Abstract
Background
Glands with glassy cells (GGCs) were recently found in 1.8% of patients showing Barrett’s mucosa in esophageal biopsies. Similar GGCs were more recently detected in a baboon having glandulo-metaplastic esophageal mucosa (GMEM). The aim was to assess the frequency of baboons with GMEM having GGCs.
Materials and Methods
GGCs were searched for in 68 consecutive baboons having GMEM. Sections were stained with H&E and with alcian blue (pH 2.5), to detect sialomucins in goblet cells (a marker of Barrett’s mucosa in GMEM).
Results
Two out of the 68 baboons with Barrett’s mucosa (2.9%) showed GGCs.
Conclusions
In similarity to humans, the Barrett’s mucosa in baboons may show GGCs. Although the significance of GGCs in baboons (and in humans) remains poorly understood, their presence might not be a fortuitous event but linked to the molecular events leading to the development of intestinal metaplasia in Barrett’s mucosa, a known pre-neoplastic mucosal change in the distal esophagus in humans.
Keywords: Nonhuman primate, pathology, disease, intestinal metaplasia
In 1991 (1, 2) we detected a new phenotype of glands in the gastric mucosa in humans, composed of cells with a homogeneously pale, eosinophilic cytoplasm. Because of their ground-glass appearance, these cells received the trivial working name of glassy cells (GCs). Years later, similar glands with GCs (GGCs) were detected in the gastric mucosa of baboons (3). Further studies indicated that the frequency of baboons having GGCs in the gastric mucosa was 11% (4). Recently, metaplastic GGCs were found in 1.8% of patients showing Barrett’s mucosa in esophageal biopsies (5). Lately, the occurrence of GGCs was also noticed in a baboon having glandulo-metaplastic esophageal mucosa (GMEM). This observation prompted us to determine the frequency of GGCs in a cohort of baboons having GMEM.
Materials and Methods
The study group comprised baboons which were members of colonies at the Southwest National Primate Research Center, Southwest Foundation for Biomedical Research, Texas, USA. The conditions of animal housing have been reported elsewhere (3, 4). Briefly, the animals were housed in metal and concrete indoor-outdoor cages and were fed commercial monkey diets, occasionally supplemented with a variety of fruit and vegetables. Water was available ad libitum.
The distal esophagus together with the proximal stomach were removed en bloc at autopsy in 74 baboons (Papio spp.). The esophagus was opened wide, adhered to cardboard, cut into longitudinal sections and fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, cut at 5 μm, stained with hematoxylin and eosin (H&E) and evaluated under light microscopy (x20 objective). Sections with GGCs were also stained with alcian blue (pH 2.5) to evidence the presence of sialomucins.
All procedures were carried out in accordance with the Institutional Animal Care and Use Committee guidelines.
Definitions
GMEM
Three phenotypes of glands with columnar-lined mucosa may occur in esophageal metaplastic mucosa: cardiac glands, fundic glands (referred to in humans as esophageal glandular mucosa type 1 and 2, respectively) and intestinal metaplastic glands (referred to in humans as esophageal glandular mucosa type 3, or Barrett’s mucosa. There is a prevailing consensus to call Barrett’s esophagus those cases having glandular mucosa type 3, as glandular dysplasia or adenocarcinoma are associated with that mucosa phenotype and not with glandular mucosa types 1 or 2).
GGCs
Glands with cells having a homogeneously pale, eosinophilic cytoplasm with a ground-glass appearance. These cells may be found in one gland or in a group of glands in the GMEM. The glassy material differs morphologically from the mucus contained in goblet cells.
Results
Of the 74 gastroesophageal specimens, 6 were autolytic and were therefore rejected from the study. The remaining 68 gastroesophageal specimens had GMEM. GGCs were recorded in two out of the 68 specimens with GMEM (Figures 1 and 2). Both the columnar epithelium and the accessory glands had alcian blue-positive goblet cells (Figure 3). Thus, GGCs were found in 2.9% of the 68 gastroesophageal specimens having GMEM with intestinal metaplastic goblet cells, a setting defined as Barrett’s mucosa in humans (6)). No glassy material was found in the sialomucin-producing goblet cells.
