Abstract
12-lipoxygenase, an arachidonic acid metabolizing enzyme of the lipoxygenase pathway, has been implicated as a major factor in promoting prostate cancer progression and metastasis. The ability of 12-LOX to aggravate the disease was linked to its proangiogenic role. Recent studies clearly demonstrated that 12-LOX enhances the expression and secretion of the angiogenic factor, vascular endothelial growth factor (VEGF) thus providing a direct link between this enzyme and its angiogenic properties. In the present study we have investigated the relationship between 12-LOX and hypoxia inducible factor-1α (HIF-1α), a transcription factor involved in the regulation of VEGF expression under hypoxic conditions in solid tumors. Our findings have revealed that HIF-1 is one of the target transcription factors regulated by 12-LOX and 12(S)-HETE, in hypoxic tumor cells of the prostate. Regulation of HIF-1α by 12-LOX adds to the complexity of pathways mediated by this enzyme in promoting prostate cancer angiogenesis and metastasis. We have evidence that 12-LOX increases the protein level, mRNA, and functional activity of HIF-1α under hypoxic conditions, one of the mechanisms by which it upregulates VEGF secretion and activity.
Keywords: 12-Lipoxygenase, Hypoxia Inducible Factor-1α (HIF-1α), angiogenesis, prostate cancer, hypoxia
Introduction
The presence of hypoxia in solid tumors arises from a defective blood supply, deteriorating diffusion geometry and generalized conditions like anemia, which is commonly seen in cancer patients [1, 2]. The most important pathway that is triggered in cells subjected to hypoxia is the Hypoxia Inducible Factor - 1 (HIF-1) pathway, orchestrated by the heterodimeric transcription factor, HIF-1α that acts on downstream pathways important in stimulating angiogenesis [1, 3–5]. The PI3 kinase–Akt pathway and the MAPK pathway play major roles in the induction of HIF-1α protein in hypoxic cells [6]. The expression level of HIF-1α is very high in high-grade prostatic intraepithelial neoplasia. The upregulation of HIF-1 can be a very important molecular event in early stages of prostate carcinogenesis, and HIF-1α may be a potential target for prostate cancer prevention [7].
We had previously reported the proangiogenic role played by the enzyme 12-lipoxygenase (12-LOX) and its arachidonic acid metabolite, 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) in prostate cancer [8, 9]. The expression of 12-LOX mRNA is highly correlated with the stage and grade of prostate cancer [10]. In comparison to 5 and 15(S)-HETE, 12(S)-HETE levels in urine samples of prostate cancer patients were found to be significantly higher and showed a drastic decrease upon radical prostatectomy suggesting the role played by this eicosanoid in promoting prostate cancer progression [11]. Studies of tumor xenografts in mice, demonstrated that prostate tumors generated from 12-LOX overexpressing prostate cancer PC-3 cells were of a highly angiogenic phenotype [9]. This phenomenon was later attributed to the enhanced secretion of VEGF induced by 12-LOX in prostate cancer cells [8, 12].
In the present study, we investigated the relationship between 12-LOX and the activation of HIF-1α in prostate cancer cells. The study stemmed from our previous observations of a stimulatory effect of 12-LOX on VEGF and the previous reports of HIF-1 serving as a major transcription factor for VEGF under hypoxic conditions (9). We hypothesized that 12-LOX may signal the transcriptional activation of VEGF in hypoxic prostate cancer cells via upregulation of HIF-1α. Experimental results from this investigation showed that 12-LOX enhances the protein level, transcription, DNA binding and transcriptional activity of HIF-1α in prostate cancer cells under hypoxic conditions.
Materials and methods
Cell Lines, Stable Transfections, and Hypoxic Incubations
PC-3 cells were purchased from ATCC and routinely tested for mycoplasma contamination using the mycoplasma detection ELISA kit (Roche) according to the manufacturer s instructions. Culture and maintenance of PC-3 prostate cancer cells were described previously. Generation and metabolic characterization of 12-LOX overexpressing PC-3 cells, namely nL-8, and the vector control cells (neo) were also described earlier (18). Hypoxic incubations were performed in a CO2 incubator, whose gaseous environment was monitored and controlled by an oxygen sensor system (Pro-Ox, Biospherix) that injects nitrogen into the incubator instead of air. For hypoxia experiments, cells were incubated under 1% oxygen, by injecting 5% CO2 and 94% N2. The culture medium used for incubations was pretreated with a gaseous mixture composed of 95% nitrogen and 5% carbon dioxide bubbled continuously, with constant stirring, to remove as much dissolved oxygen as possible and filter sterilized before use.
Protein Isolation and Immunoblotting
For protein isolation, cells in culture flasks after incubation under hypoxic conditions, were transferred immediately to a hypoxia work hood flushed with nitrogen to maintain an anaerobic environment. Cells were briefly rinsed with hypoxic PBS and proteins were isolated using the mammalian protein extraction reagent (M-PER, Pierce). Total protein concentration was assessed by BCA protein assay. Immunoblotting was performed essentially as described previously [8]. Protein concentration of 30 μg was used for immunoblotting for each experiment. Antibodies against PTEN, Akt, p-Akt (Cell Signaling Inc.), GLUT-1 (Chemicon), HIF-1α (BD Biosciences) and 12-LOX (Oxford Biomedicals), were used to probe the membranes.
RNA Isolation, Northern Blotting, and Real Time PCR
RNA isolation and real time PCR (RT-PCR) were performed essentially as described previously (18). For Northern blotting, adding formalin based loading dye denatured the total RNA isolated. The mixture was then heated to 70°C. After denaturation the RNA was subjected to electrophoretic separation on a 1% agarose gel and transferred to Duralon membranes for 12 h at room temperature. RNA on the membrane was cross-linked and the membrane was prehybridized in QuickHyb solution (Stratagene). HIF-1α probe was radiolabeled using the 5 End-Labeling Kit from Ambion according to manufacturer s instructions, and allowed to hybridize with the membrane for 6 h. The membrane was rinsed successively 3x with a low stringency buffer followed by a high stringency buffer. Washing was stopped when the background readings estimated by a Geiger counter were found to be minimal. The target band was imaged by autoradiography.
