Abstract
There is a need for neonatal screening tools to improve the long-term clinical outcome of patients with primary immunodeficiency diseases (PID). Recently, a PCR-based screening method for both TRECs and KRECs using Guthrie card samples has been developed. However, the applicability of these excision circle assays is limited to patients with severe T or B cell lymphopenia (SCID, XLA and A-T), whereas the most common forms of PID are not detected. Absence of serum IgA is seen in a major fraction of patients with immunological defects. As serum IgA in newborns is considered to be of fetal origin, eluates from routinely collected dried blood spot samples might thus be suitable for identification of children with PID. To assess the applicability of such screening assays, stored Guthrie card samples were obtained from 47 patients with various forms of primary immunodeficiency diseases (SCID, XLA, A-T, HIGM and IgAD), 20 individuals with normal serum IgA levels born to IgA-deficient mothers and 51 matched healthy newborns. Surprisingly, normal serum IgA levels were found in all SCID, XLA, A-T and HIGM patients and, additionally, in all those IgAD patients born to IgA-sufficient mothers. Conversely, no serum IgA was found in any of the 16 IgAD patients born by IgA-deficient mothers. Moreover, half of the IgA-sufficient individuals born by IgA-deficient mothers also lacked IgA at birth whereas no IgA-deficient individuals were found among the controls. IgA in neonatal dried blood samples thus appears to be of both maternal and fetal origin and precludes its use as a reliable marker for neonatal screening of primary immunodeficiency diseases.
Introduction
During pregnancy, the fetus depends on maternal transfer of specific antibodies for protection against pathogens. Humans produce five major immunoglobulin classes (IgG, IgA, IgM, IgE, IgD) and IgG is the only isotype that is actively transported from mother to child [1]–[9]. Several studies have previously demonstrated the presence of IgA in cord blood [1], [10]–[15] and IgA-positive B cells have also been reported in fetal tissues [16], [17] as well as in cord blood [18]–[21], suggesting that the IgA detected in neonatal blood is exclusively of fetal origin.
Primary immunodeficiency diseases (PID) comprise a group of more than 200 inherited genetic disorders caused by defects of innate and adaptive immune function [22]. The clinical severity ranges from non-symptomatic to recurrent, and potentially fatal, infections. Major efforts are currently undertaken to develop methods for neonatal PID screening, as early diagnosis and treatment would prevent subsequent tissue damage and premature death.
Defects in humoral immunity account for more than 60% of all forms of PID. The most common disorder, selective IgA deficiency (IgAD), is defined as serum IgA levels at or below 0.07 g/L with normal IgM and IgG levels in individuals of four years of age or older [23]. The estimated prevalence of IgAD is one in 600 in Caucasians [24]. Low or absent serum IgA is also included in the phenotype of a majority of other forms of PID (Table 1). Thus, lack of serum IgA at birth could potentially serve as a condition that would allow neonatal screening of various forms of PID.
Table 1. IgA levels and total T cell count for a selection of PID with IgA deficiency included in the phenotype.
| Disease | Estimated incidence | T lymphopenia present at birth‡ | IgA deficiency observed in the clinical phenotype† | |
| IgAD | 1∶600* [24] | No [23] | Yes [23] | |
| CVID | 1∶20–50.000* [29] | No [23] | Yes [30] | |
| HIGM | 1∶300.000 [31] | No [23] | Yes [23] | |
| XLA | 1∶70–100.000 [32] | No [22], [23] | 68 in 103 reported cases (66%) [33]–[45] | |
| A-T | 1∶100.000 [46] | Yes [26], [27] | 243 in 420 reported cases (58%) [47]–[79] | |
| SCID | IL2RG (X-SCID) | 1∶200.000 [46] | Yes [26] | 11 in 12 reported cases (92%) [80]–[89] |
| SCID | JAK3 | 1∶500.000 [46] | Yes [26] | 12 in 15 reported cases (80%) [90]–[94] |
| SCID | IL7R | not known [46] | Yes [26] | 15 in 24 reported cases (62%) [91], [95]–[100] |
| SCID | RAG1 | 1∶100.000 [46] | Yes [26] | 20 in 45 reported cases (44%) [89], [101]–[109] |
| SCID | RAG2 | 1∶100.000 [46] | Yes [110] | 15 in 20 reported cases (75%) [102], [111] |
| SCID | ADA | 1∶200–1.000.000 [46] | Yes [110] | 23 in 40 reported cases (57%) [112]–[123] |
| DiGeorge syndrome with relevantfeatures of immunodeficiency‡ † | 1∶15.000 [27] | Yes [27] | 9 in 17 reported cases (53%) [124]–[133] | |
Adapted from the prevalence seen in adults.
Clinically relevant T cell lymphopenia defined as CD3+ T cell counts <500/mm3.
Serum IgA levels <0.07 g/L.
In the 1960s, several countries introduced newborn screening programmes (NBS) for phenylketonuria, using eluates from dried blood spot samples (DBSS) of Guthrie cards. Other metabolic disorders have subsequently been added to the NBS programmes and today this screening constitutes an established form of preventive healthcare. In Sweden, DBSS have been used for NBS since 1965 and samples have been stored since 1975. As shown in our previous study [25], serum proteins can easily be eluted from stored DBSS and the corresponding levels be determined by sandwich ELISA or serum microarray techniques.
Although current neonatal PCR-based screening methods, using DNA extracted from Guthrie cards to quantify T-cell receptor excision circles (TRECs) and kappa-deleting recombination excision circles (KRECs), identify a majority of patients with severe combined immunodeficiencies (SCID) and X-linked agammaglobulinemia (XLA) [26], [27], patients suffering from the most prevalent forms of PID cannot be diagnosed using this method.
The aim of the present study was therefore to evaluate if lack of serum IgA in routinely collected DBSS eluates could serve as a condition to screen for PID.
Results
Elution Efficacy of IgA from Dried Blood Spot Samples
To determine the most efficient elution procedure for IgA from DBSS, serum IgA levels of freshly collected blood specimens were compared with the IgA levels of eluates from dried blood filter cards, prepared using the same blood samples. The serum levels of IgA were determined by routine nephelometry and ranged between 1.5–4.5 g/L, while the IgA levels in the DBSS eluates were determined by ELISA. On average, 70–85% of the serum IgA could be recovered from DBSS eluted at 4°C for 7 days (Table 2). Similar results were obtained for IgG levels in DBSS eluates (data not shown). For reasons of practicability and considering the potential risk of protein degradation at elevated temperatures, we chose to elute the DBSS at 4°C.
