Abstract
Introduction
The epidemiology and antibiotic resistance of Staphylococcus aureus have evolved, underscoring the need for novel antibiotics, particularly against methicillin-resistant S. aureus (MRSA). Telavancin is a bactericidal lipoglycopeptide with potent activity against Gram-positive pathogens.
Objective
To systematically review and synthesize the available evidence from randomized controlled trials (RCTs) evaluating telavancin in the treatment of patients with infections due to Gram-positive organisms with the methodology of meta-analysis.
Results
Six RCTs comparing telavancin with vancomycin were included; 4 (2229 patients) referred to complicated skin and soft tissue infections (cSSTIs) and 2 (1503 patients) to hospital-acquired pneumonia (HAP). Regarding cSSTIs, telavancin and vancomycin showed comparable efficacy in clinically evaluable patients (odds ratio [OR] = 1.10 [95% confidence intervals: 0.82–1.48]). Among patients with MRSA infection, telavancin showed higher eradication rates (OR = 1.71 [1.08–2.70]) and a trend towards better clinical response (OR = 1.55 [0.93–2.58]). Regarding HAP, telavancin was non-inferior to vancomycin in terms of clinical response in two Phase III RCTs; mortality rates for the pooled trials were comparable with telavancin (20%) and vancomycin (18.6%). Pooled data from cSSTIs and HAP studies on telavancin 10 mg/kg indicated higher rates of serum creatinine increases (OR = 2.22 [1.38–3.57]), serious adverse events (OR = 1.53 [1.05–2.24]), and adverse event-related withdrawals (OR = 1.49 [1.14–1.95]) among telavancin recipients.
Conclusion
Telavancin might be an alternative to vancomycin in cases of difficult-to-treat MRSA infections. The potent antistaphylococcal activity of telavancin should be weighted against the potential for nephrotoxicity.
Introduction
Antimicrobial resistance of Staphylococcus aureus poses a major threat to public health [1]. In 2005, methicillin-resistant S. aureus (MRSA) in the United States (US) accounted for 48% and 60% of S. aureus isolates from outpatients and inpatients, respectively [2]. Strains of vancomycin-intermediate S. aureus (VISA), heteroresistant VISA (hVISA) and vancomycin-resistant S. aureus (VRSA) have also emerged leading to an increase in vancomycin treatment failures [3]–[8], while newer antibiotics have not been superior to vancomycin in double-blind randomized controlled trials (RCTs) [9]. This shift in S. aureus susceptibility, along with an increase in community-acquired MRSA infections [10], underscore the need for novel antimicrobial agents with potent antistaphylococcal activity [11].
Telavancin, a semisynthetic derivative of vancomycin (VAN), is a member of the lipoglycopeptide class of antibiotics. It is a bactericidal concentration-dependent antibiotic with a dual mechanism of action, involving inhibition of cell wall synthesis and disruption of membrane integrity [12]. Telavancin exhibits potent in vitro activity against a variety of Gram-positive organisms, including S. aureus (MRSA, hVISA, VISA), coagulase-negative staphylococci and Streptococcus spp. It is also active against VAN-susceptible and VanB VAN-resistant enterococci, as well as various Gram-positive anaerobic organisms [13]–[15]. In the US and Canada, telavancin is approved for the treatment of patients with complicated skin and soft tissue infections (cSSTIs). In Europe, this drug has been approved for hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), caused by MRSA, when other alternatives are not suitable [16].
In this study, we systematically reviewed the available clinical and microbiologic outcomes of RCTs that compared the efficacy and safety of telavancin with that of other antibiotics, and pooled them, if possible, in a meta-analytic framework.
Methods
Data sources
PubMed, Scopus, Cochrane Central Register of Controlled Trials (Central) and LILACS databases were searched for relevant studies published up to March 2012. The search term applied was “telavancin” or “TD-6424”. Abstracts from the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (2005–2011) and the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (2003–2012), as well as references of relevant articles, were hand-searched. No restrictions in the language or year of publication were imposed.
Study selection
Three investigators (KAP, MNM and MCM) independently searched the literature and examined relevant studies for potential inclusion in this meta-analysis. To be considered eligible, a study should be an RCT examining the efficacy or safety of telavancin compared to any other antibiotic regimen for the treatment of patients with any type of infection. Animal studies and pharmacokinetic or pharmacodynamic studies were excluded. Additional antimicrobial agents (mainly those with effectiveness against Gram-negative pathogens involved in polymicrobial infections) could be used in the RCTs.
Data extraction
Three reviewers (KAP, MNM and MCM) independently extracted the following data from each study: year of publication; study design; study population; number of patients (intention to treat [ITT], clinically evaluable [CE], microbiologically evaluable [ME], ME with S. aureus infection [ME-S. aureus], and ME with MRSA infection [ME-MRSA]); antimicrobial agents and doses used; clinical and microbiologic outcomes; and data on safety. Any disagreement was resolved by consensus in meetings that involved all authors. When trial data was not fully available, we contacted the corresponding authors asking for additional information or used conference reports and data reported to the US Food and Drug Administration and European Medicines Agency.
The ITT population comprised patients who received at least one dose of the study medication. The CE patients were those who met all inclusion and exclusion criteria and had a clinical response of either cure or failure; patients were excluded from this population if they had only a Gram-negative pathogen or a polymicrobial infection including a Gram-negative isolate resistant to aztreonam. The ME population was a subset of patients in the CE population with a Gram-positive isolate at baseline.
The quality of the included RCTs was evaluated with a modified Jadad score, which considers randomization, generation of random numbers, allocation concealment, details of double-blinding procedure and information on withdrawals [17]. One point was awarded for the specification of each criterion, with a maximum score of 5. A score higher than 2 points was used to denote a trial of good methodological quality.
