Abstract
The combination of Pt nanoparticles and graphene was more effective in enhancing biosensing than either nanomaterial alone according to previous reports. Based on the structural similarities between water soluble graphene oxide (GrOx) and graphene, we report the fabrication of an aqueous media based GrOx/Pt-black nanocomposite for biosensing enhancement. In this approach GrOx acted as a nanoscale molecular template for the electrodeposition of Pt-black, an amorphously nanopatterned isoform of platinum metal. Scanning electron microscopy (SEM) images and energy-dispersive X-ray spectroscopy (EDS) showed that Pt-black was growing along GrOx. The effective surface area and electrocatalytic activity towards H2O2 oxidation of GrOx/Pt-black microelectrodes were significantly higher than for Pt-black microelectrodes. When used to prepare a bio-nanocomposite based on protein functionalization with the enzyme glucose oxidase (GOx), the GrOx/Pt-black microbiosensors exhibited improved sensitivity over the Pt-black microbiosensors. This suggested that the GrOx/Pt-black nanocomposite facilitated an increase in electron transfer, and/or minimized mass transport limitations as compared to Pt-black used alone. Glucose microbiosensors based on GrOx/Pt-black exhibited high sensitivity (465.9±48.0 nA/mM), a low detection limit of 1 μM, a linear response range of 1 μM – 2 mM, and response time of ~4 s. Additionally the sensor was stable and highly selective over potential interferents.
Keywords: graphene oxide, Pt-black, glucose oxidase, bionanocomposite, biosensor
1. Introduction
Nanomaterials, including carbon-based materials and metallic nanostructures, have been widely used to enhance biosensor performance due to their unique electrocatalytic properties (McLamore et al. 2010a; McLamore et al. 2010b; McLamore et al. 2009; McLamore et al. 2011; Shi 2011a, b; Shi et al. 2011; Shi 2011c). Graphene (Shao et al.) and carbon nanotubes (CNTs) (Wang 2005) have received a lot of attention because of the capacity of these materials to increase electron transfer during the oxidation or reduction of the electroactive intermediate species in biosensing (e.g., H2O2 and NADH). This is associated with the edge-plane or edge-plane-like defect sites, which function as active electron transfer sites (Banks et al. 2005). Graphene, the basic structural element of graphite and carbon nanotubes (Liu et al. 2009) is a one-atom thick planar sheet of sp2-bonded carbon (Stankovich et al. 2006). Compared with CNTs, graphene modified electrodes have shown higher electrochemical sensitivity towards dopamine detection attributed to the increased sp2 bonding content of graphene and the associated electrical conductivity. The application of graphene to biosensor fabrication requires depositing graphene on electrode surfaces and the major obstacle for graphene deposition is graphene restacking and agglomeration in aqueous solutions by van der Waals forces (Yang et al.). Agglomeration decreases the efficiency of graphene because the electrocatalytic properties of graphene are associated with individual sheets (Yang et al.). Polymers such as chitosan have been used to suspend graphene, and the polymer/graphene mixture (sometimes containing enzymes) is then deposited on electrodes (Alwarappan et al. 2009; Kang et al. 2009; Wu et al. 2009). One potential drawback with polymer immobilization is that the non-conductive polymer layer forms a diffusion barrier for analytes, and the barrier may decrease the active area of the biosensors, resulting in low sensitivity (Shi 2011a).
Platinum black (Pt-black), a layer of amorphous clusters of Pt nanoparticles (Chang et al. 2007), has been reported in the literature to enhance the performance of various biosensors, due to its electrocatalytic activities and excellent biocompatibility (Jaffe and Nuccitelli 1974; McLamore et al. 2010a; McLamore et al. 2010b; McLamore et al. 2011; Shi 2011a; Shi et al. 2011). Previous works showed that the combination of Pt/Pd nanoparticles and graphene was more effective in enhancing biosensing than using either material alone (Lu et al. 2011; Sun et al. 2011; Xu et al. 2011). However, polymer (such as Nafion and polyvinylpyrrolidone) was used to suspend graphene in these reports, but the residual polymer on the biosensor after graphene immobilization may limit mass analyte transport (Shi 2011a, b).