Table 2. Percentage agreement of the IgA elution from DBSS by ELISA compared to reference serum IgA levels measured by nephelometry.
| Elutioncondition | 4°Ctemperature | 22°Ctemperature | 37°Ctemperature |
| 1 hour | 78% | 82% | 61% |
| 24 hours | 87% | 93% | 90% |
| 48 hours | 84% | 85% | 86% |
| 7 days | 86% | 86% | 69% |
Long-time Storage does not Preclude Successful Elution of IgA from DBSS
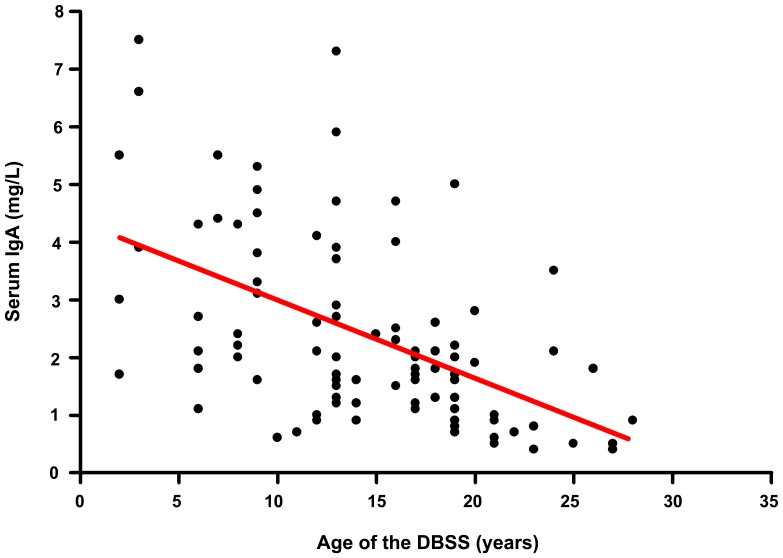
Ninety-two of the 118 DBSS included in the study had detectable IgA levels (51 controls, 31 PID patients and 10 IgA-sufficient children born to IgAD mothers) and could be analyzed to assess the effect of storage time prior to elution on the amount of eluted IgA (Figure 1). All DBSS were taken from original Guthrie cards routinely kept in sealed bags at 4°C–8°C for long-time storage. Correlation analysis of IgA levels and storage times indicates that the amount of eluted and/or detected IgA slightly decreases as a function of longer storage time (ρ = −0.54, p<0.0001). However, considerable IgA levels could be detected even in samples stored for 28 years, providing evidence that long-time storage does not exclude samples from analysis, nor would markedly influence the results obtained. Similar results were obtained previously for other serum proteins eluted from DBSS, that had been stored for long time [25].
Figure 1. Impact of the storage time on the detection of IgA levels in DBSS eluates.
The overall correlation is shown as a red trend line (ρ = −0.54). DBSS with undetectable IgA levels are not shown.
Reference Values for IgA Levels in DBSS Eluates from Healthy Newborns
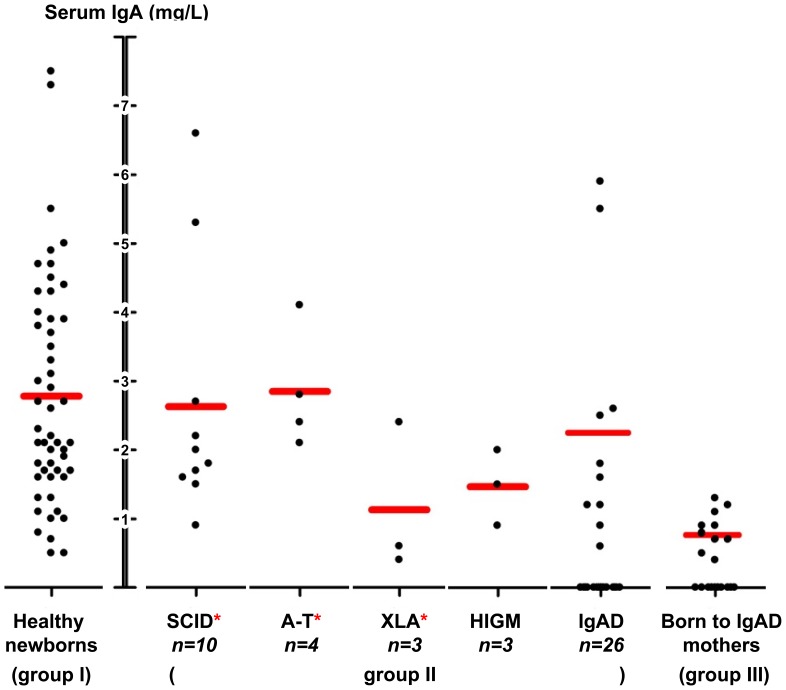
All DBSS eluates had detectable IgA levels in healthy newborns (group I), with a mean of 2.8 mg/L (range: 0.5–7.5 mg/L, Figure 2). These levels are comparable to those obtained from umbilical cord blood at delivery, as reported by Malek and colleagues [14]. For 8 individuals from this group, a separate elution from a second punch of the original Guthrie card was performed to re-assess the initially measured IgA levels, returning consistent results with a high degree of correlation (r2 = 0.97).
Figure 2. IgA levels in eluates from neonatal DBSS. Mean IgA levels per group are marked as horizontal red bars.
One SCID patient was excluded due to extremely high levels of IgA in the DBSS eluate. SCID, A-T and XLA DBSS have previously shown to result in abnormal test results in the TREC/KREC assay and have been indicated by red asterisks [26], [27].
Presence of IgA in Neonatal DBSS from Patients with SCID, XLA, HIGM or A-T
Within the group of PID patients (group II), all the patients diagnosed with SCID, XLA, Hyper IgM syndrome (HIGM) or Ataxia-telangiectasia (A-T) (n = 21) had detectable levels of serum IgA at birth, although all of them are IgA-deficient today (n = 10) or at the time of hematopoietic stem cell transplantation (n = 11). Moreover, all IgAD patients born to mothers with normal serum IgA levels also had detectable levels of IgA in neonatal DBSS (n = 10). The mean serum IgA level of these PID patients with detectable IgA levels at birth was 2.3 mg/L (range: 0.4–6.6 mg/L). A summary of the IgA levels based on specific disorders in group II is given in Figure 2, including previously reported classification results of these patients in the TREC/KREC assay [26], [27].
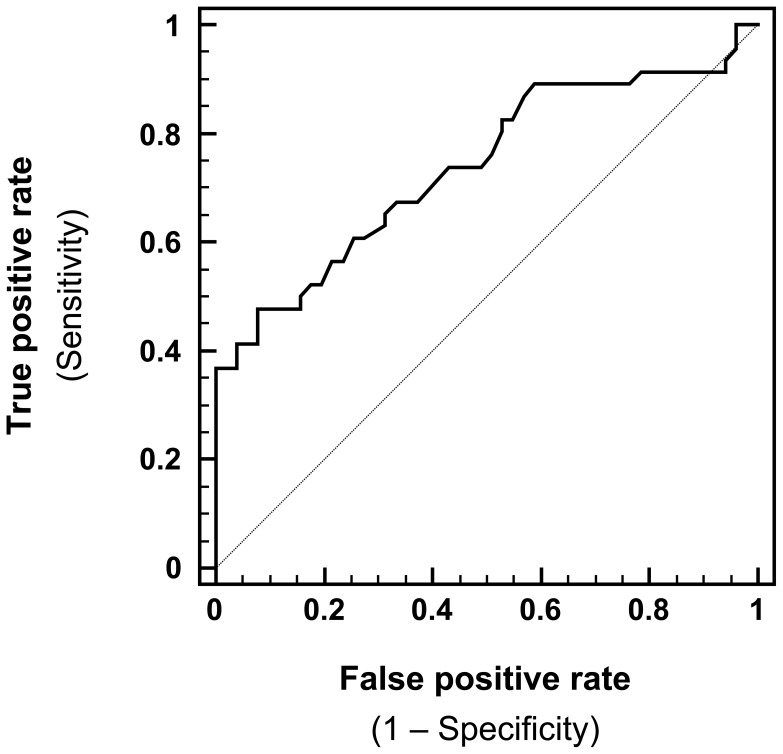
The test characteristics for measuring IgA levels in neonatal DBSS to predict IgA-deficient PID patients are shown in Table 3 based on cutoff values that were chosen by receiver operating characteristic (ROC) curve analysis (Figure 3).