Plasmids and Transient Transfections
The following plasmids were used in the study: a) PTEN expression and control vectors were purchased from Origene Technologies; b) The 5xHRE vector containing five HRE elements fused upstream of the luciferase gene in a PGL3 vector (Promega) was a kind gift from Dr. Amato Giaccia of Stanford University (19); c) The VEGF promoter vector used in this study was described previously [8] and reference 19 from Milanini et al. therein. The wild type VEGF promoter vector included +54 to −1176 of the VEGF promoter region. In addition, a VEGF promoter deletion construct (p888) with a targeted deletion of the region encompassing the HRE (between −1176 and −888) was from Dr. Pages of Nice University, France.
For transient transfection experiments, cells were plated in 6 well dishes at an initial density of 1.5 × 105 per well. The reporter vectors containing luciferase gene (5xHRE and VEGF promoter vector constructs) were cotransfected with the lacZ vector for normalization. Transient transfections were achieved using the TransIT prostate transfection kit (Mirus Bio). 48 h post transfection, cells were incubated for 6 h under hypoxia. Lysates were prepared from harvested cells using the reporter lysis buffer (Promega). Luciferase activity of the lysates was assayed using a scintillation counter and was normalized to beta-galactosidase values estimated using a beta galactosidase assay system (Invitrogen). Similarly, the PTEN expression and control vectors were also transfected transiently into nL-8 and neo cells. The transfection efficiency and the impact on HIF-1α expression were analyzed by immunoblotting.
Reagents, Inhibitors, and Cell Treatment
The PI3 kinase inhibitor LY294002 and the mTOR inhibitor rapamycin were purchased from Cell Signaling Inc. The lipoxygenase inhibitor 5,8,11,14-eicosatetraynoic acid (ETYA) and the eicosanoid, 12(S)-HETE were from Cayman Chemicals. For treatment with these compounds, 7.5 × 105 nL-8 and neo cells were plated in 10 cm dishes. The inhibitors were dissolved in DMSO and added to the cells in serum free hypoxic medium for the indicated period of time. 12(S)-HETE used for the experiment was taken to dryness by evaporating the vehicle using gaseous N2. It was resuspended in culture medium and used at indicated concentrations to treat cells. Following addition of the compounds, the cells were incubated under hypoxic conditions for the time period indicated.
Nuclear protein isolation, Gel Shift Assay, and Transcription Factor ELISA
Nuclear protein was isolated using the nuclear extract kit as per the manufacturer s instructions (Active Motif Inc.). The isolated nuclear extract was tested for HIF-1α protein level by immunoblotting and the extent of DNA binding by transcription factor ELISA and gel shift assay. Gel shift assay was performed using the gel shift assay kit based on the manufacturer’s instructions (Promega). Synthetic oligonucleotides constructed from VEGF promoter sequences encompassing the HRE region (between −975 and −968) were used to test the DNA binding activity of HIF-1α in nuclear extracts prepared from neo and nL-8 cells incubated under hypoxic conditions. Transcription factor ELISA (Active Motifs Inc.) was also performed to confirm DNA binding activity of HIF-1α in nL-8 and neo cells under hypoxic conditions. Following incubation under hypoxic conditions, nuclear extracts were prepared and incubated in 96-well plates coated with immobilized oligonucleotide. Competition experiments were run in parallel and involved co-incubation with a soluble wild type competing oligo, which would prevent the binding of HIF-1α to the immobilized target oligonucleotide.
Immunofluorescence
Parental PC3, neo and nL-8 cells and were seeded on glass coverslips in 24 well plates at an equal density of 7 × 104 cells per well in complete RPMI medium (10 % fetal calf serum, 1 X Anti-Anti antibiotic from Gibco) 24 h prior to hypoxic treatment (described above). Cells were washed twice with and then covered with 1 ml of ‘hypoxic’, serum-free RPMI and immediately returned to the hypoxic conditioned incubator for 6 h. Following incubation cells were washed twice with hypoxic conditioned PBS, followed by fixation in 3.7 % formaldehyde in PBS for 5 min at 37°C. Cells were subsequently permeabilized and blocked with antibody dilution buffer (ABD: 0.1 % Triton X-100 in Tris buffered saline with 2 % bovine serum albumin) for 10 min. Cells were stained for 1 h at room temperature in a humidifying chamber with mouse HIF-1α antibody (BD Transduction Labs) diluted 1:100 in ABD. Coverslips were washed three times with 0.1% Tx-100 in TBS followed by incubation for 30 min in a 1:500 dilution in ABD of Alexa-488 conjugated secondary antibody (Molecular Probes-Invitrogen). After three washes (as above), cell nuclei were stained for an additional 30 min in 1 μg/ml DAPI (Molecular Probes) to aid in localizing cells during microscopy. After the final three washes, cells were permanently mounted in MOWIOL (Calbiochem) containing an antifade agent and imaged on the Zeiss Axioplan2 with the ApoTome module. Images were captured with the AxioCam MRm near infrared CCD camera (Dynamic range > 1:2200, 12 bit; CCD sensor 6.45 micron square pixels) and data were processed running Windows XP Pro, SP2. Magnification was at 63X for all samples.
siRNA Knockdown of HIF-1α and measurement of VEGF
siRNA for HIF-1α, scrambled siRNA sequence, and the siRNA transfection reagent Dharmafect 2 were obtained from Dharmacon Inc. For transfection, nL-8 and neo cells were plated at 1 × 105 cells per well of a 6-well plate. After 24 h, cells were transfected either with the HIF-1α specific siRNA or the control siRNA according to the manufacturer s protocol. Post transfection, cells were incubated in hypoxic serum free medium under hypoxic conditions for 6 h. HIF-1α protein level was measured by immunoblotting. The impact of siRNA knockdown on VEGF secretion was studied by ELISA (R & D Systems) on spent medium collected at the end of the 6 h hypoxia incubation.
Statistical analysis
The experiments described were performed at least in triplicates, unless mentioned otherwise. The results are represented as mean ± standard deviation (SD), and the significance was assessed by estimating p-value using student s T-test.