Considering the disadvantages including the requirement of polymer suspension associated with graphene preparation/immobilization, water soluble graphene oxide (GrOx) may be an alternative that will enhance the electrocatalytic activity and sensitivity of biosensors. The structure of GrOx mainly consists of graphene-like sheets (Rourke et al. 2011). GrOx can be synthesized from the oxidative treatment of graphite by sulfuric acid, sodium nitrate and potassium permanganate (Hummers and Offeman 1958). The structural model of GrOx is still ambiguous (Dreyer et al. 2009), but according to the Lerf-Klinowski model (He et al. 1998a; He et al. 1998b), GrOx consists of unoxidized benzene rings, and the rings are separated by aliphatic 6-membered rings. GrOx is water soluble due to the hydroxyl and epoxide groups on the basal planes and the carbonyl and carboxyl groups at the sheet edges (He et al. 1998b; Stankovich et al. 2006; Stankovich et al. 2007). Thus, GrOx can be dissolved and manipulated in aqueous media, which allows for electrode modification based on simple drop-coating methods using a GrOx aqueous solution (Zhang et al. 2011). As a nanomaterial that contains graphene-like sheets, GrOx has exhibited electrocatalytic activities on the electro-oxidation of NADH, and the oxidation potential for NADH detection for GrOx modified electrodes has been reported to be lower compared with reduced GrOx (rGrOx) modified electrodes (+410 mV for GrOx vs. +510 mV for rGrOx) (Zhang et al. 2011). Another potential advantage of GrOx over graphene is that the oxygenated species, which have been shown to possibly contribute to the catalytic activity of graphene (Pumera et al. 2009), are completely preserved. Our previous studies have shown that Pt-black electrodeposited on water-soluble single-stranded DNA-CNT-modified electrodes grew along the CNTs, which significantly increased the sensitivity of the biosensors for glucose and adenosine-5′-triphosphate measurements, as compared with direct Pt-black deposition on the sensor surface without single-stranded DNA-CNT pre-modification (Shi 2011a). It is possible that the water soluble GrOx may also serve as a molecular template for the electrodeposition of Pt-black, and the resulting GrOx/Pt-black nanocomposite will increase sensitivity towards glucose and H2O2 detection, as the templated growth of Pt-black may significantly increase the effective surface area of the biosensor. In addition, GrOx entrapped by Pt-black will not dissolve back into solution when the electrodes are stored over time.
Glucose plays an important role in metabolism. Glucose biosensors have contributed significantly to cancer research (e.g., Wharburg effect), diabetes, and clinical monitoring (McLamore et al. 2011; Shi et al. 2011; Wang 1999). Glucose biosensing usually involves two steps. In the first step, the immobilized glucose oxidase (GOx) converts glucose and oxygen into gluconic acid and H2O2. In the second step, H2O2 is oxidized to O2 when a working potential is applied to the biosensor. An electric current proportional to glucose concentration is measured to quantify glucose. GOx has emerged as one of the most widely used enzymes to demonstrate the efficiency of novel nanomaterials in enhancing biosensing (McLamore et al. 2011; Shi 2011a, b; Shi et al. 2011; Shi 2011c) due to its robustness and the ease of attaching GOx to electrode surfaces via glutaraldehyde via covalent formation of Schiff bases (Makino et al. 1988; McLamore et al. 2010b).
In this study, we report the design of an aqueous media based process for the fabrication of GrOx/Pt-black nanocomposites for the detection of glucose. Microelectrodes modified with GrOx/Pt-black were studied using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), cyclic voltammetry and DC potential amperometry to characterize the surface morphology, composition, surface area expansion, and the electrocatalytic activities of the nanocomposite. By attaching GOx to the GrOx/Pt-black modified electrodes via glutaraldehyde, we developed a bio-nanocomposite microbiosensor as a model biosensor. The major parameters of the model biosensor were characterized to demonstrate the potential of the GrOx/Pt-black material as a universal platform for a wide variety of physiological sensing applications.