Table 3. Descriptive test characteristics of measuring IgA levels in DBSS to predict IgA-deficient newborns (group II).
| Cutoff value* | Sensitivity | Specificity | PPV | NPV |
| ≤0.35 | 0.39 | 1.0 | 100% | 63% |
| ≤0.6 | 0.41 | 0.96 | 90% | 64% |
| ≤0.9 | 0.47 | 0.92 | 84% | 66% |
| ≤1.2 | 0.5 | 0.84 | 74% | 65% |
Serum IgA [mg/L]; PPV: positive predictive value; NPV: negative predictive value. PPV and NPV calculations are based on cumulative prevalence estimates from adults with diseases represented in group II.
Figure 3. ROC curve plot depicting the performance of serum IgA levels in DBSS to predict IgA-deficient newborns (group II) compared with healthy newborns (group I).
Maternal IgA-deficiency is Associated with Lack of IgA in DBSS Eluates
Sixteen of 26 samples (62%) from patients diagnosed with IgAD were found to have undetectable levels of IgA at birth (Figure 2), yet all featured normal IgG levels (9–18 g/L; data not shown), indicating that the samples were eluted properly. Interestingly, all these IgAD patients identified with undetectable IgA levels in DBSS eluates were born to IgA-deficient mothers, suggesting an impact of maternal IgAD on the IgA levels in the newborns. All of these IgAD patients were previously shown to have normal TREC and KREC copy numbers (Figure 2) [26], [27].
To test for a maternal influence on fetal IgA levels, DBSS from 20 children with normal IgA levels at follow-up (1 to 16 years of age), born to IgAD mothers, were collected and analyzed (group III). Ten of these children (50%) also showed undetectable serum IgA levels at birth.
In the remaining 10 samples from healthy children born to IgAD mothers (group III), IgA could be detected with a mean level of 0.9 mg/L (range: 0.4–1.3 mg/L, Figure 2). This constitutes about 33% of the mean level in healthy newborns (group I), suggesting that maternal IgA contributes at least 67% of the IgA found in DBSS eluates.
Discussion
Novel neonatal screening methods for simultaneous detection of SCID and XLA patients, based on quantitation of TRECs and KRECs, have recently been proposed [26], [27]. However, these methods only identify a small proportion of all PID patients. As IgA-deficiency is included not only in the phenotype of most antibody deficiencies, albeit to varying degrees (Table 1), but also in a majority of other forms of PID, determination of serum IgA levels could potentially serve as a basis for screening programmes of inborn immune defects.
Although such a proposed screening for neonatal IgA-deficiency would result in the identification of patients with IgAD, these patients are not the primary target group for this screening as it represents a clinically mild form of PID. A screening test for IgAD would rather constitute a first step in the immunological evaluation, to be followed by second-tier tests and more in-depth analyses to establish the correct diagnosis.
The material for such a screening might preferably be a dried blood spot sample from regular Guthrie cards, as these are already routinely collected in a number of countries. In one of our previous studies [25], we investigated the possibility of identifying patients with primary immunodeficiency diseases of the complement system using DBSS. Complement C3 was readily detected in DBSS eluates from healthy controls using both sandwich ELISA and reverse phase serum microarrays. In contrast, DBSS eluates from patients with C3 deficiency lacked the protein. Thus, proteins eluted from Guthrie card samples may well be used in a high-throughput setting to identify individuals with selected primary immunodeficiency diseases.
As IgG is the only class of immunoglobulins that is actively being transported from mother to child during pregnancy [1]–[9], serum IgA in newborns is considered to be of fetal origin.
To investigate the possibility to diagnose PID at birth, we thus measured serum IgA levels in DBSS eluates from patients with various forms of PID. None of the patients with SCID, HIGM, XLA or A-T lacked IgA at birth and IgAD patients born to IgA-sufficient mothers all had detectable IgA levels at birth. In contrast, serum IgA was absent in the DBSS of IgAD children born to IgA-deficient mothers, indicating that IgA deficiency is present already at birth. In addition, an association between maternal and fetal levels of IgA was also seen in IgA-sufficient children born to IgA-deficient mothers, as 10 out of 20 children lacked IgA at birth.
Based on our results, we therefore propose the existence of a hitherto not fully appreciated mechanism allowing diffusion or active transport of maternal IgA to the child during pregnancy.
This notion is supported by previous work of Malek and colleagues [14], who investigated the transport of various proteins across human placenta by comparing the concentrations in maternal and fetal blood and where a correlation between fetal and maternal levels of serum IgA was found, suggesting that diffusion transfer does take place. Furthermore, Gurevich and colleagues [17] reported that endocrine precursor cells of 4–7 weeks old embryos were positive for surface IgA, but IgA-secreting lymphocytes did not appear until 10–11 weeks of pregnancy, suggesting a placental transfer of maternal IgA. Similarly, Ben-Hur and colleagues [28] found that components of the placental barrier were positive for IgA surface staining in 3.5–8 weeks old embryos, yet fetal, IgA-positive lymphocytes were first seen after 9–11 weeks of gestation. These findings collectively suggest that maternal IgA might be transferred to the embryo during pregnancy.
Some of the IgA-sufficient children born to IgAD mothers (group III) had detectable serum IgA levels at birth, implying that both the mother and the fetus contribute to the serum IgA detected in neonatal blood. Furthermore, the Am2 allotype has been shown to be present in the cord blood of some children, even though the mother lacks this marker, indicating a fetal origin of at least part of the neonatal serum IgA [12]. Based on our findings among the ten children in group III with detectable IgA levels, we suggest that the mother contributes at least 67% of the serum IgA detected in the DBSS eluates.
In summary, a screening approach for PID based on the determination of serum IgA levels in neonatal Guthrie card samples seems unlikely to be effective. However, it should be mentioned that the assay characteristics would be influenced by the prevalence of both the target diseases and those individuals with IgAD that give birth to healthy newborns; potentially proving the test to be of value following prospective evaluation. Alternatively, it remains to be determined if other markers, indicative of fetal IgA production, can be successfully applied to future neonatal screening programmes for PID.
Materials and Methods
Sample Collection
Guthrie card samples, generated within the first 72 hrs of life, were obtained from the PKU laboratory at the Centre for Inherited Metabolic Diseases, Karolinska University Hospital, Huddinge, Sweden. The samples were divided into three groups depending on origin. Group I: storage-time matched healthy individuals from the national neonatal biobank (n = 51). Group II: PID patients born to mothers with normal immunoglobulin levels (n = 31) or mothers with IgAD (n = 16). Group III: individuals with normal serum IgA levels born to IgA-deficient mothers (n = 20). Group II included 26 patients with IgAD, 11 newborns diagnosed with SCID (RAG1 n = 3, IL2RG n = 3, Jak3 n = 1, unknown etiology n = 4), 3 with HIGM (all CD40L mutations), 3 with XLA and 4 with A-T. The samples from these patients had all undergone TREC/KREC testing and were reported in our previous publications [26], [27]. Although all SCID, A-T and XLA patients could be readily identified, no IgAD or HIGM patients showed abnormal TREC or KREC copy numbers when compared to healthy newborns. The IgAD patients included in the present study had been referred to the immunodeficiency unit at Karolinska University Hospital Huddinge for evaluation of their immune status. As part of the investigation, all family members, including mothers, were also screened for humoral immunodeficiencies. All patients in group II lacked serum IgA at the time of investigation (n = 36) or at the time of hematopoietic stem cell transplantation (n = 11). Overall, the 118 DBSS in total had been stored for 2–28 years prior to analysis.