Analyzed outcomes
The primary outcome measures for this meta-analysis were treatment success (as defined in individual studies) in the ITT and CE populations, clinically significant increase in serum creatinine (defined as serum creatinine level ≥1.5 mg/dl at the end-of-therapy visit with an increase of at least 50% or 0.5 mg/dl over the pretreatment level), and all-cause mortality. Secondary outcomes comprised clinical success in ME-S. aureus and ME-MRSA patients, microbiologic eradication, time to clinical improvement or cure, overall adverse events (AE), serious AE [18], and AE-related withdrawals.
Data analysis and statistical methods
Statistical analyses were performed using Review Manager [19]. Pooled odds ratios (OR) and 95% confidence intervals (95% CI) for all primary and secondary outcomes were calculated by use of the Mantel-Haenszel fixed effect (FEM) and random effects model (REM) as appropriate [20], [21]. Results from the FEM were presented only when there was no heterogeneity between studies; otherwise, results from the REM were presented. Heterogeneity was assessed by using both χ2 and I2 tests; p<0.1 (χ2) or I2≥40% were considered to denote significant heterogeneity. Publication bias was not assessed due to the small number of the included studies [22].
Results
Included studies and their main characteristics
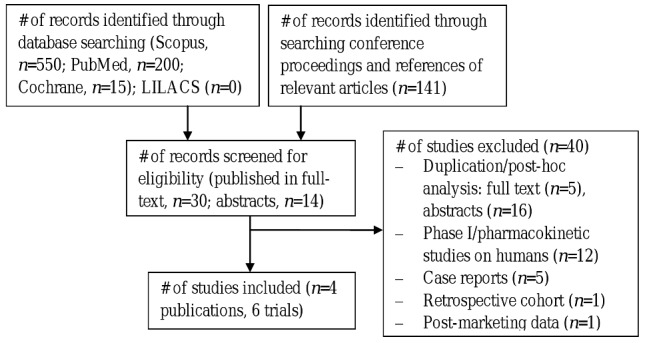
The study flow chart is presented in Figure 1. The database search generated a total of 765 records, whereas hand-searching of conference proceedings identified 141 additional records. After removing duplicates only 44 records were screened for eligibility, of which 40 were excluded. Overall, four publications describing 6 RCTs comparing telavancin with standard therapy were included in the systematic review [23]–[26]. Additional information was extracted from 2 abstracts [27], [28].
Figure 1. Flow diagram of the selection process of the included studies.
The main characteristics of the analyzed studies are shown in Table 1. All 6 RCTs were sponsored by the pharmaceutical industry and were multicenter double-blind trials (Jadad score ≥4) evaluating adult men or non-pregnant women with cSSTIs (2229 subjects) [23]–[25] or HAP (1503 subjects) [26] due to suspected or confirmed Gram-positive pathogens. Regarding cSSTIs, two Phase II (FAST) trials compared telavancin IV at 7.5 mg/kg/24 h or 10 mg/kg/24 h with standard therapy (vancomycin or antistaphylococcal penicillin, chosen prior to randomization) [23], [24], whereas two Phase III (ATLAS) non-inferiority trials compared telavancin IV at10 mg/kg/24 h with vancomycin [25]. Regarding HAP, two Phase III (ATTAIN) non-inferiority trials compared telavancin IV at 10 mg/kg/24 h with vancomycin [26]. The dose of vancomycin could be individually adjusted and could be switched to an antistaphylococcal β-lactam in case of infection due to methicillin-susceptible S. aureus [23]–[28].
Table 1. The main characteristics of the included studies.
| Study [ref] | Study design (Jadad score) | Participants | Comparatora | Populations (pts) | Notes |
| FAST 1 [23] | MC DB Phase II RCT (4) | Men or non-pregnant women with cSSTIs (abscess, 48%; cellulitis, 37%; wound infection, 11%); mean age (yr): 44/44; US, South Africa, 2003–2004 | VAN iv 1 g q12h: 76%; antistaph. PCNb iv q6h: 24% | ITT: 84 vs 83; ITT-S. aureus: 50 vs 52; ITT-MRSA: 22 vs 26; CE: 72 vs 69; ME: 56 vs 56 | CrCL<50 ml/min, QTcF>470 ms = exclusion; AZT: 35% vs 29%; MET: 24 vs 25% |
| FAST 2 [24] | MC DB Phase II RCT (4) | Men or non-pregnant women with cSSTIs (abscess, 58%; cellulitis, 29%; wound infection, 11%); mean age (yr): 45/42; US, South Africa, 2004 | VAN iv 1 g q12h: 93%; antistaph. PCNb iv q6h: 7% | ITT: 100 vs 95; CE: 77 vs 77; ME: 64 vs 57; ME-S. aureus: 50 vs 41; ME-MRSA: 26 vs 19 | QTcF>500 ms = exclusion; AZT: 41% vs 37%; MET: 44% vs 38% |
| ATLAS 1 [25], [27] | MC DB Phase III RCT (5) | Men or non-pregnant women with cSSTIs (abscess, 44%; cellulitis, 37%; wound infection, 15%); mean age (yr): 49/48; multinational, 2005–2006 | VAN iv 1 g q12h | ITT: 426 vs 429; CE: 346 vs 349; ME: 237 vs 255; ME-MRSA: 116 vs 138 | Prespecified pooled analysis design; QTcF>500 ms = exclusion; AZT: 32% vs 33%; MET: 23% vs 22% |
| ATLAS 2 [25], [28] | MC DB Phase III RCT (5) | Men or non-pregnant women with cSSTIs (abscess, 41%; cellulitis, 37%; wound infection, 13%); mean age (yr): 49/50; multinational, 2005–2006 | VAN iv 1 g q12h | ITT: 502 vs 510; CE: 399 vs 395; ME: 290 vs 281; ME-MRSA: 162 vs 163 | |
| ATTAIN 1 [26] | MC DB Phase III RCT (5) | Men or non-pregnant women with HAP; mean age (yr): 62/63; multinational, 2005–2007 | VAN iv 1 g q12h | ITT: 372 vs 374; CE: 141 vs 172; ME-S. aureus: 98 vs 109; ME-MRSA: 70 vs 84 | Prespecified pooled analysis design; QTcF>500 ms = exclusion; AZT: 60%; PIP-TAZ: 21%; MET: 23% |
| ATTAIN 2 [26] | MC DB Phase III RCT (5) | VAN iv 1 g q12h | ITT: 377 vs 380; CE: 171 vs 170; ME-S. aureus: 121 vs 105; ME-MRSA: 69 vs 70 |
Abbreviations: ref = reference; MC = multicenter; DB = double-blinded; vs = versus; RCT = randomized controlled trial; TLV = telavancin; VAN = vancomycin; PCN = penicillin; AZT = aztreonam; MET = metronidazole; PIP-TAZ = piperacillin-tazobactam; yr = years; q12h = every 12 hours; pts = patients; CrCL = creatinine clearance; QTcF = Fridericia-corrected QT; iv = intravenous; cSSTI = complicated skinn and soft tissue infection; HAP = hospital acquired-pneumonia; ITT = intention-to-treat; CE = clinically evaluable; ME = microbiologically evaluable.
