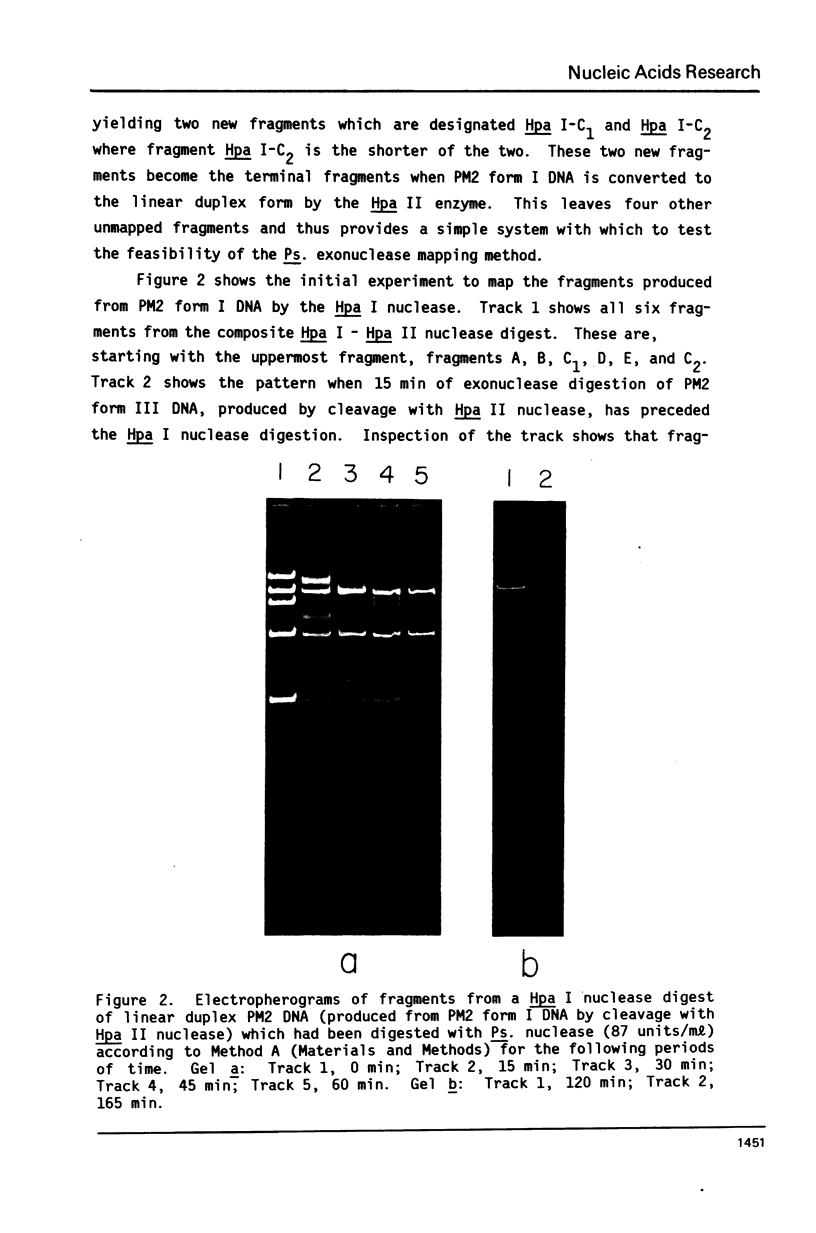
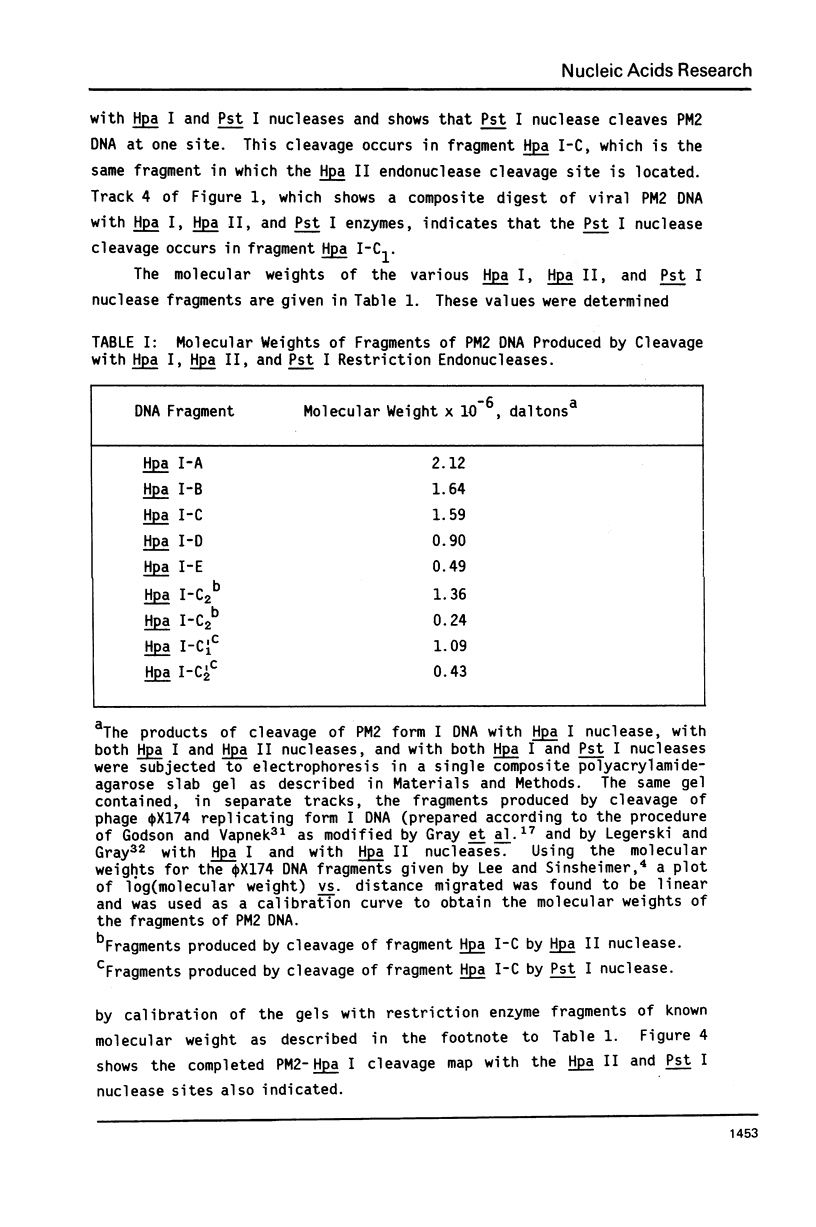
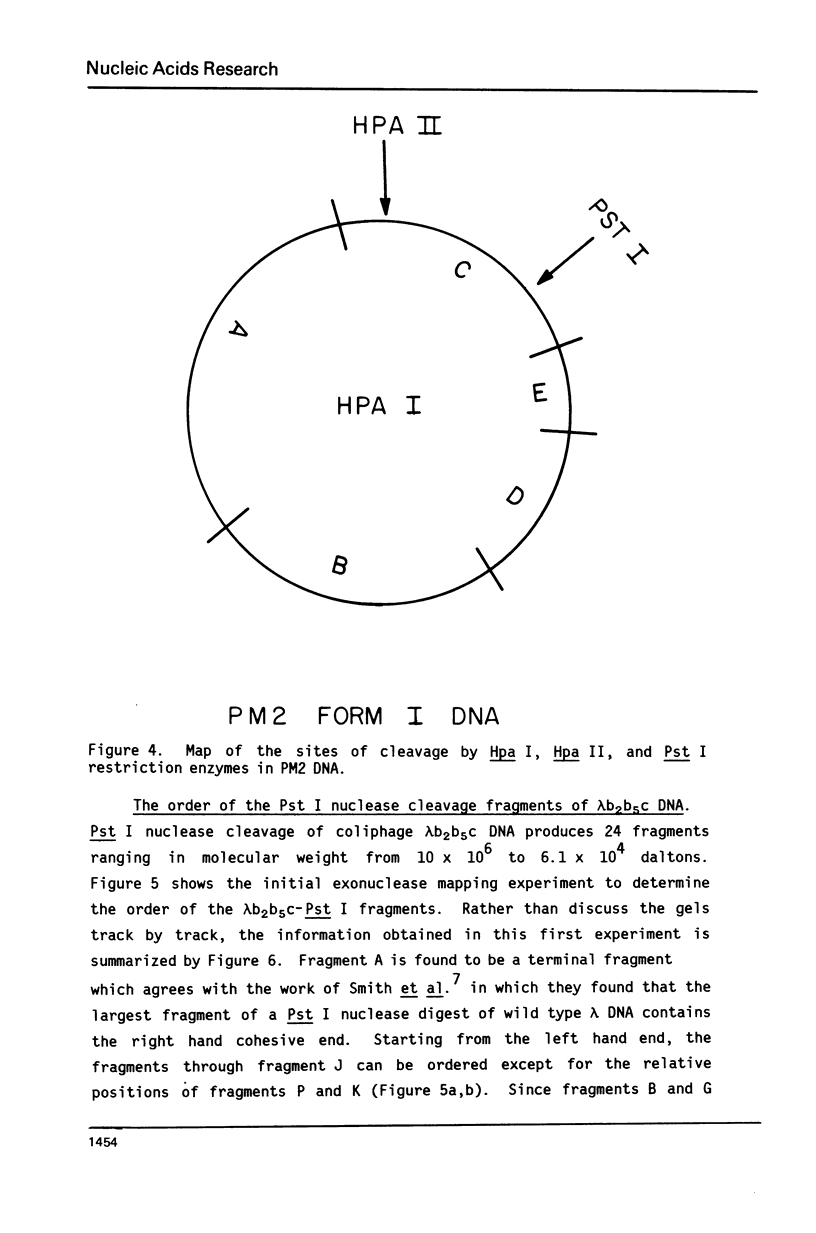
Abstract
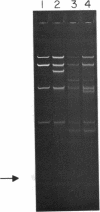
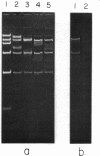
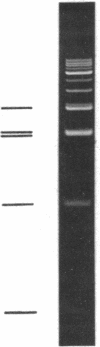
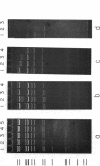
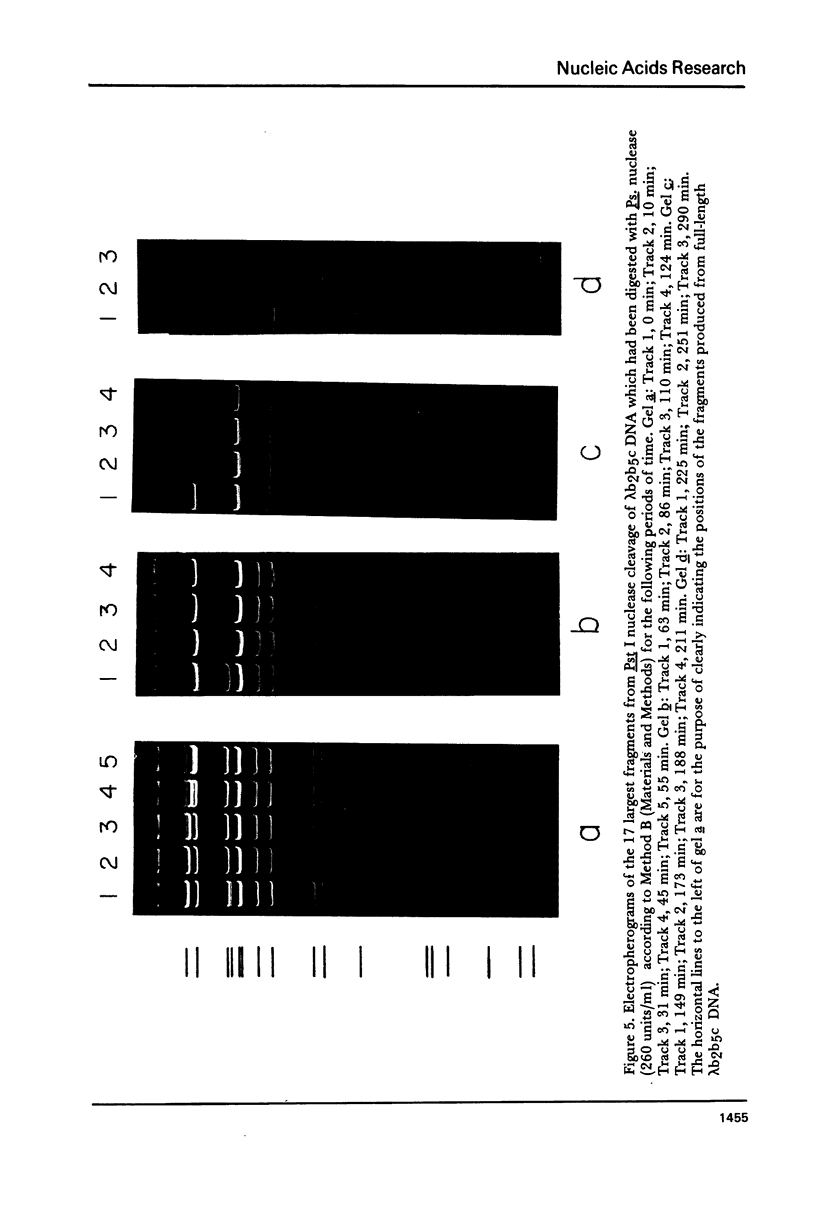
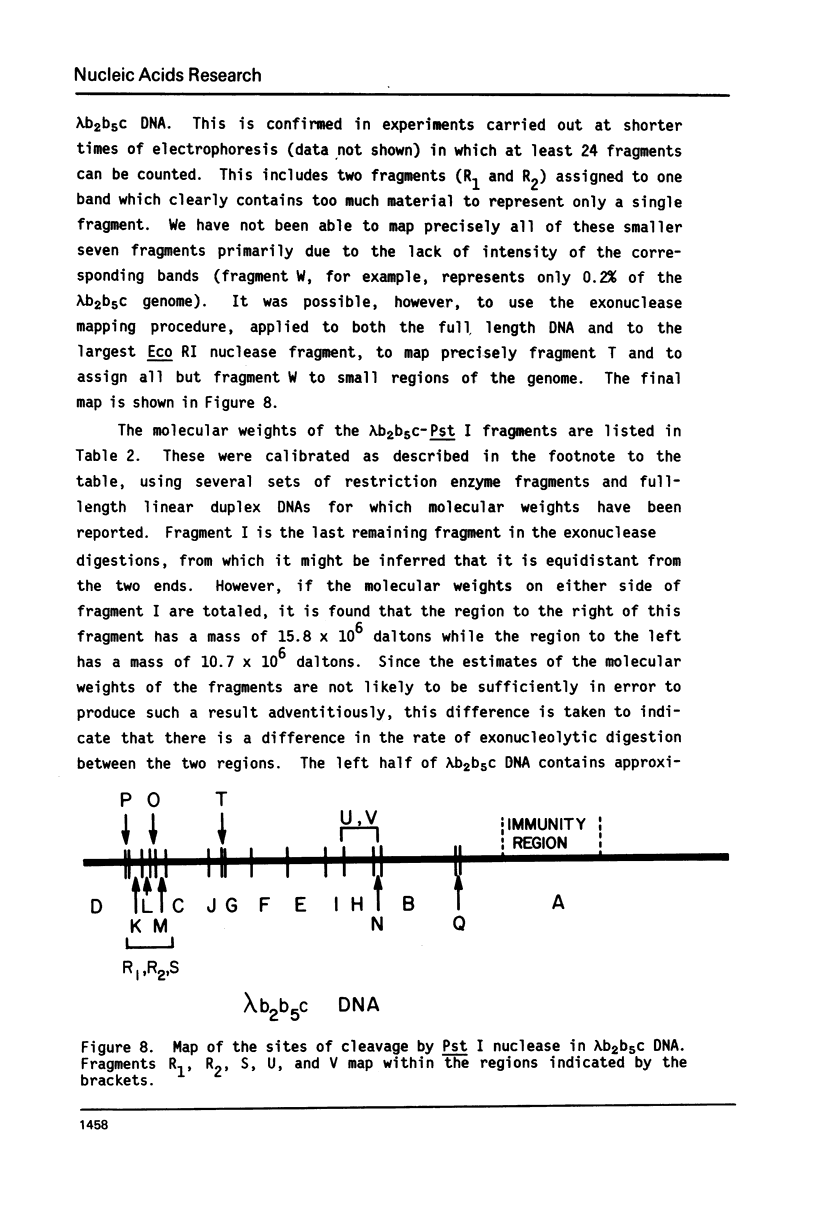
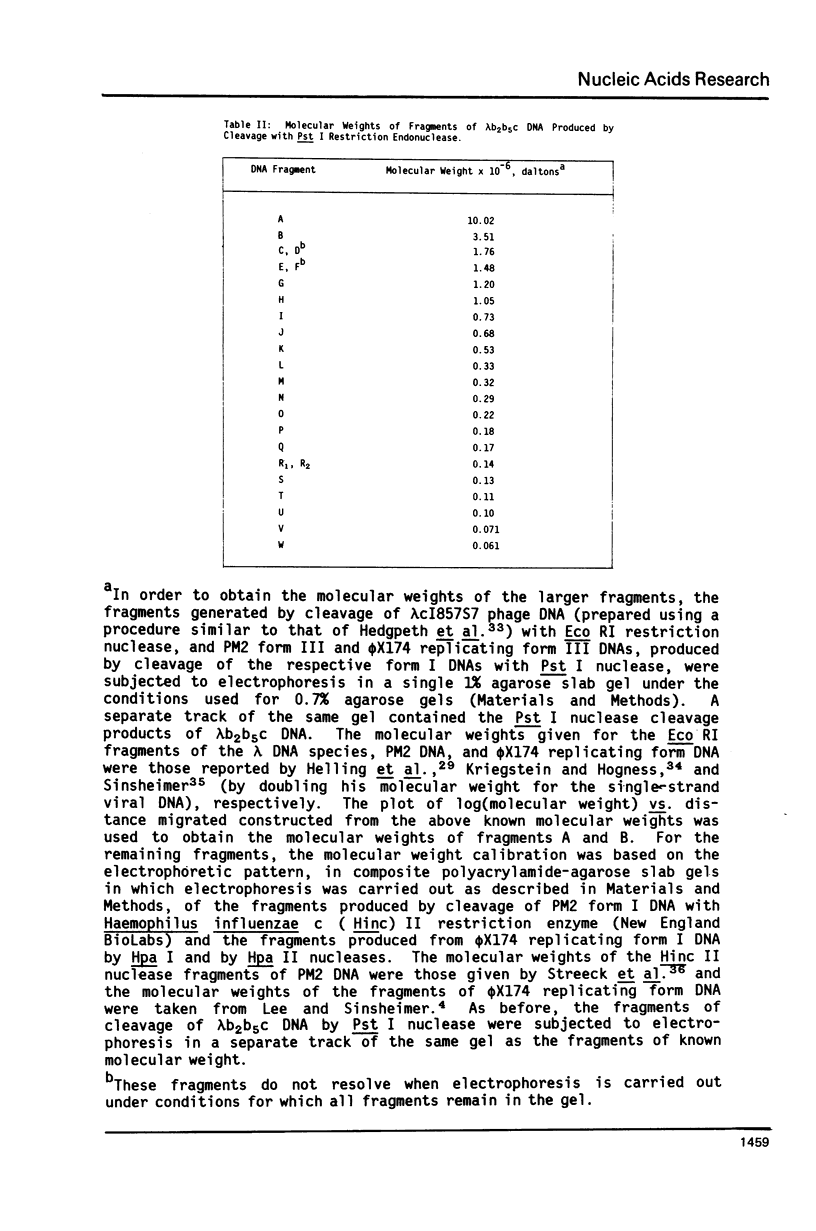
We have previously characterized an extracellular nuclease from Pseudomonas BAL 31 which, in addition to other activities, displays a double-strand exonuclease activity which progressively shortens both strands of linear duplex DNA molecules from both termini. This degradation is accomplished without the introduction of detectable scissions away from the ends of the duplexes. When this nuclease is used to produce a series of progressively shortened samples from a linear duplex DNA, subsequent digestion of these samples with a site-specific