Results
HIF-1α protein level is upregulated by 12-LOX under hypoxia
Previously we had reported that 12-LOX overexpressing PC-3 cells generated highly angiogenic tumors in nude mice [9], and subsequent investigations demonstrated that 12-LOX and 12-HETE enhance VEGF expression and secretion in prostate cancer cells [8]. In the current study, we determined the role played by 12-LOX in regulating HIF- the transcription factor involved in the modulation of VEGF expression, chiefly under hypoxic conditions. A dramatic increase in the level of HIF-1α protein was seen in the nL-8 cells compared to the neo-α cells after 6 h of hypoxic incubation (Fig 1A). Conversely, it was also found that inhibition of 12-LOX activity using a lipoxygenase inhibitor ETYA (50 μM) for 6 h resulted in a marked reduction in the level of HIF-1α protein (Fig 1B). Adding back 12(S)-HETE to the cells together with ETYA reversed the effects of inhibition and restored the levels of HIF-1α (Fig 1B), indicating that 12-LOX enzymatic activity is required for increasing the HIF-1α protein level under hypoxic conditions. Inhibition of 12-LOX activity by specific 12-LOX inhibitors baicalein and BMD 122 (BHPP; N-benzyl-N-hydroxy-5-phenylpentamide) [13] also resulted in a decrease in HIF-1α protein level in the nL-8 cells (data not shown). Wild-type PC-3 cells incubated with 12(S)-HETE at concentrations that stimulate VEGF production [8] resulted in an increase in the level of HIF-1α under hypoxic conditions suggesting that 12(S)-HETE can positively influence HIF-1α protein level during hypoxia (Fig 1C).
Figure 1. 12-LOX increases HIF-1α protein level in prostate cancer cells under hypoxia.
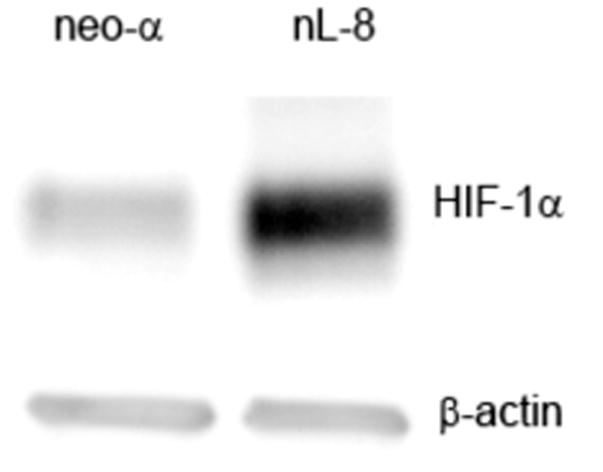
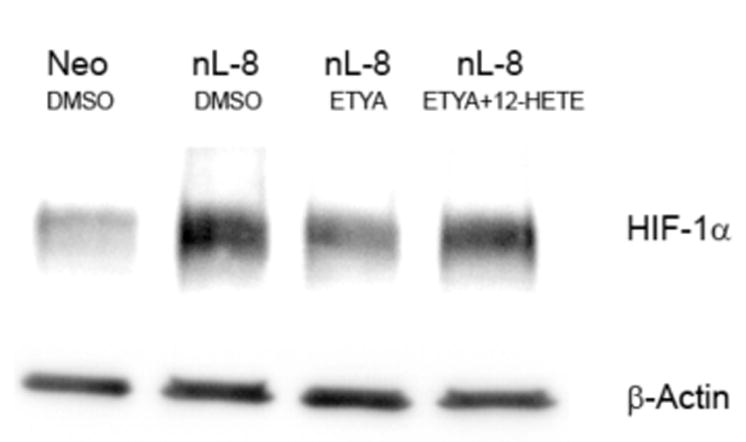
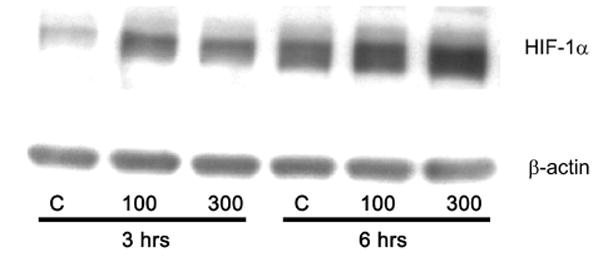
A. Total protein harvested from the nL-8 and neo cells (1 × 106 cells each) following 6 h incubation under hypoxia, was subjected to immunoblotting (30 μg per sample) and the membrane was probed with anti-HIF-1α antibody. B. nL-8 cells (1 × 106) were treated with the lipoxygenase inhibitor ETYA (50 μM) or vehicle. Also, an addback experiment was performed in which ETYA treated nL-8 cells (1 × 106) were simultaneously given exogenous 12(S)-HETE (300 nM). Cell lysates isolated after the incubation were tested for the levels of HIF-1α by immunoblotting (30 μg total protein per sample). C. Wild-type PC-3 cells (1 × 106 cells each) were treated with 12(S)-HETE (100 and 300 nM) or vehicle, and incubated under hypoxic conditions for 3 and 6 h. The level of HIF-1α in the treated cells was determined by immunoblotting (30 μg total protein per sample). Each result in this figure is representative of three independent observations performed under essentially identical conditions.
12-LOX enhances the level of HIF-1α transcript under hypoxic conditions
Real time PCR analyses showed an increase in HIF-1α message in the 12-LOX overexpressing nL-8 cells compared to neo cells under hypoxic conditions (Table 1). Northern blotting performed using the total RNA samples harvested from neo and nL-8 cells grown under hypoxic conditions showed a definite increase in the level of HIF-1α transcript in nL-8 cells confirming the real time PCR results (Fig 2). These observations clearly demonstrated that 12-LOX regulates HIF-1α transcription.
Table 1. Real-time PCR analysis of HIF-1α transcript levels in nL-8 and neo cells cultured under hypoxic conditions.
The relative abundance of the message was calculated using the ΔCT method as explained previously [8]. Shown here is representative data from three independent experiments performed under identical conditions.
| Cell type | ΔCT | ΔΔCT | T test | nL-8:neo |
|---|---|---|---|---|
| nL-8 | 5.84 | −1.48 | P = 0.013* | 2.79:1 |
| neo-α | 7.32 |
Figure 2. 12-LOX upregulates HIF-1α mRNA in prostate cancer cells under hypoxia.