Figure 1.
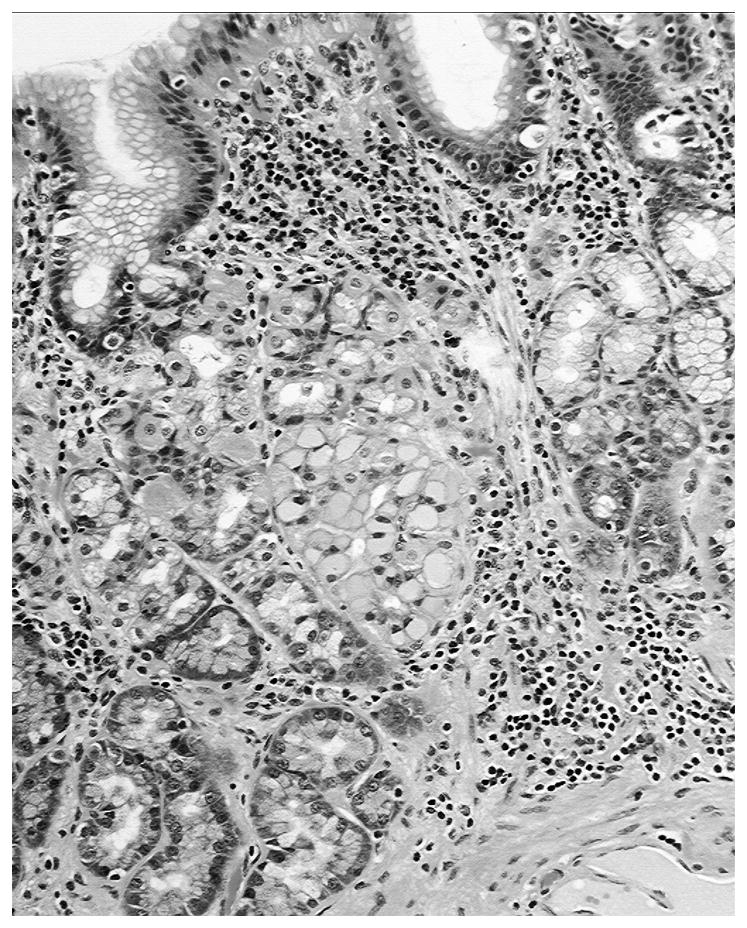
Glandulo-metaplastic esophageal mucosa in a baboon showing a gland with glassy cells (H&E, Barrett’s esophagus, x10)
Figure 2.
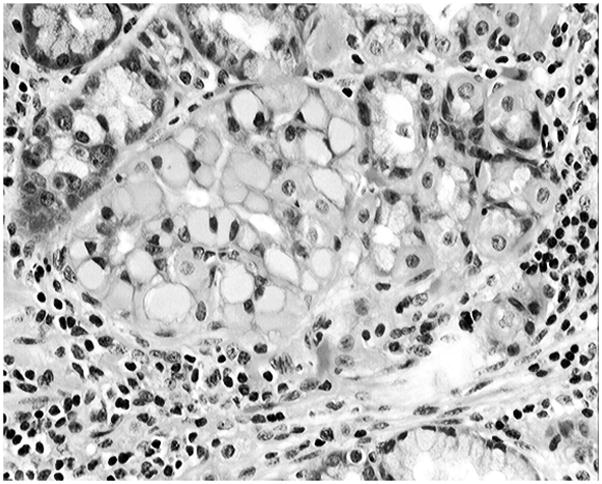
Detail from Figure 1, showing a gland composed of cells with glassy cytoplasm (H&E, Barrett’s esophagus, x40, baboon)
Figure 3.
