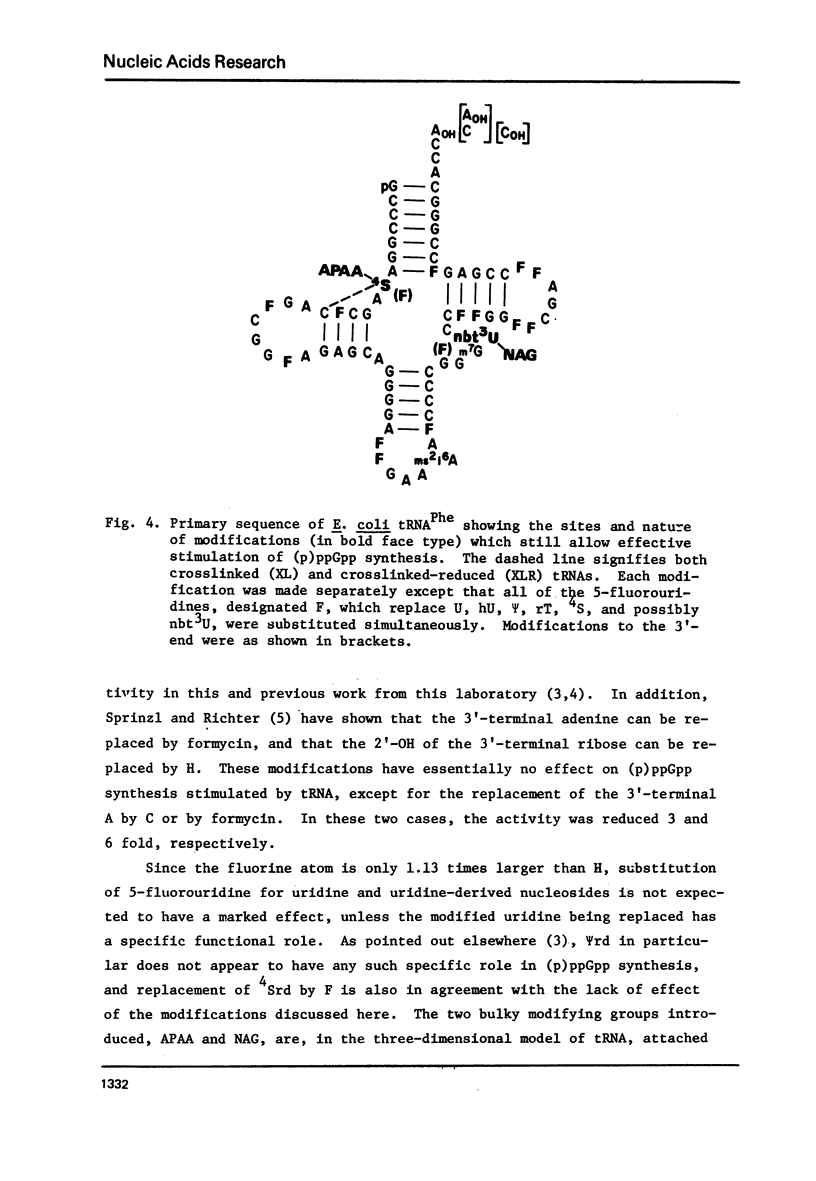
tRNAPhe of E. coli, modified at its 4-thiouridine (4Srd) and 3-(3-amino-3-carboxypropyl)uridine (nbt3Urd) residues, was tested for its ability to induce (p)ppGpp synthesis. The 4Srd residue was derivatized with the p-azido-phenacyl group, cross-linked to Cyd13, and the borohydride reduction product of the cross-link was prepared. The nbt3Urd residue was derivatized with the N-(4-azido-2-nitrophenyl)glycyl group. None of these derivatives had more than a minor effect on the affinity of the tRNA for the stringent factor-ribosome complex, and no effect at all on the maximum velocity of (p)ppGpp synthesis, either at 2 or 82 mM NH4Cl. These two regions of the tRNA which are on opposite faces of the tRNA molecule do not appear to be structurally important for recognition by the stringent factor-ribosome complex. They may provide useful sites, therefore, for the introduction of photoaffinity or fluorescent probes with which to study tRNA-stringent factor recognition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthelot F., Gros F., Favre A. Biological activity of cross-linked Escherichia coli tRNA f Met. Eur J Biochem. 1972 Sep 18;29(2):343–347. doi: 10.1111/j.1432-1033.1972.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Carré D. S., Thomas G., Favre A. Conformation and functioning of tRNAs: cross-linked tRNAs as substrate for tRNA nucleotidyl-transferase and aminoacyl synthetases. Biochimie. 1974;56(8):1089–1101. doi: 10.1016/s0300-9084(74)80097-0. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Friedman S. Acylation of transfer ribonucleic acid with the N-hydroxysuccinimide ester of phenoxyacetic acid. Biochemistry. 1972 Aug 29;11(18):3435–3443. doi: 10.1021/bi00768a017. [DOI] [PubMed] [Google Scholar]
- Kim S. H. Three-dimensional structure of transfer RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:181–216. doi: 10.1016/s0079-6603(08)60070-7. [DOI] [PubMed] [Google Scholar]
- Krauskopf M., Chen C. M., Ofengand J. Interaction of fragmented and cross-linked Escherichia coli valine transfer ribonucleic acid with T u factor-guanosine triphosphate complex. J Biol Chem. 1972 Feb 10;247(3):842–850. [PubMed] [Google Scholar]
- Kumar S. A., Krauskopf M., Ofengand J. Effect of intramolecular photochemical cross-linking and of alkylation of 4-thiouridine in E. coli tRNA1val. On the heterologous mischarging by yeast phenylalanyl-tRNA synthetase. J Biochem. 1973 Aug;74(2):341–353. [PubMed] [Google Scholar]