2. Material and methods
2.1 Chemicals and Reagents
GOx (E.C.1.1.3.4, 100,000–250,000 units/g, from Aspergillus niger), D-Glucose, potassium chloride (99%), potassium ferricyanide (K3Fe(CN)6), chloroplatinic acid solution (8% wt/wt), lead acetate (reagent grade, 95%), sodium chloride (NaCl), magnesium chloride (•99%), bovine serum albumin, glutaraldehyde (Grade II, 25% aqueous Solution), ascorbic acid, uric acid and acetaminophen were purchased from Sigma-Aldrich (St. Louis, MO). Sodium phosphate (Na2HPO4·7H2O) and potassium phosphate (KH2PO4, monobasic) were purchased from Fisher chemicals (Pittsburg, PA). Phosphate buffered saline (PBS; 0.01 M) was prepared by dissolving 8.0 g NaCl, 1.2 g Na2HPO4, 0.2 g KCl and 0.2 g KH2PO4 in 1.0 L deionized water.
2.2 Synthesis of Graphene Oxide
GrOx was synthesized from natural graphite powder (325 mesh, Alfa Aesar) by the modified Hummers method (Hummers and Offeman 1958). GrOx was then suspended in water to give a brown dispersion, which was subjected to separation by centrifugation 5 times to completely remove residual salts and acids. The purified GrOx was then dispersed in deionized water (0.5 mg/mL). Exfoliation of GrOx was achieved by ultrasonication of the dispersion using an ultrasonic bath (Branson 2510).
2.3 Construction of Microelectrodes and Microbiosensors
All microsensors were constructed based on a Pt/Ir wire microelectrode (PI20033.0A10, 51 mm length, 0.256 mm diameter, 1–2 μm tip diameter according to the manufacturer’s specifications). The design schemes that used nanomaterials were: Pt-black microelectrodes: The microelectrode was connected to a potentiostat (cathode) against a bare Pt wire (0.5 mm in diameter; Alfa Aesar, Ward Hill, MA), which acted as the anode. Pt-black was electrodeposited by applying a voltage of 10 V for 1 min using a potentiostat (Applicable Electronics) in a solution of 0.36% (w/w%) chloroplatinic acid and 0.0005% lead acetate.
GrOx/Pt-black microelectrodes: 2 μL GrOx was applied to the microelectrode and air-dried for 30 min. Pt-black was then electrodeposited following the previous protocol.
Glucose microbiosensors: 60 μL 50 mg GOx/mL PBS was mixed with 40 μL 25 mg BSA/mL PBS and 20 μL 2.5% glutaraldehyde. A volume of 2 μL of the mixture was applied to unmodified microelectrodes or microelectrodes modified with Pt-black, or GrOx/Pt-black, respectively, and air-dried for 30 min. When not used, the glucose microbiosensors were stored at −20°C.
2.4 Sensor Calibration
Three replicates were prepared for all the microbiosensors/microelectrodes. DC potential amperometry was performed to characterize the sensitivity, and the difference in performance (experimental error) was reflected by the standard error of the mean in reported sensitivity. DC potential amperometry was conducted with a 3 electrode electrochemical (C-3) cell stand (BASi, West Lafayette, IN) at a working potential of +500 mV versus a Ag/AgCl reference electrode with a sampling rate of 1 kHz. Reference electrodes (Ag/AgCl) and Pt auxiliary electrodes were purchased from BASi. Amperometric sensitivity towards glucose was determined by measuring current at a constant working potential while sequentially adding glucose to mixed solutions (pH 7.4, room temperature and all solutions stirred at 300 rpm). Following each glucose addition, measured current signal was allowed to reach steady state (defined as less than a 3% fluctuation for 10 s). Average current values represented the arithmetic mean of observed current (n=10,000 data points). Response time (t95) was calculated as the time for the sensor to reach 95% of its maximum amperometric response following addition of 100 μM glucose. Cyclic voltammetry (CV) was performed with a 3 electrode electrochemical (C-3) cell stand (BASi, West Lafayette, IN) in 4 mM Fe(CN)63− with 1 M KNO3 as a supporting electrolyte. A sweep range of 0 to +650 mV was used with a 10-s quiet time. The detection limit was determined using the minimum detectable concentration. Sensitivity density, K (in μA mM−1 cm−2), is calculated (Shi 2011b) based on
| (1) |
where i is the amperometric sensitivity (μA mM−1) and A is the effective surface area (cm2) of the tip of the biosensor.