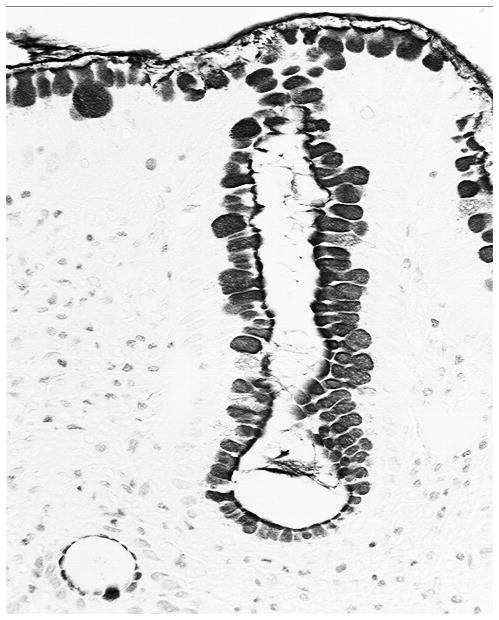
Detail, showing sialomucin-paositive goblet cell in the glandulo-metaplastic esophageal mucosa (H&E, Barrett’s esophagus, x40, baboon)
Discussion
The results demonstrated that about 3% of the esophageal specimens from baboons having Barrett’s mucosa also included GGCs. Glassy material was not found in the cytoplasm of goblet cells.
The mechanism(s) leading to the development of the glassy material in GGCs remain poorly understood. Previous histochemical and immunohistochemical studies in GGCs in human gastric mucosa (7–10) revealed that except for a faintly positive periodic acid -Schiff (PAS) reaction, the glassy material was negative for PAS-diastase (PAS-D) reaction, Alcian blue pH 2.5 (without counter stain), high iron diamine, small intestinal mucinous antigen (SIMA), oil red staining on wet sections, alkaline Congo red, chromogranin A, prussian blue, lysozyme, hepatitis B core antigen, cystatin C, orcein and hepatitis B surface antigen, suggesting that that the material is not lipoid, amyloid, sialomucin, sulphomucin, iron, lysozyme, or neuroendocrine. The negative reaction with cystatin C indicated that this material was not the same as the cystatin C-positive, strongly eosinophilic material found in the cytoplasm of human duodenal cells (11). Hence, despite the fact that GGCs can easily be recognized in H&E-stained sections, the histochemical and/or immunohistochemical nature of the glassy material remains unidentified. It is, however, apparent that the proteinaceous glassy material secreted by the Golgi organelle is, for unknown reason(s), retained in the cytoplasm of these cells. In this respect, Kopito and Sitia (12) claim that all cells are equipped with a protelytic apparatus that eliminates misfolded and damaged proteins. The 26S proteasome, the principal engine of cytoplasmic proteolysis, requires unfolded substrates but is ineffective at degrading aggregated proteins. When the production of aggregated proteins exceeds the cell capacity to eliminate them, a phenomenon of cellular indigestion of the endoplasmic reticulum (ER) occurs. The condensation of non-secreted product suggests that the mechanism of protein transport in the ER is incompetent and that the proteins are neither degraded nor secreted and, thus, remain stored in dilated cisternae (12).
Nevertheless, the cause(s) for the accumulation of intracellular glassy material in metaplastic glands and the ultimate fate of such cells remains at present elusive. As in humans (5, 7–10), the GGCs in baboons were surrounded by well-preserved connective tissue amidst other well preserved metaplastic esophageal glands, implying that GCs are not a structural artifact arising from poor fixation or maltreatment of the tissues before fixation, but are a genuine morphological phenomenon.
In conclusion, GGCs, a cell phenotype initially detected in the gastric mucosa in humans (1, 2, 10) and in non-human primates (3, 4), also identified in Barrett’s mucosa in humans (5), were also shown here to be present in Barrett’s mucosa in baboons. Although the significance of GGCs in baboons remains elusive, their presence might not be a fortuitous event, but may be related to the evolution of intestinal metaplasia, a suggestion previously given for human Barrett’s mucosa (5), a known pre-neoplastic mucosal change, as well as for the human gastric mucosa having both intestinal metaplasia and carcinoma (13).