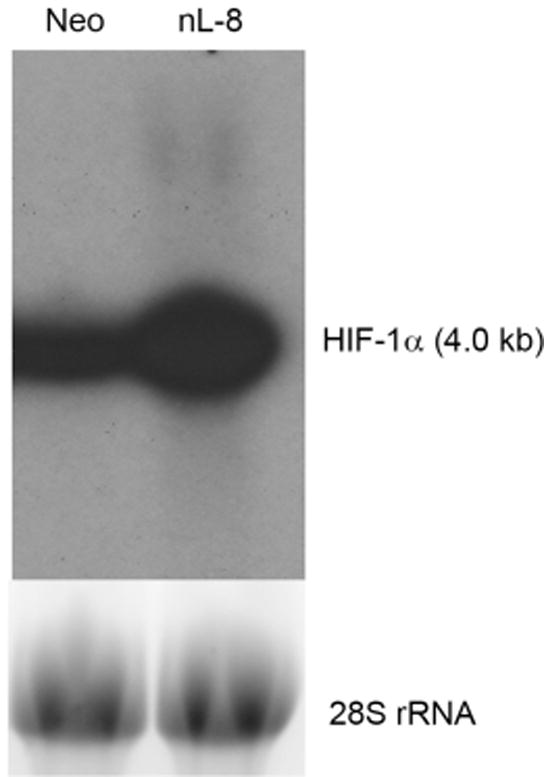
Northern blotting was performed to measure the abundance of HIF-1α message in nL-8 and neo cells as outlined in “materials and methods”, using total mRNA isolated from nL-8 and neo cells incubated under hypoxic conditions for 6 h. Shown here is a representative result from two independent experiments.
12-LOX regulates HIF-1α via the PI3K-Akt pathway
Previously we had reported that 12-LOX regulates the expression and secretion of VEGF in a PI3K-Akt dependent manner and that inhibition of the MAPK pathway had no influence on VEGF expression levels [8]. In the present study, immunoblotting analysis in nL-8 cells incubated with the PI3K inhibitor, LY294002 decreased the level of HIF-1α protein (Fig 3A). Affirmative to our previous reports, MAPK inhibition did not have any effect on HIF-1α levels in nL-8 cells compared to untreated controls (data not shown). One of the downstream targets of the PI3K-Akt pathway, mTOR, has been demonstrated to have a regulatory effect on HIF-1α protein level in PC-3 cells [14]. Immunoblotting on cell lysates prepared from nL-8 cells incubated with the mTOR inhibitor rapamycin, showed a decrease in HIF-1α protein level compared to vehicle treated cells (Fig 3A).
Figure 3. 12-LOX regulates HIF-1α via the PI3K-Akt-mTOR pathway under hypoxia.
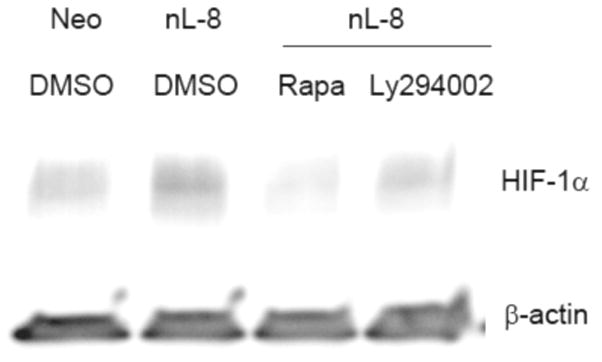
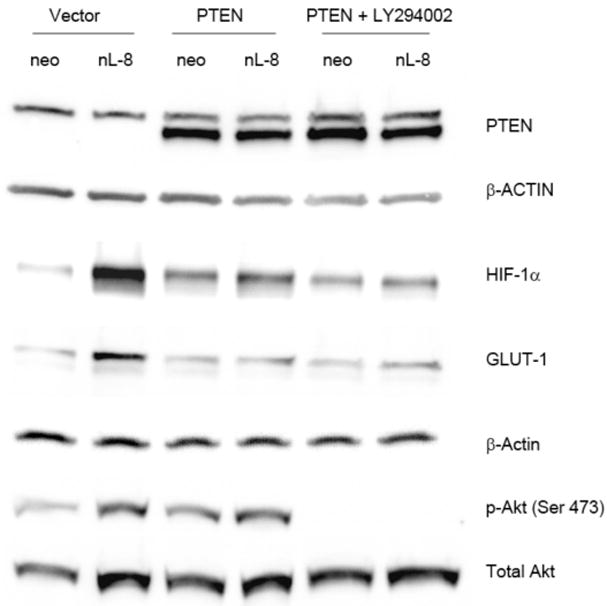
A. nL-8 cells (1 × 106 cells each) were treated either with the PI3K inhibitor LY294002 (25 μM) or the mTOR inhibitor rapamycin (10 nM) or vehicle and incubated under hypoxic conditions for 6 h, following which total protein was isolated and tested for the levels of HIF-1α by immunoblotting (30 μg total protein per sample). Shown here is a representative result from three independent experiments performed under essentially identical conditions. B. nL-8 and neo cells were transiently transfected either with a PTEN expression construct or with the empty vector. PTEN transfected cells (1 × 106 cells per incubation) were treated with LY294002 (25 μM) or vehicle (DMSO) and incubated under hypoxia for 6 h. Total protein (30 μg total protein per sample) harvested after incubation was tested for the levels of PTEN, HIF-1α, GLUT-1, p-Akt, and Akt by immunoblotting. Results shown here are from a representative experiment of n = 3; sample from one experiment was used for each protein tested and the membrane was reprobed for loading controls, i.e. actin and total Akt.
To confirm the functional significance of the PI3K-Akt pathway in 12-LOX regulation of HIF-1 we chose to transiently overexpress PTEN, a phosphatase that dephosphorylates the lipids that are phosphorylated by PI3K, in the nL-8 and neo cells. As shown in Fig 3B, transient overexpression of PTEN resulted in a decrease in the level of HIF-1α protein in nL-8 and treatment with LY294002, resulted in a further decrease. Also, GLUT-1, a downstream target of HIF-1α showed a similar decrease confirming that the PI3K-Akt pathway plays a pivotal role in 12-LOX mediated regulation of HIF-1α levels and its transcriptional activity. The effect of the inhibitor on PI3K activity was confirmed by comparing the levels of phosphorylated Akt (Ser 473) to the level of total Akt protein.