All studies used telavancin iv at 10 mg/kg q24h, except for FAST 1 (7.5 mg/kg q24h).
Nafcillin 2 g or oxacillin 2 g or cloxacillin 0.5–1 g.
Regarding cSSTIs, 1112 patients received telavancin, whereas 1117 patients received standard therapy (vancomycin [1090/1117] or antistaphylococcal β-lactam [27/1117]) for 4–14 days. Among 1678 patients with a baseline pathogen, S. aureus was isolated in 1353 (81%) subjects, of which 818 (60%) had MRSA infection. Mixed infection (≥2 isolated bacteria) was present in 16% of the ITT populations [23]–[25]. Regarding HAP, 749 and 754 patients received telavancin and vancomycin for 7–21 days, respectively. Among 1089 patients with a baseline pathogen, S. aureus was isolated in about 66% of subjects, of which two-thirds had MRSA infection. Infection due to both Gram-positive and Gram-negative bacteria was documented in 27% of patients [26].
Efficacy in complicated skin and soft tissue infections
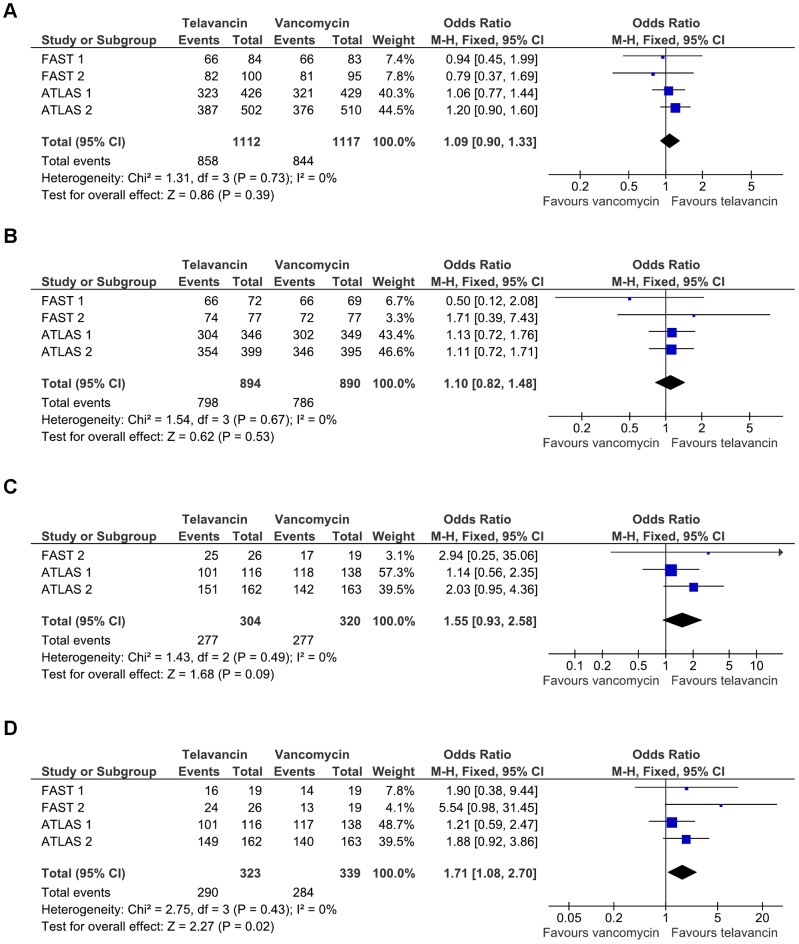
All 4 trials on cSSTIs reported data on clinical response regarding both the ITT and CE populations (Table 2). There was no significant difference regarding clinical success between telavancin and vancomycin in the ITT (2229 patients, 77% vs 76%; OR 1.09 [95% CI 0.90–1.33], FEM; 4 RCTs; figure 2A) or the CE population (1784 patients, 89% vs 88%; OR 1.10 [0.82–1.48], FEM; 4 RCTs; figure 2B) [23]–[25], [27], [28]. When only studies using telavancin at the recommended dose (10 mg/kg) were pooled, similar results were found for the above-mentioned endpoints (data not shown). Clinical response in ME patients with S. aureus infection was comparable between telavancin and vancomycin (1027 patients, 90% vs 87%; OR 1.38 [0.93–2.04], FEM; 3 RCTs). However, among subjects with MRSA infection, telavancin was associated with a trend towards higher cure rates (624 patients, 91% vs 87%; OR 1.55 [0.93–2.58], FEM; 3 RCTs; figure 2C) [24], [25], [27], [28].