For this study, two spots (3.2 mm in diameter), each equaling the plasma fraction of ∼3 µl of whole blood, were punched from the original stored Guthrie cards. Approval for this study was obtained from the regional research ethical board in Stockholm.
Comparison of different Elution Procedures
Peripheral venous blood was collected from four adult individuals and serum IgA levels determined by routine nephelometry. 15 µl of blood, corresponding to ∼9 µl of plasma, was spotted on twelve Whatman 903 protein saver cards (GE Healthcare, USA) per individual. The cards were left to dry and DBSS were subsequently eluted in 450 µl (1∶50) phosphate-buffered saline (PBS) with 0.5% Tween20 under varying conditions for temperature (4°C–37°C) and elution time (1 h –7 days). Serum IgA levels were determined by sandwich ELISA as described below. The ratio of IgA levels obtained after elution and the serum IgA levels determined by nephelometry was calculated and compared for each individual.
Determination of IgA and IgG Levels in DBSS Eluates
The DBSS were soaked in 150 µl of PBS supplemented with 0.5% Tween20 (PBS-Tween, Sigma-Aldrich, USA) for 7 days at 4°C, giving a dilution of approximately 1∶50. 96-well polystyrene plates (Corning Incorporated, USA) were coated at room temperature overnight with 100 µl/well of polyclonal rabbit anti-human IgA or IgG antibodies (DAKO, Denmark) added at a dilution of 1∶4000. The DBSS eluates were diluted at 1∶5 and quantified using a serially diluted standard. Alkaline phosphatase-conjugated rabbit anti-human serum IgA or IgG antibodies (Jackson ImmunoResearch Laboratories, USA) were subsequently added at a 1∶2000 dilution, followed by the addition of a p-Nitrophenylphosphate solution (Sigma-Aldrich). The absorbance was measured at 405 nm on a Vmax microplate reader (Molecular Devices, USA) and the mean concentration was calculated for each sample using Deltasoft JV 1.8 (Biometallics Inc, USA). All included samples were tested 2–6 times.
Statistical Analysis
The elution efficiency from DBSS was assigned by indicating a percentage agreement of those IgA levels measured by ELISA and the reference serum IgA levels measured by nephelometry. As the IgA levels in DBSS are not normally distributed, the data on the influence of storage time on IgA levels were analyzed according to Spearma?s rank correlation coefficient, using XLSTAT (Addinsoft, France). ROC curve analysis and calculation of test sensitivity, specificity, positive and negative predictive value was done using MedCalc (MedCalc Software, Belgium).
Acknowledgments
We are indebted to Ms Karen Lo for technical assistance during the initial ELISA experiments on the dried blood samples.
Funding Statement
The herein presented research was supported in part by the Jeffrey Modell Foundation (to S.B. and L.H.), the Swedish Primary Immunodeficiency Organisation (PIO), the Gillbergska Foundation, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet, the Swedish Research Council, the European Union (Euro-Gene-Scan, project 223293) and the German Federal Ministry of Education and Research (BMBF, PtJ-Bio 0315883). M.J. was supported by an educational grant from the National Research School in Health and Caring Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Avrech OM, Samra Z, Lazarovich Z, Caspi E, Jacobovich A, et al. (1994) Efficacy of the placental barrier for immunoglobulins: correlations between maternal, paternal and fetal immunoglobulin levels. Int. Arch. Allergy Immunol 103: 160–165. [DOI] [PubMed] [Google Scholar]
- 2. Billington WD (1992) The normal fetomaternal immune relationship. Baillieres Clin Obstet Gynaecol 6: 417–438. [DOI] [PubMed] [Google Scholar]
- 3. Bjerkli IH, Myklebust R, Räisänen S, Telimaa S, Stenfors LE (1996) Bacterial attachment to oropharyngeal epithelial cells in breastfed newborns. Int J Pediatr Otorhinolaryngol 36: 205–213. [DOI] [PubMed] [Google Scholar]
- 4. Gusdon JP (1969) Fetal and maternal immunoglobulin levels during pregnancy. Am J Obstet Gynecol 103: 895–900. [DOI] [PubMed] [Google Scholar]
- 5. Quan CP, Watanabe S, Forestier F, Bouvet JP (1998) Human amniotic IgA inhibits natural IgG autoantibodies of maternal or unrelated origin. Eur J Immunol 28: 4001–4009. [DOI] [PubMed] [Google Scholar]
- 6. Quie PG (1990) Antimicrobial defenses in the neonate. Semin Perinatol 14: 2–9. [PubMed] [Google Scholar]
- 7. Saji F, Samejima Y, Kamiura S, Koyama M (1999) Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod 4: 81–89. [DOI] [PubMed] [Google Scholar]
- 8. Wedgwood JF, Palmer R, Weinberger B (1994) Development of the ability to make IgG and IgA in newborns and infants. Mt Sinai J Med 61: 409–415. [PubMed] [Google Scholar]
- 9. Vetro SW, Bellanti JA (1989) Fetal and neonatal immunoincompetence. Fetal Ther 4 Suppl 182–91. [DOI] [PubMed] [Google Scholar]
- 10. Cederqvist LL, Merkatz IR, Litwin SD (1977) Fetal immunoglobulin synthesis following maternal immunosuppression. Am J Obstet Gynecol 129: 687–690. [DOI] [PubMed] [Google Scholar]
- 11. Cederqvist LL, Ewool LC, Litwin SD (1978) The effect of fetal age, birth weight, and sex on cord blood immunoglobulin values. Am J Obstet Gynecol 131: 520–525. [DOI] [PubMed] [Google Scholar]
- 12. Cederqvist LL, Litwin SD (1974) Production of alpha 1 and alpha 2 immunoglobulin heavy chains during fetal life. J Immunol 112: 1605–1608. [PubMed] [Google Scholar]
- 13. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H (1996) Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol 36: 248–255. [DOI] [PubMed] [Google Scholar]
- 14. Malek A, Sager R, Schneider H (1998) Transport of proteins across the human placenta. Am J Reprod Immunol 40: 347–351. [DOI] [PubMed] [Google Scholar]
- 15. Ueda H, Nakanishi A, Ichijo M (1980) Immunoglobulins in newborns, particularly in term SFD infants. Tohoku J Exp Med 132: 31–35. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Hur H, Gurevich P, Huszar M, Ziv-Sokolovsky N, Zion H, et al. (1997) Immunoglobulin A in the epithelium of the respiratory tract and intrahepatic bile ducts of fetuses and newborns with pneumonia and sepsis. Hum Antibodies 8: 119–123. [PubMed] [Google Scholar]
- 17. Gurevich P, Ben-Hur H, Moldavsky M, Szvalb S, Shperling I, et al. (2001) An immunohistochemical study of the secretory immune system in human fetal endocrine glands and their precursors. Early Pregnancy 5: 191–200. [PubMed] [Google Scholar]