Nuclear localization and DNA binding activity of HIF-1α are enhanced by 12-LOX
To study the pattern of nuclear localization of HIF-1α in response to 12-LOX, nuclear fractions of nL-8 and neo cells cultured under hypoxic conditions were isolated. As shown in Fig 4A, immnoblotting for HIF-1α revealed that this protein is more abundant in the nuclear fraction compared to the cytoplasmic fraction of hypoxic cells. Of interest, the nuclear abundance of HIF-1α was increased in the nuclear fraction obtained from the nL-8 cells compared to the neo cells and this was confirmed by immunofluorescence, Fig 4D. To test whether the enhanced nuclear localization of HIF-1α translates to a higher DNA binding activity, gel shift assays were performed. As shown in fig 4B, the binding of HIF-1α to DNA was higher in nL-8 cells compared to neo. To confirm the findings from the gel shift assay, a transcription factor ELISA was also performed, using nuclear extracts prepared from hypoxically incubated cells, as described in materials and methods. Results demonstrated that the amount of HIF-1α DNA binding is enhanced in nL-8 cells (Fig 4C). Incubation of the nuclear extract with a competing oligonucleotide abolished the DNA binding ability of HIF-1α to the immobilized oligonucleotide, which indicates the specificity of the binding reaction (Fig 4C). These results confirmed that both nuclear localization and DNA binding activity of HIF-1α are increased by 12-LOX under hypoxic conditions.
Figure 4. Nuclear localization and DNA binding of HIF-1α are higher in 12-LOX overexpressing cells under hypoxia.
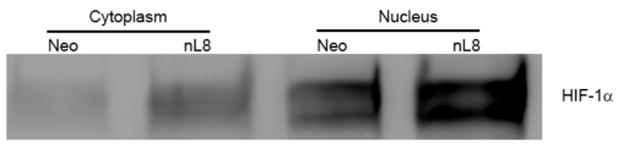
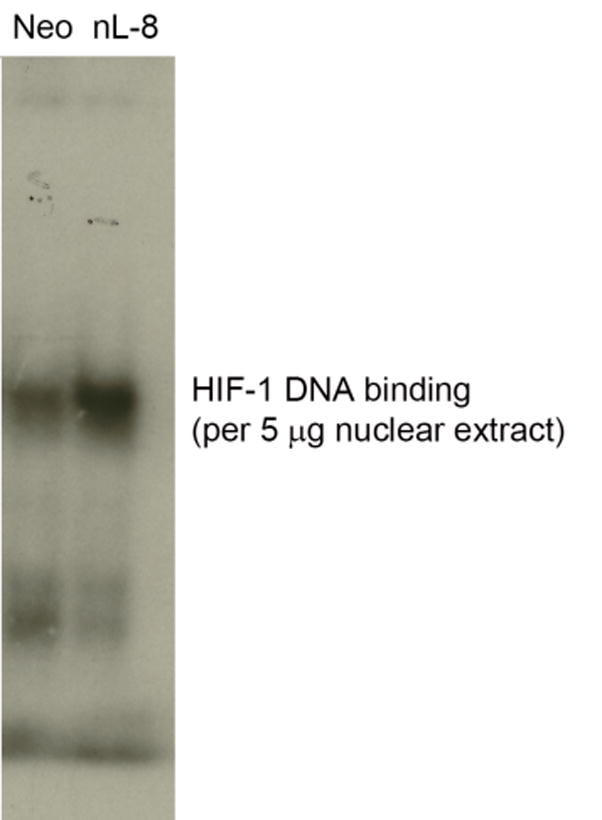
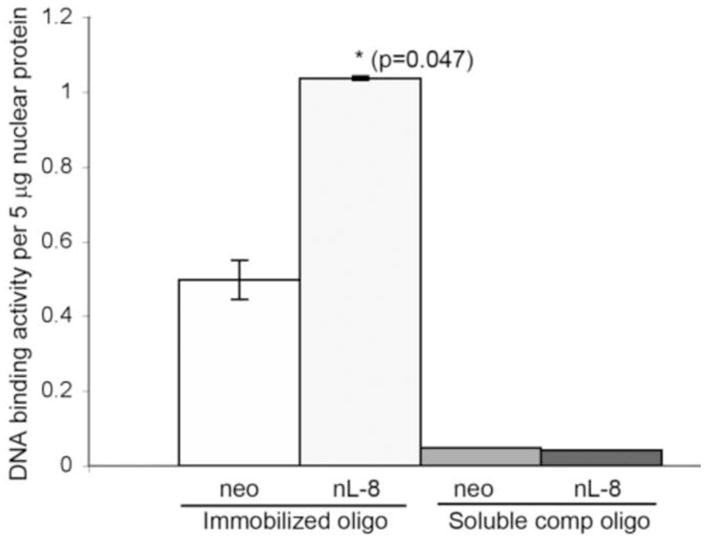
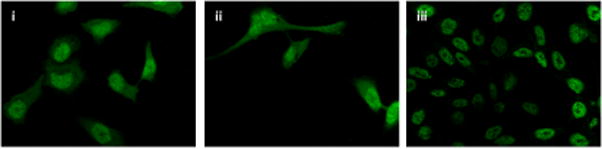
A. Nuclear fractions of neo and nL-8 cells incubated under hypoxic conditions were prepared using a nuclear isolation kit based on manufacturer s instructions. Both nuclear and cytoplasmic fractions (30 μg protein per sample) were subjected to Immunoblotting and the membrane was probed with antibody to HIF-1α. Shown here is a representative blot from three independent experiments. B. DNA binding activity of HIF-1α in nL-8 and neo cells was studied by gel shift assay as described in materials and methods. Shown here is a representative autoradiogram from two separate experiments performed under essentially identical conditions. C. Transcription factor ELISA for quantification of the extent of DNA binding of HIF-1α in nL-8 and neo cells, was performed based on the manufacturer s instructions. 5 μg of nuclear protein was incubated with immobilized HIF-1α DNA binding oligonucleotide sequences in the presence or absence of either a competing soluble oligonucleotide or a non-competing mutant oligonucleotide. The extent of DNA binding was then measured by specific primary and secondary antibodies. Results shown here are mean ± SD of n = 3 experiments performed under essentially identical conditions. * represents p-value assessed from student s t-test. D. Immunofluorescence localization of HIF-1α in PC3 (i), PC3 neo (ii), and PC3 nL8 (iii) incubated under hypoxic conditions, magnification is 63X in all instances. An overexposed version of fig D (iii) is included in the supplement, to show that magnification at which the cells were visualized was identical to the controls.