Table 2. Efficacy and safety.
| Study ID | Clinical response, n/N (%) | Microbiologic eradication, n/N (%) | Adverse events, n/N (%) |
| FAST 1 | ITT: 66/84 (79) vs 66/83 (80); ITT-S. aureus: 40/50 (80) vs 40/52 (77); ITT-MRSA: 18/22 (82) vs 18/26 (69); CE: 66/72 (92) vs 66/69 (96); ME: 52/56 (93) vs 53/56 (95) | Total: 44/56 (79) vs 46/56 (82); MRSA: 16/19 (84) vs 14/19 (74) | TEAE: 47/84 (56) vs 50/83 (60); SAE: 4/84 (5) vs 9/83 (11); withdrawals: 5/84 (6) vs 4/83 (5); Cr elevationa: 7/84 (8) vs 2/83 (2); mortality: NR |
| FAST 2 | ITT: 82/100 (82) vs 81/95 (85); CE: 74/77 (96) vs 72/77 (94); ME: 62/64 (97) vs 53/57 (93); ME-S. aureus: 48/50 (96) vs 37/41 (90); ME-MRSA: 25/26 (96) vs 17/19 (89) | Total: 60/64 (94) vs 47/57 (82); S. aureus: 46/50 (92) vs 32/41 (78); MRSA: 24/26 (92) vs 13/19 (68) | TEAE: 56/100 (56) vs 54/95 (57); SAE: 7/100 (7) vs 3/95 (3); withdrawals: 6/100 (6) vs 3/95 (3); Cr elevationb: 3/100 (3) vs 0/95 (0); mortality: 0/100 (0) vs 1/95 (1) |
| ATLAS 1 | ITT: 323/426 (76) vs 321/429 (75); CE: 304/346 (88) vs 302/349 (87); ME: NR; ME-S. aureusc: 412/459 (90) vs 414/477 (87); ME-MRSA: 101/116 (87) vs 118/138 (86) | Total: 212/237 (89) vs 219/255 (86); S. aureusc: 411/459 (90) vs 414/477 (87); MRSA: 101/116 (87) vs 117/138 (85) | TEAE: 358/426 (84) vs 335/429 (78); SAE: 31/426 (7) vs 27/429 (6); withdrawalsc: 73/929 (8) vs 53/938 (6); Cr elevationb , c: 52/822 (6) vs 19/856 (2); mortality: 5/426 (1) vs 5/429 (1) |
| ATLAS 2 | ITT: 387/502 (77) vs 376/510 (74); CE: 354/399 (89) vs 346/395 (88); ME-MRSA: 151/162 (93) vs 142/163 (87) | Total: 261/290 (90) vs 249/281 (89); MRSA: 149/162 (92) vs 140/163 (86) | TEAE: 377/503 (75) vs 341/509 (67); SAE: 38/503 (8) vs 15/509 (3); mortality: 3/503 (0.6) vs 3/509 (0.6) |
| ATTAIN 1 | ITT: 214/372 (58) vs 221/374 (59); CE: 118/141 (84) vs 138/172 (80); MEd: 192/243 (79) vs 182/237 (77); ME-S. aureus: 80/98 (82) vs 81/109 (74); ME-MRSA: 57/70 (81) vs 63/84 (75) | Total: 86/108 (79.6) vs 85/113 (75.2) | TEAEd: 616/751 (82) vs 613/752 (82); SAEd: 234/751 (31) vs 197/752 (26); withdrawalsd: 60/751 (8) vs 40/752 (5); Cr elevationb , d: 111/716 (16) vs 69/723 (10); mortality: 80/372 (22) vs 62/374 (17) |
| ATTAIN 2 | ITT: 227/377 (60) vs 228/380 (60); CE: 139/171 (81) vs 138/170 (81); ME-S. aureus: 91/121 (75) vs 80/105 (76); ME-MRSA: 47/69 (68) vs 52/70 (74) | Total: 103/135 (76.3) vs 96/124 (77.4) | Mortality: 70/379 (18) vs 78/378 (21) |
Abbreviations: ITT = intention-to-treat; CE = clinically evaluable; ME = microbiologically evaluable; TEAE = treatment-emergent adverse events; SAE = serious adverse events; Cr = creatinine.
Compared to baseline.
Serum creatinine level ≥1.5 mg/dl at the end-of-therapy visit with an increase of at least 50% or 0.5 mg/dl over the pretreatment level.
Pooled data from both ATLAS studies.
Pooled data from both ATTAIN studies.
Figure 2. Odds ratios of clinical and microbiologic outcomes with telavancin versus vancomycin in cSSTIs.
Panel A: Clinical response in the intention-to-treat population. Panel B: Clinical response in the clinically evaluable population. Panel C: Clinical response in the microbiologically evaluable population with MRSA infection. Panel D: MRSA eradication.
Overall microbiologic eradication rates were similar between telavancin and standard therapy (1296 patients, 89% vs 86%; OR 1.29 [0.92–1.80], FEM; 4 RCTs), whereas those for MRSA were significantly higher with telavancin (662 strains, 90% vs 84%; OR 1.71 [1.08–2.70], FEM; 4 RCTs; figure 2D) [23]–[25], [27], [28]. Median duration of treatment was identical between treatment groups in FAST 1 study (7 days) [23], whereas in ATLAS studies it was 1 day shorter in the telavancin group (10 vs 11, and 8 vs 9 days) [25].