- 18. Andersson U, Bird G, Britton S (1980) Cellular mechanisms of restricted immunoglobulin formation in the human neonate. Eur J Immunol 10: 888–894. [DOI] [PubMed] [Google Scholar]
- 19. Gathings WE, Lawton AR, Cooper MD (1977) Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol 7: 804–810. [DOI] [PubMed] [Google Scholar]
- 20. Gudmundsson KO, Thorsteinsson L, Gudmundsson S, Haraldsson A (1999) Immunoglobulin-secreting cells in cord blood: effects of Epstein-Barr virus and interleukin-4. Scand J Immunol 50: 21–24. [DOI] [PubMed] [Google Scholar]
- 21. Miyawaki T, Kubagawa H, Butler JL, Cooper MD (1988) Ig isotypes produced by EBV-transformed B cells as a function of age and tissue distribution. J Immunol 140: 3887–3892. [PubMed] [Google Scholar]
- 22. Notarangelo LD, Fischer A, Geha RS, Casanova J, Chapel H, et al. (2009) Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol 124: 1161–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conley ME, Notarangelo LD, Etzioni A (1999) Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol 93: 190–197. [DOI] [PubMed] [Google Scholar]
- 24. Yel L (2010) Selective IgA deficiency. J Clin Immunol 30: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janzi M, Sjöberg R, Wan J, Fischler B, von Döbeln U, et al. (2009) Screening for C3 deficiency in newborns using microarrays. PLoS ONE: e5321. [DOI] [PMC free article] [PubMed]
- 26. Borte S, von Döbeln U, Fasth A, Wang N, Janzi M, et al. (2012) Neonatal screening for severe primary immunodeficiency diseases using high-throughput triplex real-time PCR. Blood 119: 2552–2555. [DOI] [PubMed] [Google Scholar]
- 27. Borte S, Wang N, Oskarsdóttir S, von Döbeln U, Hammarström L (2011) Ann N Y Acad Sci. 1246: 118–130. [DOI] [PubMed] [Google Scholar]
- 28. Ben-Hur H, Gurevich P, Elhayany A, Avinoach I, Schneider DF, et al. (2005) Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int J Mol Med 16: 401–407. [PubMed] [Google Scholar]
- 29. Pan-Hammarström Q, Hammarström L (2008) Antibody deficiency diseases. Eur J Immunol 38: 327–333. [DOI] [PubMed] [Google Scholar]
- 30. Hammarström L, Vorechovsky I, Webster D (2000) Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID). Clin Exp Immunol 120: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Durandy A, Revy P, Imai K, Fischer A (2005) Hyper-immunoglobulin M syndromes caused by intrinsic B-lymphocyte defects. Immunol Rev 203: 67–79. [DOI] [PubMed] [Google Scholar]
- 32. Fried AJ, Bonilla FA (2009) Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin Microbiol Rev 22: 396–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hunter HL, McKenna KE, Edgar JDM (2008) Eczema and X-linked agammaglobulinaemia. Clin Exp Dermatol 33: 148–150. [DOI] [PubMed] [Google Scholar]
- 34. Oxelius VA (1979) Quantitative and qualitative investigations of serum IgG subclasses in immunodeficiency diseases. Clin Exp Immunol 36: 112–116. [PMC free article] [PubMed] [Google Scholar]
- 35. Sigmon JR, Kasasbeh E, Krishnaswamy G (2008) X-linked agammaglobulinemia diagnosed late in life: case report and review of the literature. Clin Mol Allergy 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang L, Yang Y, Lin Y, Chiang B (2004) Immunological and clinical features of pediatric patients with primary hypogammaglobulinemia in Taiwan. Asian Pac J Allergy Immunol 22: 25–31. [PubMed] [Google Scholar]
- 37. Wang Y, Kanegane H, Wang X, Han X, Zhang Q, et al. (2009) Mutation of the BTK gene and clinical feature of X-linked agammaglobulinemia in mainland China. J Clin Immunol 29: 352–356. [DOI] [PubMed] [Google Scholar]
- 38. Mitsui T, Tsukamoto N, Kanegane H, Agematsu K, Sekigami T, et al. (2006) X-linked agammaglobulinemia diagnosed in adulthood: a case report. Int J Hematol 84: 154–157. [DOI] [PubMed] [Google Scholar]
- 39. Graziani S, Di Matteo G, Benini L, Di Cesare S, Chiriaco M, et al. (2008) Identification of a Btk mutation in a dysgammaglobulinemic patient with reduced B cells: XLA diagnosis or not? Clin Immunol 128: 322–328. [DOI] [PubMed] [Google Scholar]
- 40. Usui K, Sasahara Y, Tazawa R, Hagiwara K, Tsukada S, et al. (2001) Recurrent pneumonia with mild hypogammaglobulinemia diagnosed as X-linked agammaglobulinemia in adults. Respir Res 2: 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quartier P, Debré M, de Blic J, de Sauverzac R, Sayegh N, et al. (1995) Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr 134: 589–596. [DOI] [PubMed] [Google Scholar]
- 42. Landreth KS, Engelhard D, Anasetti C, Kapoor N, Kincade PW, et al. (1985) Pre-B cells in agammaglobulinemia: evidence for disease heterogeneity among affected boys. J Clin Immunol 5: 84–89. [DOI] [PubMed] [Google Scholar]
- 43. van der Meer JW, Weening RS, Schellekens PT, van Munster IP, Nagengast FM (1993) Colorectal cancer in patients with X-linked agammaglobulinaemia. Lancet 341: 1439–1440. [DOI] [PubMed] [Google Scholar]
- 44. Berlucchi M, Soresina A, Redaelli De Zinis LO, Valetti L, et al. (2008) Sensorineural hearing loss in primary antibody deficiency disorders. J Pediatr 153: 293–296. [DOI] [PubMed] [Google Scholar]
- 45. Sirianni MC, Atzori C, de Santis W, Milito C, Esposito A, et al. (2006) A case of Pneumocystis jiroveci pneumonia in X-linked agammaglobulinaemia treated with immunosuppressive therapy: a lesson for immunologists. Int Arch Allergy Immunol 140: 82–88. [DOI] [PubMed] [Google Scholar]
- 46. Samarghitean C, Väliaho J, Vihinen M (2007) IDR knowledge base for primary immunodeficiencies. Immunome Res 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Noordzij JG, Wulffraat NM, Haraldsson A, Meyts I, van’t Veer LJ, et al. (2009) Ataxia-telangiectasia patients presenting with hyper-IgM syndrome. Arch Dis Child 94: 448–449. [DOI] [PubMed] [Google Scholar]
- 48. Staples ER, McDermott EM, Reiman A, Byrd PJ, Ritchie S, et al. (2008) Immunodeficiency in ataxia telangiectasia is correlated strongly with the presence of two null mutations in the ataxia telangiectasia mutated gene. Clin Exp Immunol 153: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moin M, Aghamohammadi A, Kouhi A, Tavassoli A, Rezaei N, et al. (2007) Ataxia-telangiectasia in Iran: clinical and laboratory features of 104 patients. Pediatr Neurol 37: 21–28. [DOI] [PubMed] [Google Scholar]