2.5 Effective Surface Area Characterization
The CV reduction peak current ip is determined by the Randles-Sevick Equation:(Kissinger and William 1983)
| (2) |
where: ip is the peak current (A), n is the number of electrons transferred during the redox reaction of Fe(CN)63−, D is the diffusion coefficient of Fe(CN)63− (6.70×10−6 cm2 s−1) (Chou et al. 1979), C is the molar concentration of ferricyanide (4 mM), A is the electroactive surface area (cm2), and v is the scan rate (V s−1). CV was carried out at different scan rates (v=20, 50, 100, 125, 150 and 200 mV/s) following previous works (Kissinger and William 1983; Shi 2011a, b) and effective surface area A was obtained from the slope in linear regression between ip and v1/2.
2.6 Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
SEM and EDS measurements were performed using an FEI Philips XL40 SEM equipped with an energy-dispersive X-ray detector from EDAX Inc.
3. Results and discussion
3.1 Surface Morphology and Composition
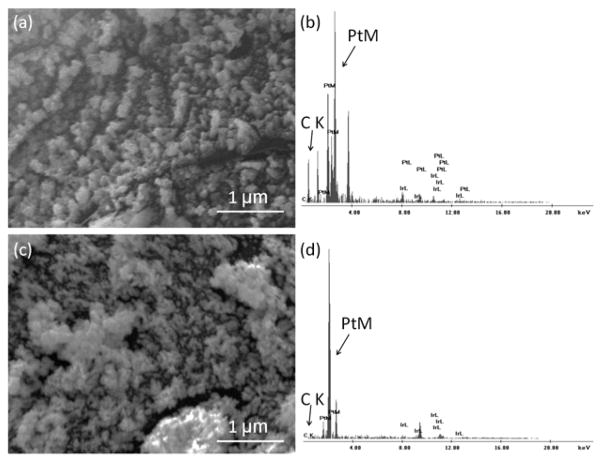
SEM and EDS were initially used to characterize the surface morphology and composition of GrOx/Pt-black-modified and Pt-black-modified microelectrodes. The micrographs and EDS spectra are shown in Fig. 1. For GrOx/Pt-black microelectrodes, several “lines” of Pt clusters were observed (Fig. 1a), which was similar to Pt-black grown on single-stranded DNA modified single-walled CNT (SWCNT) templates (Shi 2011a), indicating a similar templated electrodeposition process for Pt-black, which followed GrOx as the molecular templates. This was further supported by the peak for carbon (noted as “C K”) in the EDS spectrum due to the immobilized GrOx (Fig. 1b). Pt-black deposited directly on the microelectrode surface showed an amorphous layer of Pt-clusters (Fig. 1c), and the EDS spectrum for the Pt-black microelectrode showed no carbon peak (Fig. 1d), indicating that when no GrOx template was applied, the pattern of Pt-black was totally amorphous.
Figure 1.
Representative SEM image and EDS spectrum of GrOx/Pt-black microelectrode ((a) and (b), respectively) and Pt-black microelectrode ((c) and (d), respectively). In EDS, the peak for carbon was noted as “C K” and for platinum as “Pt M” (“K” and “M” represented the electron shells).