restriction endonuclease and analysis of the resulting fragments by gel electrophoresis permits the rapid establishment of the order of the restriction enzyme fragments through the entire genome. This is accomplished by noting from the electropherograms the order in which the various restriction enzyme fragments become noticeably shortened or disappear. Using this method, the five cleavage sites for the endonuclease Hpa I and the single cleavage sites for the nucleases Hpa II and Pst I have been mapped in PM2 bacteriophage DNA. In a more stringent test of the method, 18 of the 24 fragments produced by cleavage of coliphage lambdab2b5c DNA with the Pst I nuclease have been mapped, and five of the six remaining fragments have been assigned to small regions of the genome.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Brack C., Eberle H., Bickle T. A., Yuan R. A map of the sites on bacteriophage PM2 DNA for the restriction endonucleases HindIII and HpaII. J Mol Biol. 1976 Jun 14;104(1):305–309. doi: 10.1016/0022-2836(76)90016-4. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkova R., Goodgal S. H. Action of haemophilus endodeoxyribonuclease on biologically active deoxyribonucleic acid. J Bacteriol. 1972 Mar;109(3):987–992. doi: 10.1128/jb.109.3.987-992.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G., Sanders L., Slocombe P. M. A restriction cleavage map of phiX 174 DNA by pulse-chase labelling using E. coli DNA polymerase. Nucleic Acids Res. 1976 May;3(5):1323–1329. doi: 10.1093/nar/3.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. THE EFFECT OF LYSOGENIC INDUCTION ON THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI K12-LAMBDA. I. APPEARANCE OF A NEW EXONUCLEASE ACTIVITY. J Biol Chem. 1963 Oct;238:3390–3394. [PubMed] [Google Scholar]
- Kiger J. A., Jr, Young E. T., 2nd, Sinsheimer R. L. Purification and properties of intracellular lamba DNA rings. J Mol Biol. 1968 Apr 28;33(2):395–413. doi: 