- 50. Forte WCN, Menezes MCSD, Dionigi PCL, Bastos CLAFE (2005) Different clinical and laboratory evolutions in ataxia-telangiectasia syndrome: report of four cases. Allergol Immunopathol (Madr) 33: 199–203. [DOI] [PubMed] [Google Scholar]
- 51. Stray-Pedersen A, Jónsson T, Heiberg A, Lindman CR, Widing E, et al. (2004) The impact of an early truncating founder ATM mutation on immunoglobulins, specific antibodies and lymphocyte populations in ataxia-telangiectasia patients and their parents. Clin Exp Immunol 137: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trimis GG, Athanassaki CK, Kanariou MM, Giannoulia-Karantana AA (2004) Unusual absence of neurologic symptoms in a six-year old girl with ataxia-telangiectasia. J Postgrad Med 50: 270–271. [PubMed] [Google Scholar]
- 53. Meyts I, Weemaes C, de Wolf-Peeters C, Proesmans M, Renard M, et al. (2003) Unusual and severe disease course in a child with ataxia-telangiectasia. Pediatr Allergy Immunol 14: 330–333. [DOI] [PubMed] [Google Scholar]
- 54. Huang KY, Shyur SD, Wang CY, Shen EY, Liang DC (2001) Ataxia telangiectasia: report of two cases. J Microbiol Immunol Infect 34: 71–75. [PubMed] [Google Scholar]
- 55. Khumalo NP, Joss DV, Huson SM, Burge S (2001) Pigmentary anomalies in ataxia-telangiectasia: a clue to diagnosis and an example of twin spotting. Br J Dermatol 144: 369–371. [DOI] [PubMed] [Google Scholar]
- 56. Sadighi Akha AA, Humphrey RL, Winkelstein JA, Loeb DM, Lederman HM (1999) Oligo−/monoclonal gammopathy and hypergammaglobulinemia in ataxia-telangiectasia. A study of 90 patients. Medicine (Baltimore) 78: 370–381. [DOI] [PubMed] [Google Scholar]
- 57. McDonald PS, Cora-Bramble D, de Palma L (1998) Monoclonal gammopathy of the immunoglobulin A class in a two-year-old girl with ataxia telangiectasia. Pediatr Dev Pathol 1: 319–321. [DOI] [PubMed] [Google Scholar]
- 58. Miyagi K, Mukawa J, Kinjo N, Horikawa K, Mekaru S, et al. (1995) Astrocytoma linked to familial ataxia-telangiectasia. Acta Neurochir (Wien) 135: 87–92. [DOI] [PubMed] [Google Scholar]
- 59. Willems PJ, van Roy BC, Kleijer WJ, van der Kraan M, Martin JJ (1993) Atypical clinical presentation of ataxia telangiectasia. Am J Med Genet 45: 777–782. [DOI] [PubMed] [Google Scholar]
- 60. Tsukahara M, Masuda M, Ohshiro K, Kobayashi K, Kajii T, et al. (1986) Ataxia telangiectasia with generalized skin pigmentation and early death. Eur J Pediatr 145: 121–124. [DOI] [PubMed] [Google Scholar]
- 61. Meshram CM, Sawhney IM, Prabhakar S, Chopra JS (1986) Ataxia telangiectasia in identical twins: unusual features. J Neurol 233: 304–305. [DOI] [PubMed] [Google Scholar]
- 62. Fiorilli M, Crescenzi M, Carbonari M, Tedesco L, Russo G, et al. (1986) Phenotypically immature IgG-bearing B cells in patients with hypogammaglobulinemia. J Clin Immunol 6: 21–25. [DOI] [PubMed] [Google Scholar]
- 63. Ozer NK, Ciliv G, Berkel AI, Sanal O, Yeğin O, et al. (1985) Studies on lymphocyte cell surface in ataxia-telangiectasia. Clin Exp Immunol 61: 118–124. [PMC free article] [PubMed] [Google Scholar]
- 64. Cohen LE, Tanner DJ, Schaefer HG, Levis WR (1984) Common and uncommon cutaneous findings in patients with ataxia-telangiectasia. J Am Acad Dermatol 10: 431–438. [DOI] [PubMed] [Google Scholar]
- 65. Nelson DL, Biddison WE, Shaw S (1983) Defective in vitro production of influenza virus-specific cytotoxic T lymphocytes in ataxia-telangiectasia. J Immunol 130: 2629–2634. [PubMed] [Google Scholar]
- 66. Fiorilli M, Businco L, Pandolci F, Paganelli R, Russo G, et al. (1983) Heterogeneity of immunological abnormalities in ataxia-telangiectasia. J Clin Immunol 3: 135–141. [DOI] [PubMed] [Google Scholar]
- 67. Waldmann TA, Broder S, Goldman CK, Frost K, Korsmeyer SJ, et al. (1983) Disorders of B cells and helper T cells in the pathogenesis of the immunoglobulin deficiency of patients with ataxia telangiectasia. J Clin Invest 71: 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bordigoni P, Faure G, Bene MC, Dardenne M, Bach JF, et al. (1982) Improvement of cellular immunity and IgA production in immunodeficient children after treatment with synthetic serum thymic factor (FTS). Lancet 2: 293–297. [DOI] [PubMed] [Google Scholar]
- 69. Mitsuya H, Matsukura M, Tomino S, Fujiwara H, Kishimoto S (1981) T-cell suppression of immunoglobulin synthesis in ataxia telangiectasia: restriction of suppressor activity to B cells from unrelated donors. Clin Immunol Immunopathol 19: 383–393. [DOI] [PubMed] [Google Scholar]
- 70. Rivat-Peran L, Buriot D, Salier JP, Rivat C, Dumitresco SM, et al. (1981) Immunoglobulins in ataxia-telangiectasia: evidence for IgG4 and IgA2 subclass deficiencies. Clin Immunol Immunopathol 20: 99–110. [DOI] [PubMed] [Google Scholar]
- 71. Otabor IA, Abdessalam SF, Erdman SH, Hammond S, Besner GE (2009) Gastric outlet obstruction due to adenocarcinoma in a patient with Ataxia-Telangiectasia syndrome: a case report and review of the literature. World J Surg Oncol 7: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Agamanolis DP, Greenstein JI (1979) Ataxia-telangiectasia. Report of a case with Lewy bodies and vascular abnormalities within cerebral tissue. J Neuropathol Exp Neurol 38: 475–489. [DOI] [PubMed] [Google Scholar]
- 73. Frais MA (1979) Gastric adenocarcinoma due to ataxia-telangiectasia (Louis-Bar syndrome). J Med Genet 16: 160–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Humphreys MW, Nevin NC, Wooldridge MA (1989) Cytogenetic investigations in a family with ataxia telangiectasia. Hum Genet 83: 79–82. [DOI] [PubMed] [Google Scholar]
- 75. Janic D, Dokmanovic L, Jovanovic N, Lazic J (2007) T-cell acute lymphoblastic leukemia in a child with ataxia-telangiectasia: case report. J Pediatr Hematol Oncol 29: 713–715. [DOI] [PubMed] [Google Scholar]
- 76. Sharma LM, Kashyap R, Gupta S, Bhargava M (2008) B-cell acute lymphoblastic leukemia in a child with ataxia telangiectasia. Pediatr Hematol Oncol 25: 473–476. [DOI] [PubMed] [Google Scholar]
- 77. Verhagen MMM, Abdo WF, Willemsen MAAP, Hogervorst FBL, Smeets DFCM, et al. (2009) Clinical spectrum of ataxia-telangiectasia in adulthood. Neurology 73: 430–437. [DOI] [PubMed] [Google Scholar]