12-LOX signals VEGF production via HIF-1α
To investigate if 12-LOX mediated elevation in HIF-1α levels and DNA binding translate to a higher transcriptional activity of the protein, we performed HIF-1α transactivation assays using a 5 × HRE luciferase vector which harbors five HRE elements fused in tandem in a luciferase vector. Luciferase readings normalized with lacZ activity demonstrated that HRE transcriptional activity is higher in nL-8 cells compared to neo cells (Fig 5A). Next, we used siRNA to knockdown HIF-1α in nL-8 and neo cells as explained in materials and methods. As shown in Fig 5B, introduction of siRNA specific for HIF-1α resulted in a drastic decrease in the level of the protein in both nL-8 and neo cells subjected to hypoxia. ELISA for VEGF performed on media isolated from these cells showed that the concentration of secreted VEGF was significantly lower in nL-8 cells transfected with HIF-1α siRNA construct, compared to those transfected with a scrambled siRNA construct (Fig 5C). This clearly shows that 12-LOX mediates the secretion of VEGF via activation of HIF-1α under hypoxic conditions.
Figure 5. 12-LOX activates downstream targets of HIF-1α under hypoxia in prostate cancer cells.
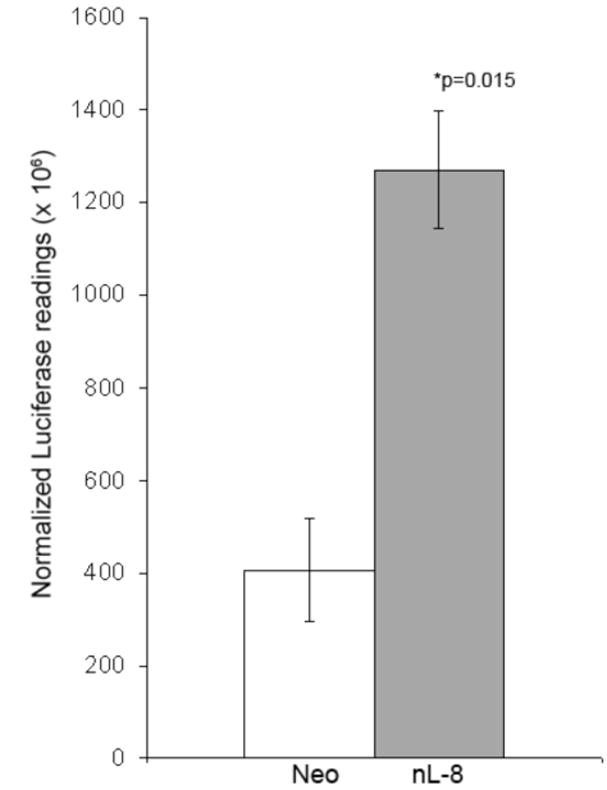
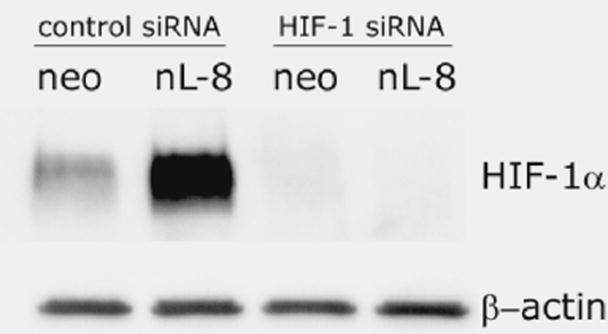
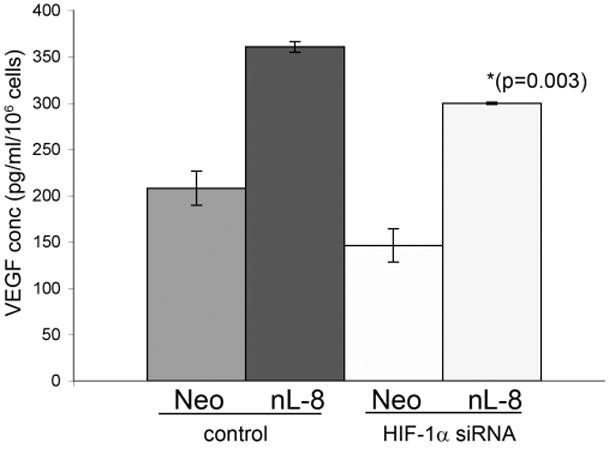
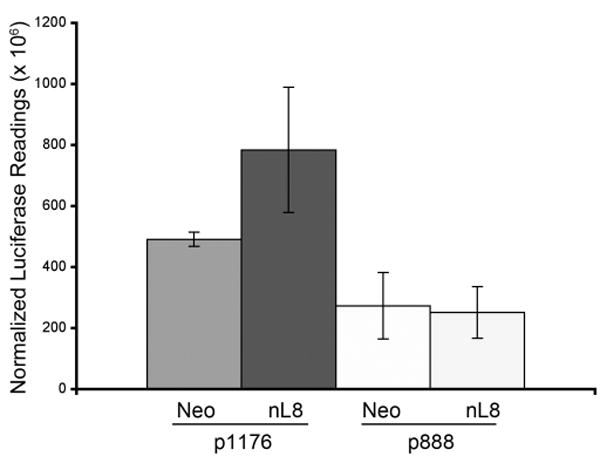
A. To test the ability of HIF-1α to mediate downstream gene transcription under the influence of 12-LOX, nL-8 and neo cells were transiently co-transfected with the 5xHRE and the lacZ expression vectors (see methods). 48 h post transfection, cells were incubated under hypoxic conditions for 6 h. Results are luciferase activity normalized to β-gal activity represented as mean from n=3 ± SD; * represents p-value assessed from student s t-test. B. nL-8 and neo cells were transiently transfected with either an siRNA construct targeted against HIF-1α or a scrambled construct. 48 h post transfection, the cells were incubated under hypoxic conditions for 6 h. Protein samples harvested from the incubated cells were tested for the levels of HIF-1α by immunoblotting. Shown here is a representative result from three independent experiments. C. Conditioned media from the HIF-1α siRNA and the control siRNA transfected cells were assayed for the levels of secreted VEGF using a VEGF ELISA kit according to the manufacturer s instructions. Values were normalized to the total number of cells. Shown here is a representative result from three independent experiments. D. nL-8 and neo cells were co-transfected with either a full length VEGF promoter vector (p1176) or a VEGF promoter vector with the HRE region deleted (p888) and a lacZ vector. 48 h post transfection, cells were washed and incubated under hypoxic conditions for 6 h, lysates were tested for luciferase and β-gal activities. Shown here are the normalized luciferase values from three independent experiments performed under essentially identical conditions. Results are expressed as mean of n=3 ± SD; * represents p value assessed using student s t-test.