Efficacy in hospital-acquired pneumonia
Two methodologically identical RCTs compared telavancin to vancomycin in the setting of HAP, using a non-inferiority margin of 20%. Telavancin proved to be non-inferior to vancomycin on the basis of clinical response in each trial. The prespecified pooled analysis of these trials failed to show superiority of telavancin in patients with MRSA infection (293 patients, 74.8% vs 74.7%). Mortality rates with telavancin versus vancomycin were 22% versus 17% (95% CI of the difference [−0.7–10.6]) in the first trial, and 18% versus 21% (95% CI of the difference [−7.8–3.5]) in the second trial. Subgroup analyses showed higher cure rates with telavancin in patients with monomicrobial S. aureus infection (298 patients, 84.2% vs 74.3%) or pneumonia caused by S. aureus with vancomycin MIC ≥1 µg/ml (190 patients, 87% vs 74%). The median duration of treatment was comparable between treatment groups in each trial (9–10 days) [26].
Safety and tolerability
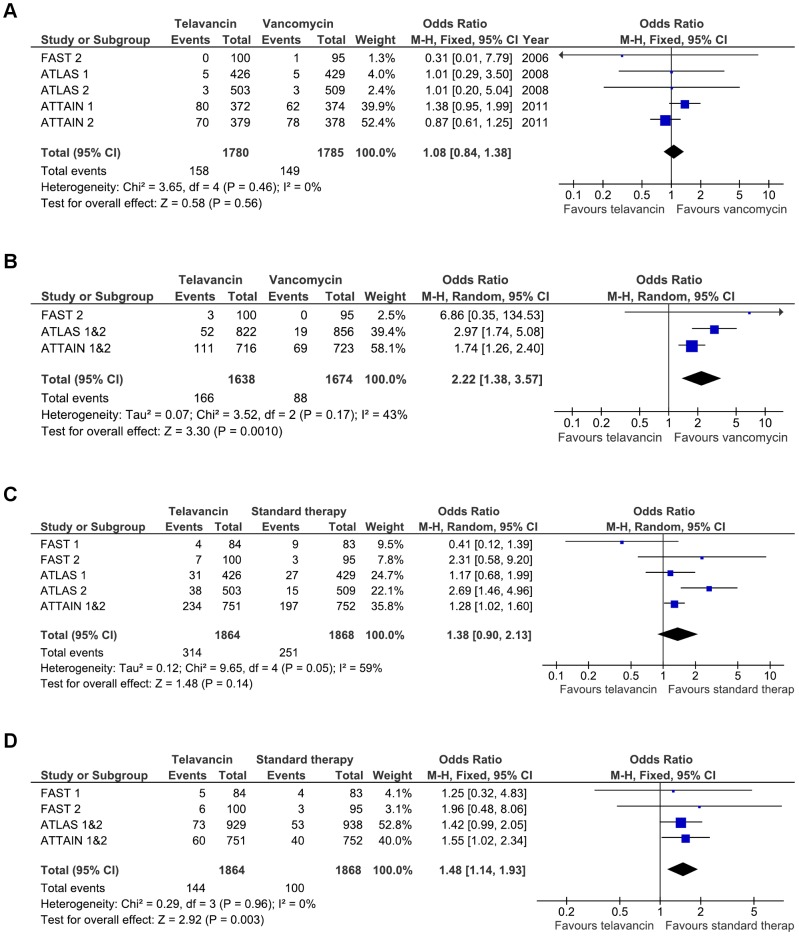
The safety analysis was based on synthesis of trial data from both cSSTIs and HAP (Table 3). Overall, mortality rates were comparable between telavancin and vancomycin (3565 patients, 8.9% vs 8.3%; OR 1.08 [0.84–1.38], FEM; 5 RCTs; figure 3A) [23]–[28]. Clinically significant increases in serum creatinine were more frequently observed with telavancin compared to vancomycin (3312 patients, 10% vs 5%; OR 2.22 [1.38–3.57], REM; 5 RCTs; figure 3B) [24]–[28]. Overall AE (3732 patients, 78% vs 75%; OR 1.20 [0.97–1.49], REM; 6 RCTs) showed a trend towards higher rates with telavancin, whereas serious AE (3732 patients, 17% vs 13%; OR 1.38 [0.90–2.13], REM; 6 RCTs; figure 3C) were comparable between telavancin and vancomycin recipients. AE-related withdrawals were more common in the telavancin group (3732 patients, 8% vs 5%; OR 1.48 [1.14–1.93], FEM; 6 RCTs; figure 3D) [23]–[28]. When only studies using telavancin 10 mg/kg were analyzed, telavancin recipients also showed higher rates of overall AE (3565 patients, 79% vs 75%; OR 1.25 [1.01–1.55], REM; 5 RCTs) and serious AE (3565 patients, 17% vs 14%; OR 1.53 [1.05–2.24], REM; 5 RCTs) [24]–[28].