- 78. Watanabe A, Hanazono H, Sogawa H, Takaya H (1977) Stomach cancer of a 14-year-old boy with ataxia-telangiectasia. Tohoku J Exp Med 121: 127–131. [DOI] [PubMed] [Google Scholar]
- 79. Ying KL, Decoteau WE (1981) Cytogenetic anomalies in a patient with ataxia, immune deficiency, and high alpha-fetoprotein in the absence of telangiectasia. Cancer Genet Cytogenet 4: 311–317. [DOI] [PubMed] [Google Scholar]
- 80. Huang L, Shyur S, Weng J, Shin-Chi, Tzen C, et al. (2005) Disseminated Bacille Calmette-Guérin disease as the initial presentation of X-linked severe combined immunodeficiency-a case report. Asian Pac J Allergy Immunol 23: 221–226. [PubMed] [Google Scholar]
- 81. Mustillo P, Bajwa RPS, Termuhlen AM, Nicol K, Scherzer R, et al. (2008) Tumor immune surveillance defect of X-linked severe combined immunodeficiency is not Epstein-Barr virus specific. Pediatr Blood Cancer 51: 706–709. [DOI] [PubMed] [Google Scholar]
- 82. Ginn SL, Smyth C, Wong M, Bennetts B, Rowe PB, et al. (2004) A novel splice-site mutation in the common gamma chain (gammac) gene IL2RG results in X-linked severe combined immunodeficiency with an atypical NK+ phenotype. Hum Mutat 23: 522–523. [DOI] [PubMed] [Google Scholar]
- 83. Dvorak CC, Sandford A, Fong A, Cowan MJ, George TI, et al. (2008) Maternal T-cell engraftment associated with severe hemophagocytosis of the bone marrow in untreated X-linked severe combined immunodeficiency. J Pediatr Hematol Oncol 30: 396–400. [DOI] [PubMed] [Google Scholar]
- 84. Trahair TN, Wainstein B, Manton N, Bourne AJ, Ziegler JB, et al. (2008) Rituximab for lymphoproliferative disease prior to haematopoietic stem cell transplantation for X-linked severe combined immunodeficiency. Pediatr Blood Cancer 50: 366–369. [DOI] [PubMed] [Google Scholar]
- 85. Kellermayer R, Hsu AP, Stankovics J, Balogh P, Hadzsiev K, et al. (2006) A novel IL2RG mutation associated with maternal T lymphocyte engraftment in a patient with severe combined immunodeficiency. J Hum Genet 51: 495–497. [DOI] [PubMed] [Google Scholar]
- 86. Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA (1968) Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 2: 1366–1369. [DOI] [PubMed] [Google Scholar]
- 87. Yount WJ, Utsinger PD, Whisnant J, Folds JD (1978) Lymphocyte subpopulations in X-linked severe combined immunodeficiency (SCID). Evidence against a stem cell defect. Transformation response to calcium inophore A23187. Am J Med 65: 847–854. [DOI] [PubMed] [Google Scholar]
- 88. Stephan V, Wahn V, Le Deist F, Dirksen U, Broker B, et al. (1996) Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N Engl J Med 335: 1563–1567. [DOI] [PubMed] [Google Scholar]
- 89. Gruber TA, Shah AJ, Hernandez M, Crooks GM, Abdel-Azim H, et al. (2009) Clinical and genetic heterogeneity in Omenn syndrome and severe combined immune deficiency. Pediatr Transplant 13: 244–250. [DOI] [PubMed] [Google Scholar]
- 90. Macchi P, Villa A, Giliani A, Sacco MG, Frattini A, et al. (1995) Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377: 65–68. [DOI] [PubMed] [Google Scholar]
- 91. Palmer K, Green TD, Roberts JL, Sajaroff E, Cooney M, et al. (2007) Unusual clinical and immunologic manifestations of transplacentally acquired maternal T cells in severe combined immunodeficiency. J Allergy Clin Immunol 120: 423–428. [DOI] [PubMed] [Google Scholar]
- 92. Roberts JL, Lengi A, Brown SM, Chen M, Zhou Y, et al. (2004) Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood 103: 2009–2018. [DOI] [PubMed] [Google Scholar]
- 93. Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, et al. (1995) Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270: 797–800. [DOI] [PubMed] [Google Scholar]
- 94. Mella P, Imberti L, Brugnoni D, Pirovano S, Candotti F, et al. (2000) Development of autologous T lymphocytes in two males with X-linked severe combined immune deficiency: molecular and cellular characterization. Clin Immunol 95: 39–50. [DOI] [PubMed] [Google Scholar]
- 95. Butte MJ, Haines C, Bonilla FA, Puck J (2007) IL-7 receptor deficient SCID with a unique intronic mutation and post-transplant autoimmunity due to chronic GVHD. Clin Immunol 125: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Puel A, Ziegler SF, Buckley RH, Leonard WJ (1998) Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet 20: 394–397. [DOI] [PubMed] [Google Scholar]
- 97. Giliani S, Mori L, de Saint Basile G, Le Deist F, Rodriguez-Perez C, et al. (2005) Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev 203: 110–126. [DOI] [PubMed] [Google Scholar]
- 98. Jo E, Kook H, Uchiyama T, Hakozaki I, Kim Y, et al. (2004) Characterization of a novel nonsense mutation in the interleukin-7 receptor alpha gene in a Korean patient with severe combined immunodeficiency. Int J Hematol 80: 332–335. [DOI] [PubMed] [Google Scholar]
- 99. Ponda P, Schuval SJ, Kaplan B, Logalbo P, Roberts JL, et al. (2006) Interleukin 7 receptor alpha-chain-mutation severe combined immunodeficiency without lymphopenia: correction with haploidentical T-cell-depleted bone marrow transplantation. Ann Allergy Asthma Immunol 97: 755–758. [DOI] [PubMed] [Google Scholar]
- 100. Giliani S, Bonfim C, de Saint Basile G, Lanzi G, Brousse N, et al. (2006) Omenn syndrome in an infant with IL7RA gene mutation. J Pediatr 148: 272–274. [DOI] [PubMed] [Google Scholar]
- 101. Pasic S, Djuricic S, Ristic R, Slavkovic B (2009) Recombinase-activating gene 1 immunodeficiency: different immunological phenotypes in three siblings. Acta Paediatr 98: 1062–1064. [DOI] [PubMed] [Google Scholar]
- 102. Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, et al. (2001) V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 97: 81–88. [DOI] [PubMed] [Google Scholar]
- 103. Ohm-Laursen L, Nielsen C, Fisker N, Lillevang ST, Barington T (2008) Lack of nonfunctional B-cell receptor rearrangements in a patient with normal B cell numbers despite partial RAG1 deficiency and atypical SCID/Omenn syndrome. J Clin Immunol 28: 588–592. [DOI] [PubMed] [Google Scholar]
- 104. Karaca NE, Aksu G, Genel F, Gulez N, Can S, et al. (2009) Diverse phenotypic and genotypic presentation of RAG1 mutations in two cases with SCID. Clin Exp Med 9: 339–342. [DOI] [PubMed] [Google Scholar]
- 105. Strauss KA, Puffenberger EG, Bunin N, Rider NL, Morton MC, et al. (2008) Clinical application of DNA microarrays: molecular diagnosis and HLA matching of an Amish child with severe combined immune deficiency. Clin Immunol 128: 31–38. [DOI] [PubMed] [Google Scholar]