3.2 Electrochemical Characterization
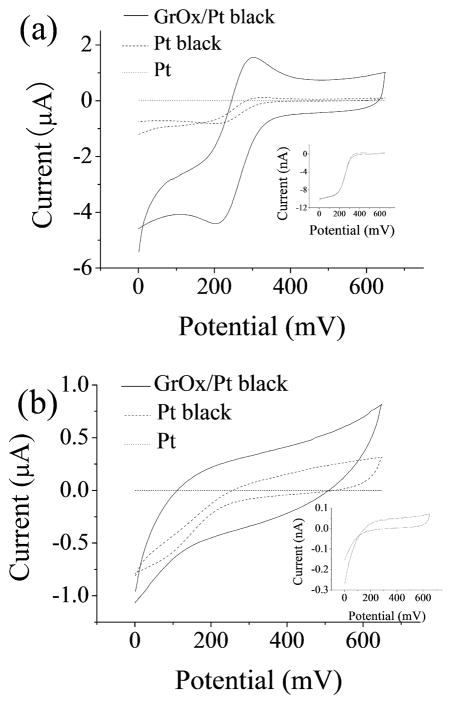
Cyclic voltammetry (CV) of potassium ferricyanide (Kissinger and William 1983; Shi 2011a, b) at an unmodified Pt microelectrode, a Pt-black modified Pt microelectrode and a GrOx/Pt-black modified Pt microelectrode in KNO3 supporting electrolyte was performed to estimate their surface area. GrOx/Pt-black electrodes exhibited well-defined redox peaks that were characteristic of macroelectrodes (Van Benschoten 1983), Pt-black electrodes showed redox peaks which were not as well-defined as GrOx/Pt-black, and the unmodified electrodes showed a sigmoidal CV curve with steady state diffusion limited currents, which was typical of microelectrodes (Fig. 2a). The difference in the shape of CV indicated that the effective surface area of the unmodified microelectrodes was significantly increased after nanomaterial modification. The peak separation in both cyclic voltammograms obtained at the GrOx/Pt-black and Pt-black modified electrodes was estimated to be 110 mV, demonstrating that the electrochemical reversibility was not affected when GrOx was incorporated. The effective surface area of the GrOx/Pt-black electrodes was estimated to be (6.0 ± 0.56) × 10−3 cm2 (n=3), and our previous work showed that the effective surface area of Pt-black electrodes was (4.7 ± 1.3) × 10−4 cm2 (Shi 2011a). GrOx/Pt-black modified electrodes exhibited a 12-time larger effective surface area than that of Pt-black modified electrodes. Compared with the unmodified microelectrodes (surface area specified as 1.8×10−8 cm2), the surface area was expanded by more than 2.6×104 times due to Pt-black, and the expansion was even more significant (over 3.3×105 times) when GrOx was incorporated. Previous studies have shown that the conductivity of GrOx changes with temperature (Jung et al. 2008). Although the resistance of GrOx was high (1–2 MΩ for GrOx sheet of approximately 400 μm2 in area and 1 nm in thickness) at room temperature (Jung et al. 2008), SEM/EDS results and the increased effective surface area indicated that GrOx could still function as a molecular template for Pt-black electrodeposition, which resulted in an overall increase of the effective surface area of the microelectrodes. CV in PBS showed that although the charging currents increased for GrOx/Pt-black and Pt-black modified electrodes compared with unmodified electrodes, the noise did not increase correspondingly (Fig. 2b).
Figure 2.
Representative CV of unmodified Pt, Pt-black and GrOx/Pt-black microelectrodes at +20 mV/s in (a) 4 mM Fe(CN)63−/ 1M KNO3 and (b) 0.01 M PBS. Insets: CV for the unmodified microelectrode.