Table 3. Adverse events and laboratory abnormalities for pooled cSSTIs and HAP studies.a .
| AE, n/N (%) | Telavancin | Vancomycin | OR (95% CI) |
| Overall AE | 1454/1864 (78) | 1393/1868 (74.6) | 1.20 (0.97–1.49) |
| Serious AE | 314/1864 (16.8) | 251/1868 (13.4) | 1.38 (0.90–2.13) |
| Withdrawals | 144/1864 (7.7) | 100/1868 (5.4) | 1.48 (1.14–1.93) |
| Nausea | 318/1864 (17.1) | 190/1868 (10.2) | 1.88 (1.54–2.29) |
| Vomiting | 143/1113 (12.8) | 78/1116 (7) | 1.97 (1.47–2.63) |
| Taste disturbance | 325/1029 (31.6) | 62/1033 (6) | 7.37 (5.52–9.85) |
| Diarrhoea | 73/1029 (7.1) | 81/1033 (7.8) | 0.90 (0.65–1.25) |
| Constipation | 174/1864 (9.3) | 144/1868 (7.7) | 1.12 (0.72–1.74) |
| Insomnia | 137/1780 (7.7) | 136/1785 (7.6) | 1.14 (0.62–2.11) |
| Pruritus | 34/1029 (3.3) | 68/1033 (6.6) | 0.48 (0.32–0.74) |
| Headache | 147/1113 (13.2) | 132/1116 (11.8) | 1.14 (0.89–1.47) |
| Chills | 47/1029 (4.6) | 23/1033 (2.2) | 2.10 (1.27–3.48) |
| Cr elevation | 166/1638 (10.1) | 88/1674 (5.3) | 2.22 (1.38–3.57) |
| Hypokalemia | 73/1528 (4.8) | 44/1521 (2.9) | 1.91 (0.91–4.00) |
| AST increase | 36/1045 (3.4) | 39/1084 (3.6) | 0.93 (0.43–2.04) |
| ALT increase | 38/1101 (3.5) | 61/1165 (5.2) | 0.64 (0.42–0.97) |
| QTcF increaseb | 59/1560 (3.8) | 49/1578 (3.1) | 1.24 (0.84–1.83) |
| Anemia | 66/1052 (6.3) | 65/1058 (6.1) | 1.01 (0.71–1.46) |
| Leukopenia | 12/1006 (1.2) | 19/989 (1.9) | 0.62 (0.30–1.28) |
| Platelet decreasec | 8/1064 (0.8) | 10/1110 (0.9) | 0.87 (0.35–2.17) |
The FAST 1 study is included in the analysis.
>60 ms.
<75×109/L.
Figure 3. Odds ratios of adverse events with telavancin versus vancomycin in cSSTIs and HAP.
Panel A: All-cause mortality. Panel B: Elevation in serum creatinine concentration. Panel C: Serious adverse events. Panel D: Adverse-event related withdrawals.
Discussion
Telavancin was non-inferior to vancomycin in the treatment of patients with cSSTIs and HAP in large Phase III RCTs [25]–[28]. Our meta-analysis indicated that telavancin was associated with significantly higher eradication rates and a trend towards better clinical response among patients with MRSA skin and soft tissue infections. All-cause mortality was similar between telavancin- and vancomycin-treated patients with cSSTIs or HAP. On the other hand, telavancin at the recommended dose was associated with higher rates of treatment-emergent adverse events, serious adverse events, adverse event-related withdrawals, and elevations in serum creatinine.
Regarding cSSTIs, our analysis found a trend towards higher cure rates with telavancin compared to standard therapy in patients with MRSA infections; microbiologic eradication was also higher with telavancin among these patients. It should be noted that the RCTs excluded patients with chronic diabetic foot, necrotizing fasciitis or osteomyelitis [23]–[25]. The more potent and rapidly bactericidal activity of telavancin against S. aureus compared to vancomycin may be an explanation for the better performance of this lipoglycopeptide antibiotic [29], [30]. The MIC90 values of telavancin against MRSA (0.25–0.5 µg/ml) in the cSSTIs trials were two- to four-fold lower than those of vancomycin (1 µg/ml) [23]–[25], [31]. A multiple-comparison meta-analysis also found higher success rates with telavancin, dalbavancin or linezolid compared to vancomycin in MRSA cSSTIs [32].
A number of post-hoc analyses of the ATLAS studies on cSSTIs have been performed [33]–[35]. Stryjewski et al. reported similar clinical response rates in patients with different types of cSSTIs (major abscess, infective cellulitis, wound infection), including infections caused by MRSA [33]. Clinical efficacy was also similar with telavancin or vancomycin in both obese and non-obese patients [34]. In addition, a recent post-hoc analysis addressing the new FDA guidance for cSSTIs found that telavancin and vancomycin demonstrated comparable efficacy in patients with baseline lesion ≥75 cm2 [35], [36]. Despite the limitations of post-hoc analyses, the consistency of their findings indicates that telavancin is effective in the treatment of cSSTIs and could be an alternative to vancomycin for MRSA infections.
Regarding HAP, clinical cure rates were similar between telavancin and vancomycin. The 20% non-inferiority margin of the ATTAIN studies is controversial; nevertheless, the lower bound of the 95% CI around the difference between treatments in clinical response exceeded −10% for both ITT and CE patients. The pooled mortality was comparable between treatment groups. However, an individual trial indicated a trend towards higher mortality among telavancin-recipients. It is not clear whether this finding is an issue of efficacy or safety of telavancin and warrants further investigation [16], [26]. Regarding VAP, post-hoc analyses showed that clinical response with telavancin and vancomycin was not significantly different in the ITT (427 patients, 49% vs 53%), CE (135 patients, 80% vs 66%) and ME (118 patients, 78% vs 61%) populations [37]. Of note, recent studies suggest that linezolid is superior to vancomycin in terms of clinical response in nosocomial pneumonia [38], [39]. In this regard, the use of telavancin in HAP may be considered in cases of infection due to Gram-positive organisms that are resistant to common antimicrobial agents.