10.1016/0022-2836(68)90197-6. [DOI] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Sinsheimer R. L. A cleavage map of bacteriophage phiX174 genome. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2882–2886. doi: 10.1073/pnas.71.7.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Sinsheimer R. L. A cleavage map of bacteriophage phiX174 genome. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2882–2886. doi: 10.1073/pnas.71.7.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Gray H. B., Jr A sedimentation velocity method for the separation of complementary strands of DNA. Biochim Biophys Acta. 1976 Aug 18;442(2):129–141. doi: 10.1016/0005-2787(76)90483-4. [DOI] [PubMed] [Google Scholar]
- Legerski R. J., Gray H. B., Jr, Robberson D. L. A sensitive endonuclease probe for lesions in deoxyribonucleic acid helix structure produced by carcinogenic or mutagenic agents. J Biol Chem. 1977 Dec 10;252(23):8740–8746. [PubMed] [Google Scholar]
- Little J. W., Lehman I. R., Kaiser A. D. An exonuclease induced by bacteriophage lambda. I. Preparation of the crystalline enzyme. J Biol Chem. 1967 Feb 25;242(4):672–678. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Modrich P., Zabel D. EcoRI endonuclease. Physical and catalytic properties of the homogenous enzyme. J Biol Chem. 1976 Oct 10;251(19):5866–5874. [PubMed] [Google Scholar]
- Mulder C., Arrand J. R., Delius H., Keller W., Pettersson U., Roberts R. J., Sharp P. A. Cleavage maps of DNA from adenovirus types 2 and 5 by restriction endonucleases EcoRI and HpaI. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):397–400. doi: 10.1101/sqb.1974.039.01.051. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Sato S., Hutchinson C. A., 3rd, Harris J. I. A thermostable sequence-specific endonuclease from Thermus aquaticus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):542–546. doi: 10.1073/pnas.74.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck R. E., Philippsen P., Zachau H. G. Cleavage of small bacteriophage and plasmid DNAs by restriction endonucleases. Eur J Biochem. 1974 Jun 15;45(2):489–499. doi: 10.1111/j.1432-1033.1974.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereijken J. M., Van Mansfeld A. D., Baas P. D., Jansz H. S. Arthrobacter luteus restriction endonuclease cleavage map of phi chi 174 RF DNA. Virology. 1975 Nov;68(1):221–233. doi: 10.1016/0042-6822(75)90163-4. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Chowdhury K., Gruss P., Sauer G. Random cleavage of superhelical SV 40 DNA S1 nuclease. Biochim Biophys Acta. 1976 Mar 4;425(2):157–167. doi: 10.1016/0005-2787(76)90021-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wheeler F. C., Fishel R. A., Warner R. C. Agarose gel electrophoresis of circular DNA of replicative form of bacteriophage G4. Anal Biochem. 1977 Mar;78(1):260–275. doi: 10.1016/0003-2697(77)90031-8. [DOI] [PubMed] [Google Scholar]