To demonstrate further that 12-LOX regulates the transcription of VEGF via HIF-1α, neo and nL-8 cells were transiently transfected either with a wild-type VEGF promoter construct (p1176) or a VEGF promoter deletion construct (p888) which lacks the HRE region of the VEGF promoter. Analyses of the normalized luciferase readings showed that deletion of the HRE region of the VEGF promoter construct significantly decreased VEGF promoter activity in 12-LOX overexpressing cells under hypoxia (Fig 5D). These findings clearly demonstrate that 12-LOX partially enhances VEGF secretion via the activation of HIF-1 in prostate cancer cells under hypoxia.
Discussion
We report for the first time about a lipoxygenase enzyme and its arachidonic metabolite positively influencing the level and activity of the transcription factor HIF-1α under hypoxic conditions. 12-LOX also enhances the nuclear localization, DNA binding activity, and transcriptional activation of HIF-1α under hypoxia. The signaling mechanism involved in the activation of HIF-1 by 12-LOX was found to be mediated by the PI3K-Akt pathway, inhibition of which diminishes the ability of 12-LOX to upregulate HIF-1α. Finally, inhibition of HIF-1α by siRNA in 12-LOX overexpressing cells affects the secretion of VEGF in these cells, clearly demonstrating the influence of 12-LOX in eliciting VEGF expression via activation of the HIF-1 pathway.
Immunoblotting performed from cell lysates of 12-LOX overexpressing PC-3 and vector control cells incubated under hypoxic conditions, showed an increase in the level of HIF-1α protein, thereby indicating the presence of a regulatory pathway connecting these molecules. Similar results were also obtained from incubation of wild-type PC-3 cells with exogenously added 12(S)-HETE. Inhibition of 12-LOX activity using a LOX inhibitor ETYA, or two chemically unrelated specific 12-LOX inhibitors baicalein or BMD-122 (data not shown), decreased the level of HIF-1α protein emphasizing the necessity for 12-LOX enzymatic activity for the HIF-1 response. Other eicosanoids have been shown to regulate the expression and function of HIF-1α in a number of cancer cells. For example, COX-2 overexpression resulted in the upregulation of HIF-1 in gastric carcinoma cells. The same study also showed that treatment of gastric carcinoma cells with exogenously added PGE2 upregulated the HIF-1α protein level [15]. However, it was also observed in prostate cancer PC-3ML cells that PGE2 treatment did not increase the amount of HIF-1α protein, but only led to a nuclear translocation of HIF-1α, enhancing its availability within the nuclear pool [14]. Studies have also revealed that indomethacin treatment inhibits HIF-1α levels in both PC-3 and Du145 prostate cancer cell lines, but on the contrary were found to be COX-2 independent [16].
Real time PCR and northern blotting demonstrated an increase in HIF-1α mRNA in 12-LOX overexpressing cells. Several mechanisms have been proposed that can enhance HIF-1α levels and most of these revolve around the posttranslational stabilization and inhibition of protein degradation [1]. The presence of Sp1 binding sites on the promoter region of the HIF-1 gene has been identified by systematic sequence analysis [17, 18]. Promoter mutagenesis experiments performed for studying VEGF expression have revealed the significance of Sp1 on VEGF transcription triggered by 12-LOX [8] and it is possible that 12-LOX could activate HIF-1α transcription via Sp1. In addition to Sp1, NF-κB binding sites also have been identified in the HIF-1 promoter region, and an NF-κB dependent increase in expression of HIF-1α has been documented in human monocytes in response to bacterial LPS under aerobic conditions [19]. We have previously reported the stimulation of NF-κB by 12-LOX and 12(S)-HETE in prostate cancer cells and hence it is possible that 12-LOX may enhance HIF-1α transcription via NF-κB [20].
Earlier studies showed that the PI3K-Akt pathway is pivotal in mediating 12-LOX induced VEGF expression and secretion [8]. The present work has revealed that 12-LOX utilizes the same pathway in upregulating HIF-1α levels under hypoxic conditions. Additionally we have demonstrated that mTOR plays a role in 12-LOX mediated upregulation of HIF-1 under hypoxia. An active PI3K-Akt pathway has been linked to the stimulation of HIF-1 under hypoxic conditions, for example in Hep G2 cells, inhibition of the pathway led to a decrease in HIF-1α protein level [21].
HIF-1α activated by 12-LOX was found to be transcriptionally active as determined by nuclear localization, DNA binding ability, and functional assays such as promoter assays using the 5xHRE vector and siRNA studies. The ability of 12-LOX to influence VEGF expression via multiple transcription factors is evident in the siRNA studies. The incomplete abolition of VEGF transcription and secretion (Fig. 5C) upon introduction of HIF-1α siRNA suggests that other transcription factors may also play a role to compensate the loss of HIF-1α. These may include Sp1 and AP2, which were shown to be involved in 12-LOX dependent upregulation of VEGF, as reported previously [8]. Collectively these findings demonstrate a link between 12-LOX and HIF-1 that may trigger angiogenesis in tumors. By upregulating HIF-1, 12-LOX cannot only turn on the angiogenic switch, but can also regulate other downstream targets of HIF-1α such as GLUT-1. This in turn improves the availability of glucose inside anaerobic cells thereby enhancing metabolism and survival under conditions of high energy demand amidst an environment of low oxygen supply. The summary of these interactions is depicted in figure 6.
Figure 6. Summary of regulation of HIF-1α by 12-LOX in hypoxic prostate cancer cells.