An issue regarding ATTAIN studies that should be mentioned is the fact that 34% of patients with available relevant data had trough vancomycin levels <10 µg/ml [26]. Although firm prospective data are not available, current guidelines suggest that trough levels of vancomycin be 15–20 µg/ml [40], [41]. In this regard, the trials' investigators reported that, in multiple subgroup analyses stratifying patients by vancomycin serum trough level, they found lower clinical response rates and higher mortality and nephrotoxicity rates in the highest trough group [42]. In addition, it has been suggested that vancomycin MIC values in the higher levels of the susceptible range (1–2 µg/ml) are associated with adverse clinical outcomes [43]; in post-hoc analysis of the ATTAIN studies, telavancin was associated with higher clinical success rates than vancomycin among patients infected by isolates with vancomycin MIC ≥1 µg/ml [16], [26]. The role of telavancin in the treatment of patients with infections by S. aureus isolates with “higher” vancomycin MIC warrants further investigation.
The analyzed data raise concerns regarding the safety profile of telavancin. The most commonly reported AE in the telavancin group were foamy urine, nausea, vomiting, taste disturbance and headache; most of them were mild to moderate in intensity and reversible [23]–[26]. In our analysis, telavancin was associated with higher rates of serious AE (17%) and AE-related withdrawals (8%). Most common serious AE in the telavancin group were renal AE (including acute renal failure, increased serum creatinine and renal insufficiency), anaemia, sepsis and multi-organ failure. The most common AE that led to discontinuation of telavancin therapy were renal AE, nausea and vomiting [37], [44].
In addition, elevation in serum creatinine concentration was more frequently observed with telavancin; the renal impairment was generally reversible [23]–[26]. A recent retrospective cohort study found that 7 of 21 patients (33%) developed acute renal insufficiency after a median time of 9 days on telavancin. Most of these patients had comorbidities and received telavancin for non-approved indications [45]. Therefore, monitoring of serum creatinine concentration seems warranted, and the administration of telavancin in patients with risk factors for renal AE should be based on a benefit-risk approach. Telavancin also has an effect on cardiac repolarization. An early study on healthy volunteers found a placebo-corrected mean change in QTcF 4.5 ms with telavancin 15 mg/kg/24 h for three days [46]. In the cSSTIs and HAP trials, respectively, the mean change from baseline in QTcF was 7 ms and 4 ms longer in the telavancin group [44]. However, no cardiac AEs associated with QT prolongation were reported [23]–[26].
Our study has specific limitations that need to be considered in the interpretation of our findings. First, only a few studies have been conducted on this issue and the Phase III trials are those that mostly influenced our results; however, the pooled sample size is relatively large (2229 patients with cSSTIs and 1503 patients with HAP) [23]–[26]. Furthermore, one of the analyzed studies was a Phase II trial evaluating telavancin at a lower than the recommended dose; however, there was no difference in our results when it was excluded from analysis [23].
In conclusion, telavancin is a potent antimicrobial agent against Gram-positive infections that proved to be non-inferior to vancomycin in the treatment of patients with cSSTIs or HAP, and might be a therapeutic alternative in cases of difficult-to-treat MRSA infections. However, the potential for nephrotoxicity of this antibiotic should be taken under consideration. Additional randomized controlled trials, optimized to assess mortality, are warranted to better clarify the role of telavancin in the treatment of patients with hospital-acquired pneumonia.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sakoulas G, Moellering RC Jr (2008) Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 46 Suppl 5: S360–367. [DOI] [PubMed] [Google Scholar]
- 2. Styers D, Sheehan DJ, Hogan P, Sahm DF (2006) Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, et al. (1997) Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40: 135–136. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (2002) Staphylococcus aureus resistant to vancomycin–United States, 2002. MMWR Morb Mortal Wkly Rep 51: 565–567. [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (2002) Vancomycin-resistant Staphylococcus aureus–Pennsylvania, 2002. MMWR Morb Mortal Wkly Rep 51: 902. [PubMed] [Google Scholar]
- 6. Center for Disease Control and Prevention (2004) Vancomycin-resistant Staphylococcus aureus–New York, 2004. MMWR Morb Mortal Wkly Rep 53: 322–323. [PubMed] [Google Scholar]
- 7. Wang G, Hindler JF, Ward KW, Bruckner DA (2006) Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 44: 3883–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML (2004) Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 38: 448–451. [DOI] [PubMed] [Google Scholar]
- 9. Vardakas KZ, Mavros MN, Roussos N, Falagas ME (2012) Meta-analysis of randomized controlled trials of vancomycin for the treatment of patients with gram-positive infections: focus on the study design. Mayo Clin Proc 87: 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stryjewski ME, Chambers HF (2008) Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46 Suppl 5: S368–377. [DOI] [PubMed] [Google Scholar]
- 11. Eckmann C, Dryden M (2010) Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res 15: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins DL, Chang R, Debabov DV, Leung J, Wu T, et al. (2005) Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 49: 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang MH, Kish TD, Fung HB (2010) Telavancin: a lipoglycopeptide antimicrobial for the treatment of complicated skin and skin structure infections caused by gram-positive bacteria in adults. Clin Ther 32: 2160–2185. [DOI] [PubMed] [Google Scholar]
- 14. Pfaller MA, Mendes RE, Sader HS, Jones RN (2010) Telavancin activity against Gram-positive bacteria isolated from respiratory tract specimens of patients with nosocomial pneumonia. J Antimicrob Chemother 65: 2396–2404. [DOI] [PubMed] [Google Scholar]
- 15. Mendes RE, Sader HS, Farrell DJ, Jones RN (2011) Update on the telavancin activity tested against European staphylococcal clinical isolates (2009–2010). Diagn Microbiol Infect Dis 71: 93–97. [DOI] [PubMed] [Google Scholar]
- 16. Rubinstein E, Corey GR, Stryjewski ME, Kanafani ZA (2011) Telavancin for the treatment of serious gram-positive infections, including hospital acquired pneumonia. Expert Opin Pharmacother 12: 2737–2750. [DOI] [PubMed] [Google Scholar]
- 17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services, Food and Drug Administration (2005) Reviewer guidance: conducting a clinical safety review of a new product application and preparing a report on the review. Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072974.pdf. Accessed 2012 July 16.