- Nauheimer U., Hedgcoth C. Activation of several tRNAs of Escherichia coli by the phenoxyacetyl derivative of N-hydroxysuccinimide. Arch Biochem Biophys. 1974 Feb;160(2):631–642. doi: 10.1016/0003-9861(74)90440-8. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Chládek S., Robilard G., Bierbaum J. Enzymatic acylation of oxidized-reduced transfer ribonucleic acid by Escherichia coli, yeast, and rat liver synthetases occurs almost exclusively at the 2'-hydroxyl. Biochemistry. 1974 Dec 17;13(26):5425–5432. doi: 10.1021/bi00723a029. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Delaney P., Bierbaum J. Photo-induced cross-linking of 4Srd and Cyd residues in Escherichia coli tRNA and its use as a conformational probe. Methods Enzymol. 1974;29:673–684. doi: 10.1016/0076-6879(74)29059-1. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Henes C. The function of pseudouridylic acid in transfer ribonucleic acid. II. Inhibition of amino acyl transfer ribonucleic acid-ribosome complex formation by ribothymidylyl-pseudouridylyl-cytidylyl-guanosine 3'-phosphate. J Biol Chem. 1969 Nov 25;244(22):6241–6253. [PubMed] [Google Scholar]
- Ofengand J., Schwartz I., Chinali G., Hixson S. S., Hixson S. H. Photoaffinity-probe-modified tRNA for the analysis of ribosomal binding sites. Methods Enzymol. 1977;46:683–702. doi: 10.1016/s0076-6879(77)46086-5. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Roe B., Michael M., Dudock B. Function of N2 methylguanine in phenylalanine transfer RNA. Nat New Biol. 1973 Dec 5;246(153):135–138. doi: 10.1038/newbio246135a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Schechter A. N. Covalent attachment of fluorescent probes to the X-base of Escherichia coli phenylalanine transfer ribonucleic acid. Nucleic Acids Res. 1977 Jul;4(7):2161–2167. doi: 10.1093/nar/4.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart L. Effect of selective chemical modification of 4-thiouridine of phenylalanine transfer ribonucleic acid on enzyme recognition. Arch Biochem Biophys. 1972 Feb;148(2):488–495. doi: 10.1016/0003-9861(72)90167-1. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Richter D. Free 3'-OH group of the terminal adenosine of the tRNA molecule is essential for the synthesis in vitro of guanosine tetraphosphate and pentaphosphate in a ribosomal system from Escherichia coli. Eur J Biochem. 1976 Dec;71(1):171–176. doi: 10.1111/j.1432-1033.1976.tb11103.x. [DOI] [PubMed] [Google Scholar]
- Thomas G., Favre A. 4-Thiouridine as the target for near-ultraviolet light induced growth delay in Escherichia coli. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1454–1461. doi: 10.1016/0006-291x(75)90522-7. [DOI] [PubMed] [Google Scholar]