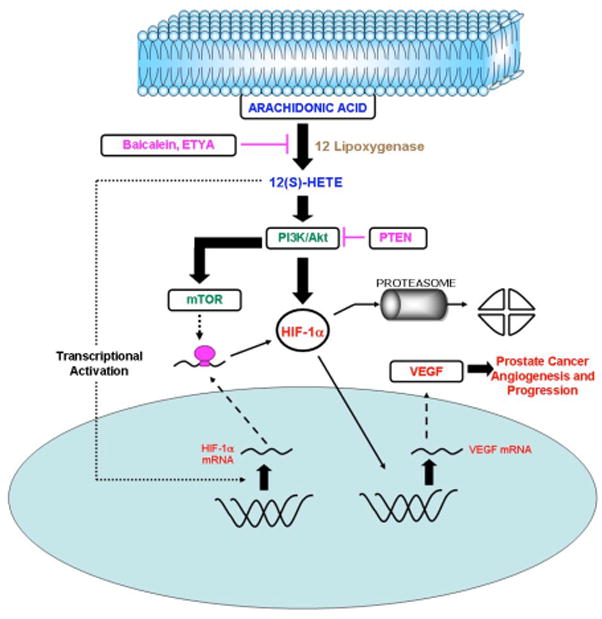
12-LOX generates 12(S)-HETE by its enzymatic activity on cell membrane arachidonic acid. 12(S)-HETE, being a lipid molecule, can freely diffuse across the cell membrane and bind to the cell membrane receptors, triggering intracellular signaling pathways. The present study has shown that 12-LOX and 12(S)-HETE are capable of upregulating HIF-1α mRNA levels, protein level, and functional activity. In addition to the effect on the transcriptional pathway, 12-LOX was also found to modulate HIF-1α via the PI3K-Akt-mTOR pathway, which is antagonized by the tumor suppressor PTEN. Transcriptional and post transcriptional pathways activated by 12-LOX may be sufficient to overcome the loss of HIF-1α protein by degradation thereby, enhancing the availability of functionally active protein, which can activate transcription of downstream genes such as VEGF and in turn prostate cancer angiogenesis.
HIF-1α expression and function was reported to be a poor prognostic indicator in tumors, because this promotes improved cell survival, angiogenesis, and resistance to therapy [5]. By triggering the HIF-1 pathway, 12-LOX overexpressing cells within a heterogeneous population of cells in a solid tumor may stimulate the angiogenic response. In the absence of tumor suppressors such as p53 and PTEN, activation of this pathway can lead to the formation of highly aggressive tumors that may have a higher potential for metastasis and resistance to therapy. Use of 12-LOX inhibitors, in combination with other therapeutic regimens may prove successful in managing solid tumors under such conditions.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grant CA-29997 (K.V.H.). These studies were done in conjunction with the Microarray and Bioinformatic Facility Core of the Environmental Health Sciences Center in Molecular and Cellular Toxicology with Human Applications at Wayne State University, the AICR, and The ligue Nationale Contre le Cancer (Equipe Labellisée).
Abbreviations
- LOX
Lipoxygenase
- HETE
Hydroxyeicosatetraenoic Acid
- HIF-1α
Hypoxia Inducible Factor–1α
- VEGF
Vascular Endothelial Growth Factor
- HRE
Hypoxia Response Element
- PI3K
Phospho Inositide 3 Kinase
- PTEN
Phosphatase and Tensin homolog deleted on chromosome ten
- mTOR
mammalian Target of Rapamycin
- GLUT-1
Glucose Transporter–1
Footnotes
Disclosure Statement- None of the authors has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 2.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends in Molecular Medicine. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 5.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 7.Zhong H, Semenza GL, Simons JW, De Marzo AM. Up-regulation of hypoxia-inducible factor 1alpha is an early event in prostate carcinogenesis. Cancer Detect Prev. 2004;28:88–93. doi: 10.1016/j.cdp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Nie D, Krishnamoorthy S, Jin R, Tang K, Chen Y, Qiao Y, Zacharek A, Guo Y, Milanini J, Pages G, Honn KV. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem. 2006;281:18601–18609. doi: 10.1074/jbc.M601887200. [DOI] [PubMed] [Google Scholar]
- 9.Nie D, Hillman GG, Geddes T, Tang K, Pierson C, Grignon DJ, Honn KV. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res. 1998;58:4047–4051. [PubMed] [Google Scholar]
- 10.Timar J, Raso E, Dome B, Li L, Grignon D, Nie D, Honn KV, Hagmann W. Expression, subcellular localization and putative function of platelet-type 12-lipoxygenase in human prostate cancer cell lines of different metastatic potential. Int J Cancer. 2000;87:37–43. doi: 10.1002/1097-0215(20000701)87:1<37::aid-ijc6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Nithipatikom K, Isbell MA, See WA, Campbell WB. Elevated 12- and 20-hydroxyeicosatetraenoic acid in urine of patients with prostatic diseases. Cancer Lett. 2006;233:219–225. doi: 10.1016/j.canlet.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 12.McCabe NP, Selman SH, Jankun J. Vascular endothelial growth factor production in human prostate cancer cells is stimulated by overexpression of platelet 12-lipoxygenase. Prostate. 2006;66:779–787. doi: 10.1002/pros.20360. [DOI] [PubMed] [Google Scholar]
- 13.Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–2727. [PubMed] [Google Scholar]
- 14.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SP, Wu MS, Shun CT, Wang HP, Hsieh CY, Kuo ML, Lin JT. Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. J Biomed Sci. 2005;12:229–241. doi: 10.1007/s11373-004-8177-5. [DOI] [PubMed] [Google Scholar]
- 16.Palayoor ST, Tofilon PJ, Coleman CN. Ibuprofen-mediated reduction of hypoxia-inducible factors HIF-1alpha and HIF-2alpha in prostate cancer cells. Clin Cancer Res. 2003;9:3150–3157. [PubMed] [Google Scholar]
- 17.Minet E, Ernest I, Michel G, Roland I, Remacle J, Raes M, Michiels C. HIF1A gene transcription is dependent on a core promoter sequence encompassing activating and inhibiting sequences located upstream from the transcription initiation site and cis elements located within the 5′UTR. Biochem Biophys Res Commun. 1999;261:534–540. doi: 10.1006/bbrc.1999.0995. [DOI] [PubMed] [Google Scholar]
- 18.Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. doi: 10.1006/geno.1998.5416. [DOI] [PubMed] [Google Scholar]
- 19.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandouz M, Nie D, Pidgeon GP, Krishnamoorty S, Maddipati KR, Honn KV. Platelet-type 12-Lipoxygenase Activates NF-kB in Prostate Cancer Cells. Prostaglandins and other lipid mediators. 2003;71:189–204. doi: 10.1016/s1098-8823(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 21.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