- 19.Review Manager (RevMan) 5.1 ed: Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- 20. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stryjewski ME, O'Riordan WD, Lau WK, Pien FD, Dunbar LM, et al. (2005) Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis 40: 1601–1607. [DOI] [PubMed] [Google Scholar]
- 24. Stryjewski ME, Chu VH, O'Riordan WD, Warren BL, Dunbar LM, et al. (2006) Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother 50: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, et al. (2008) Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis 46: 1683–1693. [DOI] [PubMed] [Google Scholar]
- 26. Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, et al. (2011) Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 52: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corey GR, Stryjewski ME, O'Riordan WD, Fowler VG Jr, Ross DP, et al.. (2007) ATLAS 1: The first Phase 3 study evaluating the new lipoglycopeptide, telavancin, for the treatment of patients with complicated skin and skin structure infections. 17th European Congress of Clinical Microbiology and Infectious Diseases ICC, 31 March–04 April, 2007, Munich, Germany.
- 28.Corey GR, Stryjewski ME, Fowler Jr VG, Teglia O, Hopkins A, et al.. (2007) ATLAS 2: A double-blind, randomized, active controlled, multinational Phase 3 study comparing telavancin with vancomycin for the treatment of patients with complicated skin and skin structure infections. 17th European Congress of Clinical Microbiology and Infectious Diseases ICC, 31 March–04 April, 2007, Munich, Germany.
- 29. Pace JL, Krause K, Johnston D, Debabov D, Wu T, et al. (2003) In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob Agents Chemother 47: 3602–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonard SN, Szeto YG, Zolotarev M, Grigoryan IV (2011) Comparative in vitro activity of telavancin, vancomycin and linezolid against heterogeneously vancomycin-intermediate Staphylococcus aureus (hVISA). Int J Antimicrob Agents 37: 558–561. [DOI] [PubMed] [Google Scholar]
- 31. Pfaller MA, Rhomberg PR, Sader HS, Mendes RE, Jones RN (2010) Telavancin Activity against gram-positive bacteria isolated from patients with skin and skin-structure infections. J Chemother 22: 304–311. [DOI] [PubMed] [Google Scholar]
- 32. Logman JF, Stephens J, Heeg B, Haider S, Cappelleri J, et al. (2010) Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections. Curr Med Res Opin 26: 1565–1578. [DOI] [PubMed] [Google Scholar]
- 33. Stryjewski ME, Barriere SL, O'Riordan W, Dunbar LM, Hopkins A, et al. (2012) Efficacy of telavancin in patients with specific types of complicated skin and skin structure infections. J Antimicrob Chemother 67: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 34.Slover CM, Azie N, Barriere S, Lu Q (2011) Telavancin (TLV) for treatment of complicated skin and skin structure infections (cSSSI) in obese patients. 51th ICAAC Interscience Conference on Antimicrobial Agents and Chemotherapy, September 17–20, 2011, Chicago, Illinois.
- 35.Stryjewski ME, Corey GR, Li Y, Barriere S (2011) Post-hoc analysis of efficacy of telavancin in patients with complicated skin and skin structure infections: Applying New FDA Guidance. 51th ICAAC Interscience Conference on Antimicrobial Agents and Chemotherapy, September 17–20, 2011, Chicago, Illinois.
- 36.U.S. Department of Health and Human Services, Food and Drug Administration (2010) Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment. Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed 2012 July 16.
- 37.European Medicines Agency (2011) Assessment report. Vibativ. International Nonproprietary Name: telavancin. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001240/WC500115363.pdf. Accessed 2012 July 16.
- 38. Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, et al. (2012) Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54: 621–629. [DOI] [PubMed] [Google Scholar]
- 39. Falagas ME, Siempos II, Vardakas KZ (2008) Linezolid versus glycopeptide or beta-lactam for treatment of Gram-positive bacterial infections: meta-analysis of randomised controlled trials. Lancet Infect Dis 8: 53–66. [DOI] [PubMed] [Google Scholar]
- 40. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, et al. (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52: e18–55. [DOI] [PubMed] [Google Scholar]
- 41. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 42. Tarchini G (2011) On a different level: telavancin versus vancomycin for hospital-acquired pneumonia. Clin Infect Dis 52: 1390; author reply 1391–1392. [DOI] [PubMed] [Google Scholar]
- 43. Haque NZ, Zuniga LC, Peyrani P, Reyes K, Lamerato L, et al. (2010) Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest 138: 1356–1362. [DOI] [PubMed] [Google Scholar]
- 44.(2008) Telavancin for the Treatment of Complicated Skin and Skin Structure Infections. FDA Briefing Document for Anti-Infective Drugs Advisory Committee Meeting. Food and Drug Administration. Available: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4394b2-01-FDA.pdf. Accessed 2012 July 16.
- 45. Marcos LA, Camins BC, Ritchie DJ, Casabar E, Warren DK (2012) Acute renal insufficiency during telavancin therapy in clinical practice. J Antimicrob Chemother 67: 723–726. [DOI] [PubMed] [Google Scholar]
- 46. Barriere S, Genter F, Spencer E, Kitt M, Hoelscher D, et al. (2004) Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J Clin Pharmacol 44: 689–695. [DOI] [PubMed] [Google Scholar]