3.3 Electrocatalytic Activity
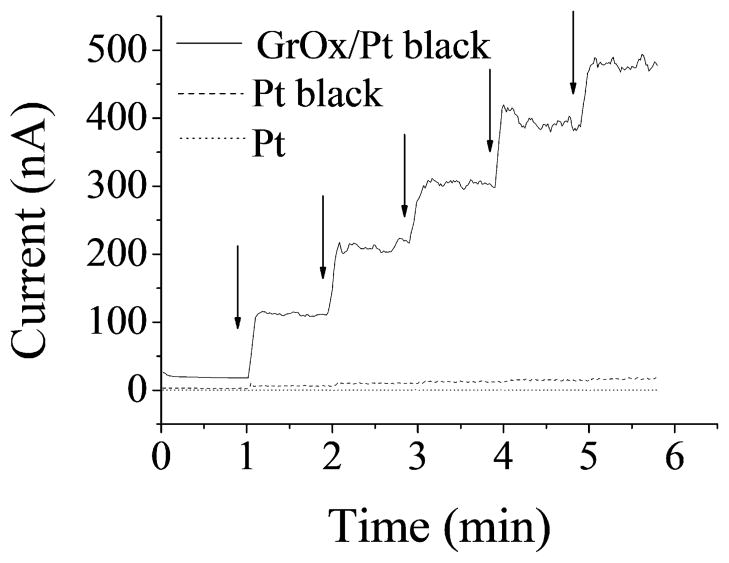
The electrocatalytic activity of the nanocomposites was characterized in terms of the amperometric sensitivity to H2O2, because H2O2 is the electroactive intermediate for oxidase based biosensors, including glucose biosensors (McLamore et al. 2010b; McLamore et al. 2011; Shi 2011a, b; Shi et al. 2011; Shi 2011c). DC potential amperometry at +500 mV showed a well-defined response to H2O2 for unmodified microelectrodes, Pt-black microelectrodes and GrOx/Pt-black microelectrodes (Fig. 3) (enlarged figures for response curves of unmodified and Pt-black microelectrodes were shown in Fig. S1.). The amperometric sensitivity was calculated using the slope in linear regression for current and H2O2 concentration. The corresponding sensitivity value of unmodified electrodes was 1.3±0.1 nA/mM, and Pt-black modification increased this value to 375.7±140.1 nA/mM according to our previous work (Shi 2011a), while the modification of the electrodes with GrOx/Pt-black nanocomposite further increased H2O2 sensitivity by more than 12 times (4.8±2.2 μA/mM) compared with Pt-black, indicating a clear advantage of GrOx incorporation in this configuration.
Figure 3.
Representative amperometric response to H2O2 for unmodified, Pt-black and GrOx/Pt-black microelectrodes at +500 mV (arrows indicated addition of 10 μM H2O2).
3.4 Biosensing Performance
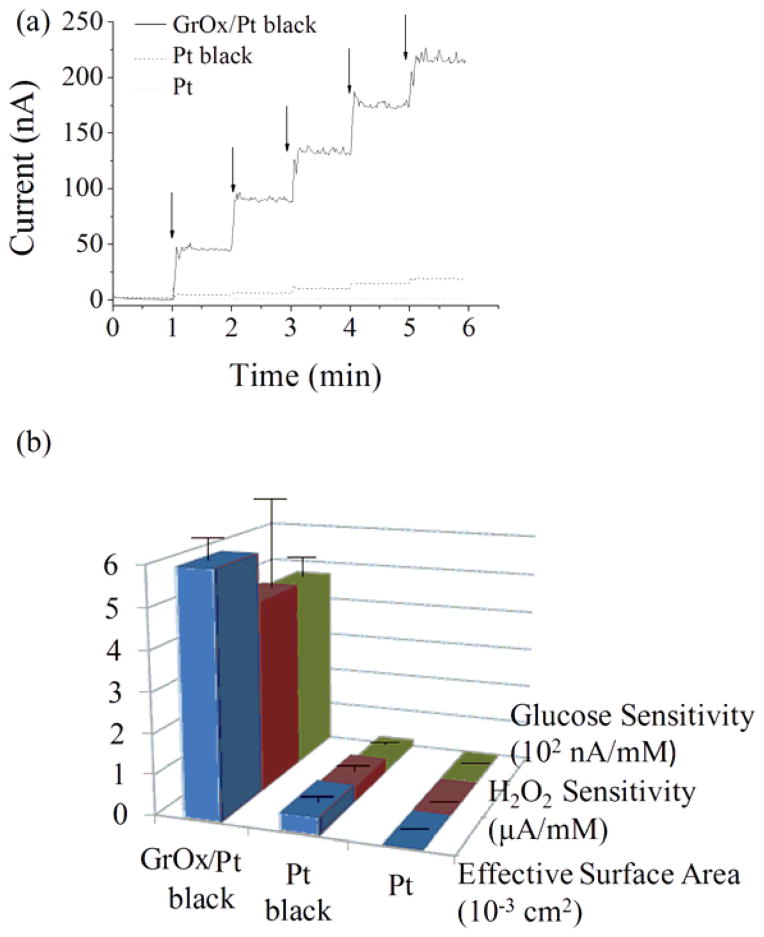
Glucose microbiosensors were fabricated by cross-linking GOx to unmodified or modified microelectrodes using glutaraldehyde. DC potential amperometry for the detection of glucose was conducted by adopting previously reported experimental conditions of working potential at +500 mV, pH 7.4, and 3 replicates (McLamore et al. 2011; Shi 2011a, b; Shi et al. 2011). As shown in Fig. 4a, a well-defined amperometric response to glucose was observed