References
- 1.Rubio CA, Hirota T, Itabashi M, Hirohashi S, Kato Y. A possible error in the interpretation of gastric carcinoma. Jpn J Cancer Res. 1991;82:1354–1355. doi: 10.1111/j.1349-7006.1991.tb01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio CA, Jessurun J, Alonso de Ruiz P. Geographic variations in the histologic characteristics of the gastric mucosa. Am J Clin Pathol. 1991;96:330–333. doi: 10.1093/ajcp/96.3.330. [DOI] [PubMed] [Google Scholar]
- 3.Rubio CA, Hubbard GB. A new phenotype of gastric pyloric cells. A study in baboons. In Vivo. 1998;12:543–546. [PubMed] [Google Scholar]
- 4.Rubio CA, Dick EJ, Jr, Miller ML, Hubbard GB. The frequency of gastric pyloric cells with glassy cytoplasm in baboons. A comparison with human subjects. In Vivo. 2008;22:9–12. [PubMed] [Google Scholar]
- 5.Rubio CA. Glands with glassy cells in Barrett’s esophagus. Anticancer Res. 2009 (in press) [PubMed] [Google Scholar]
- 6.Faller G, Berndt R, Borchard F, Ell C, Fuchs KH, Geddert H, Gossner L, Gunther T, Kirchner T, Koch HK, Langner C, Luttges J, May A, Muller S, Oberhuber G, Seitz G, Stolte M, Tannapfel A, Vieth M, Walch A, Ruschoff J Working Group for Gastroenterological Pathology, German Society for Pathology. Histopathological diagnosis of Barrett’s mucosa and associated neoplasias. Pathologe. 2003;24:9–14. doi: 10.1007/s00292-002-0600-y. [DOI] [PubMed] [Google Scholar]
- 7.Rubio CA. Five types of pyloric cells in the antral mucosa of the stomach. Pathol Res Pract. 1992;188:157–161. doi: 10.1016/S0344-0338(11)81173-8. [DOI] [PubMed] [Google Scholar]
- 8.Rubio CA, Stemmermann G. Geographic variations in the phenotype of ciliated gastric cells. J Environ Pathol Toxicol Oncol. 1993;12:93–99. [PubMed] [Google Scholar]
- 9.Rubio CA, Jass JR, King A. Gastric cell phenotypes and intestinal metaplasia in Polynesian and non-Polynesian residents of New Zealand. J Environ Pathol Toxicol Oncol. 1994;13:243–249. [PubMed] [Google Scholar]
- 10.Rubio CA, Mandai K, Jonasson JG. Gastric glassy cells: a study of 3202 gastrectomy specimens from dwellers of the Atlantic and Pacific basins. J Environ Pathol Toxicol Oncol. 2005;24:281–289. doi: 10.1615/jenvironpatholtoxicoloncol.v24.i4.50. [DOI] [PubMed] [Google Scholar]
- 11.Rubio CA, Hirota T, Itabashi M, Jacobsson B, Lignelid H. Eosinophilic bodies in pyloric and Brunner’s gland cells. J Clin Pathol. 1992;45:1119–1120. doi: 10.1136/jcp.45.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopito RR, Sitia R. Aggresomes and Russell bodies. Symptoms of cellular indigestion? EMBO Rep. 2000;1:225–231. doi: 10.1093/embo-reports/kvd052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio CA, Jonasson JG, Nesi G. Extensive intestinal metaplasia in gastric carcinoma and in other lesions requiring surgery: a study of 3421 gastrectomy specimens from dwellers of the Atlantic and Pacific basins. J Clin Pathol. 2005;58:1271–1277. doi: 10.1136/jcp.2005.029587. [DOI] [PMC free article] [PubMed] [Google Scholar]