- 106. de Villartay J, Lim A, Al-Mousa H, Dupont S, Déchanet-Merville J, et al. (2005) A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest 115: 3291–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, et al. (2005) A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest 115: 3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wada T, Toma T, Okamoto H, Kasahara Y, Koizumi S, et al. (2005) Oligoclonal expansion of T lymphocytes with multiple second-site mutations leads to Omenn syndrome in a patient with RAG1-deficient severe combined immunodeficiency. Blood 106: 2099–2101. [DOI] [PubMed] [Google Scholar]
- 109. Pirovano S, Mazzolari E, Pasic S, Albertini A, Notarangelo LD, et al. (2003) Impaired thymic output and restricted T-cell repertoire in two infants with immunodeficiency and early-onset generalized dermatitis. Immunol Lett 86: 93–97. [DOI] [PubMed] [Google Scholar]
- 110. Cossu F (2010) Genetics of SCID. Ital J Pediatr 36: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tabori U, Mark Z, Amariglio N, Etzioni A, Golan H, et al. (2004) Detection of RAG mutations and prenatal diagnosis in families presenting with either T-B- severe combined immunodeficiency or Omenn’s syndrome. Clin Genet 65: 322–326. [DOI] [PubMed] [Google Scholar]
- 112. Gaspar HB, Bjorkegren E, Parsley K, Gilmour KC, King D, et al. (2006) Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol Ther 14: 505–513. [DOI] [PubMed] [Google Scholar]
- 113. Hönig M, Albert MH, Schulz A, Sparber-Sauer M, Schütz C, et al. (2007) Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood 109: 3595–3602. [DOI] [PubMed] [Google Scholar]
- 114. Härtel C, Strunk T, Bucsky P, Schultz C (2004) Failure to thrive in a 14-month-old boy with lymphopenia and eosinophilia. Klin Padiatr 216: 24–25. [DOI] [PubMed] [Google Scholar]
- 115. Ozdemir O (2006) Severe combined immune deficiency in an adenosine deaminase-deficient patient. Allergy Asthma Proc 27: 172–174. [PubMed] [Google Scholar]
- 116. Ozsahin H, Arredondo-Vega FX, Santisteban I, Fuhrer H, Tuchschmid P, et al. (1997) Adenosine deaminase deficiency in adults. Blood 89: 2849–2855. [PubMed] [Google Scholar]
- 117. Onodera M, Ariga T, Kawamura N, Kobayashi I, Ohtsu M, et al. (1998) Successful peripheral T-lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood 91: 30–36. [PubMed] [Google Scholar]
- 118. Jiang C, Hong R, Horowitz SD, Kong X, Hirschhorn R (1997) An adenosine deaminase (ADA) allele contains two newly identified deleterious mutations (Y97C and L106V) that interact to abolish enzyme activity. Hum Mol Genet 6: 2271–2278. [DOI] [PubMed] [Google Scholar]
- 119. Hirschhorn R, Chakravarti V, Puck J, Douglas SD (1991) Homozygosity for a newly identified missense mutation in a patient with very severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID). Am J Hum Genet 49: 878–885. [PMC free article] [PubMed] [Google Scholar]
- 120. Hirschhorn R, Yang DR, Insel RA, Ballow M (1993) Severe combined immunodeficiency of reduced severity due to homozygosity for an adenosine deaminase missense mutation (Arg253Pro). Cell Immunol 152: 383–393. [DOI] [PubMed] [Google Scholar]
- 121. Weinberg K, Hershfield MS, Bastian J, Kohn K, Sender L, et al. (1993) T lymphocyte ontogeny in adenosine deaminase-deficient severe combined immune deficiency after treatment with polyethylene glycol-modified adenosine deaminase. J Clin Invest 92: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ochs HD, Buckley RH, Kobayashi RH, Kobayashi AL, Sorensen RU, et al. (1992) Antibody responses to bacteriophage phi X174 in patients with adenosine deaminase deficiency. Blood 80: 1163–1171. [PubMed] [Google Scholar]
- 123. Silber GM, Winkelstein JA, Moen RC, Horowitz SD, Trigg M, et al. (1987) Reconstitution of T- and B-cell function after T-lymphocyte-depleted haploidentical bone marrow transplantation in severe combined immunodeficiency due to adenosine deaminase deficiency. Clin Immunol Immunopathol 44: 317–320. [DOI] [PubMed] [Google Scholar]
- 124. Borzy MS, Ridgway D, Noya FJ, Shearer WT (1989) Successful bone marrow transplantation with split lymphoid chimerism in DiGeorge syndrome. J Clin Immunol 9: 386–392. [DOI] [PubMed] [Google Scholar]
- 125. Goldsobel AB, Haas A, Stiehm ER (1987) Bone marrow transplantation in DiGeorge syndrome. J Pediatr 111: 40–44. [DOI] [PubMed] [Google Scholar]
- 126. Janda A, Sedlacek P, Mejstrikova E, Zdrahalova K, Hrusak O, et al. (2007) Unrelated partially matched lymphocyte infusions in a patient with complete DiGeorge/CHARGE syndrome. Pediatr Transplant 11: 441–447. [DOI] [PubMed] [Google Scholar]
- 127. Sanka M, Tangsinmankong N, Loscalzo M, Sleasman JW, Dorsey MJ (2007) Complete DiGeorge syndrome associated with CHD7 mutation. J Allergy Clin Immunol 120: 952–954. [DOI] [PubMed] [Google Scholar]
- 128. Ramos JT, López-Laso E, Ruiz-Contreras J, Giancaspro E, Madero S (1999) B cell non-Hodgkin’s lymphoma in a girl with the DiGeorge anomaly. Arch Dis Child 81: 444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Al-Tamemi S, Mazer B, Mitchell D, Albuquerque P, Duncan AMV, et al. (2005) Complete DiGeorge anomaly in the absence of neonatal hypocalcemia and velofacial and cardiac defects. Pediatrics 116: e457–460. [DOI] [PubMed] [Google Scholar]
- 130. Collard HR, Boeck A, Mc Laughlin TM, Watson TJ, Schiff SE, et al. (1999) Possible extrathymic development of nonfunctional T cells in a patient with complete DiGeorge syndrome. Clin Immunol 91: 156–162. [DOI] [PubMed] [Google Scholar]
- 131. Markert ML, Kostyu DD, Ward FE, McLaughlin TM, Watson TJ, et al. (1997) Successful formation of a chimeric human thymus allograft following transplantation of cultured postnatal human thymus. J Immunol 158: 998–1005. [PubMed] [Google Scholar]
- 132. Markert ML, Hummell DS, Rosenblatt HM, Schiff SE, Harville TO, et al. (1998) Complete DiGeorge syndrome: persistence of profound immunodeficiency. J Pediatr 132: 15–21. [DOI] [PubMed] [Google Scholar]
- 133. Matsumoto T, Amamoto N, Kondoh T, Nakayama M, Takayanagi T, et al. (1998) Complete-type DiGeorge syndrome treated by bone marrow transplantation. Bone Marrow Transplant 22: 927–930. [DOI] [PubMed] [Google Scholar]