for the microbiosensors (enlarged figures for response curves of biosensors based on unmodified and Pt-black microelectrodes were shown in Fig. S2.). Glucose microbiosensors based on unmodified microelectrodes exhibited a glucose sensitivity of 0.8±0.2 nA/mM, and Pt-black increased the sensitivity by more than 22 times (18.3±5.7 nA/mM) according to our previous study (Shi 2011a). Significantly increased glucose sensitivity was observed with GrOx/Pt-black microbiosensors (465.9±48.0 nA/mM), which was more than 25 times that of Pt-black based biosensors. The relatively small standard error of the mean in sensitivity indicated desirable reproducibility. We reported in Section 3.2 and Section 3.3 that the surface area and the electrocatalytic activities were both increased by the GrOx/Pt black nanocomposite. Since drop-coating was used for enzyme immobilization, the total enzyme loading was not significantly different for all the microbiosensors. Thus, we propose that the enhanced glucose biosensing performance could be mainly attributed to increased effective surface area and electrochemical activities due to the hybrid nanocomposite. A summary of the major electrochemical parameters (effective surface area, H2O2 sensitivity and glucose sensitivity) of unmodified Pt microelectrodes, Pt-black microelectrodes and GrOx/Pt-black microelectrodes is shown in Fig. 4b. GrOx/Pt-black microelectrodes exhibited superior performance over Pt-black and unmodified microelectrodes in all the aforementioned aspects.
Figure 4.
(a) Representative amperometric response of glucose microbiosensors based on unmodified, Pt-black and GrOx/Pt-black microelectrodes at +500 mV (arrows indicated addition of 100 μM glucose). (b) Histogram showing the major electrochemical parameters of unmodified Pt, Pt-black and GrOx/Pt-black microelectrodes (error bars represented standard error of the mean).
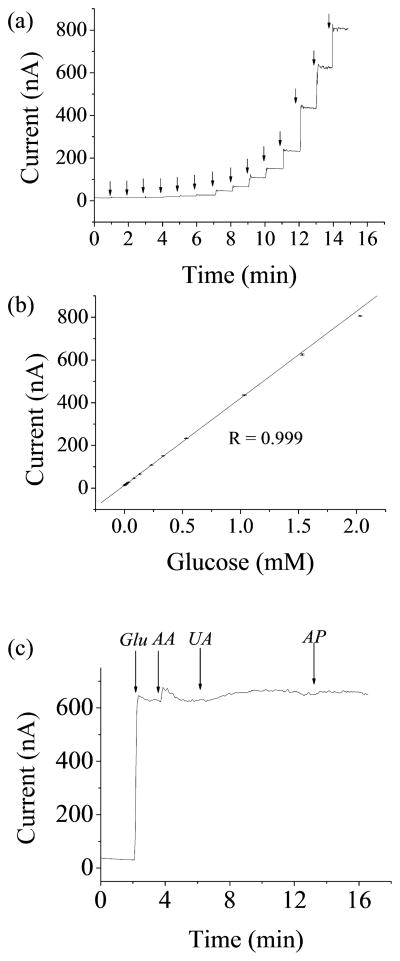
Glucose microbiosensors based on the GrOx/Pt-black nanocomposite showed a detection limit of 1 μM, a linear response range of 1 μM – 2 mM (R=0.999), and a response time (t95) of ~ 4 sec (Fig. 5a and b). The low concentration part (1 μM – 32 μM) of Fig. 5a was shown in Fig. S3. The limited linear range was due to saturated enzyme kinetics and saturated diffusion of substrates and products (Mauko et al. 2009; Mell and Maloy 1975). Selectivity tests for GrOx/Pt-black microbiosensors were carried out with the common interferents in human blood including ascorbic acid, uric acid and acetaminophen following a previously published protocol (Shi 2011a). DC potential amperometry tests (+500 mV working potential) were performed with 2 mM glucose (concentration in the middle of the linear range), followed by additions of 125 μM ascorbic acid (physiological concentration), 330 μM uric acid (physiological concentration), and 130 μM acetaminophen (therapeutic concentration) (Fig. 5c). The current response to ascorbic acid, uric acid and acetaminophen was 0.2%, 4.8% and 0.8%, respectively, compared to the current response to glucose, demonstrating excellent selectivity towards the common interferents. This showed that the microbiosensors based on the GrOx/Pt-black nanocomposite could be readily used for physiological studies. The working potential (+500 mV) was chosen to ensure a desirable amperometric sensitivity and a low probability of oxidizing interference compounds (Claussen et al. 2009; Shi 2011a, b; Sun et al. 2010). The high sensitivity and the desirable selectivity as shown above justified the working potential used. A 4.8±0.2% change in sensitivity was observed with repeated glucose tests on the same microbiosensor, demonstrating desirable repeatability. The stability of the GrOx/Pt-black microbiosensors was characterized by measuring the sensitivity over 7 days. The sensitivity was decreased by 9% over 7 days storage in air at a temperature of −20°C. The stability of the sensor was mainly determined by the enzyme immobilization approach used, where glutaraldehyde was for cross-linking and BSA was for protecting enzymes. Thus, the stability can be improved and the shelf life of the sensor can be extended to more than one year by optimizing the concentration of glucose oxidase, glutaraldehyde and BSA (House et al. 2007). Considering the desirable performance, the biosensors based on GrOx/Pt-black nanocomposites will possess a wide variety of clinical and research applications, e.g., cancer cell glucose metabolism studies.
Figure 5.
(a) Representative amperometric response to glucose in the whole linear range (1 μM – 2 mM) for GrOx/Pt-black microbiosensors at +500 mV (arrows (from left to right) indicated addition of 1, 1, 5, 5, 10, 10, 50, 50, 100, 100, 200, 500, 500, and 500 μM glucose). (b) Linear regression for (a) (error bars represented standard error of the mean). (c) Representative amperometric response to 2 mM glucose (noted as “Glu”), 125 μM ascorbic acid (AA), 330 μM uric acid (UA), and 130 μM acetaminophen (AP) for GrOx/Pt-black microbiosensors.
The efficiency of nanocomposites/nanomaterials in biosensor enhancement reported by different works should be characterized by sensitivity density instead of sensitivity, because different works used different substrate electrodes to construct biosensors, and the calculation of sensitivity density standardized the comparison by normalizing the sensitivity based on the effective surface area (Shi 2011b). A comparison of the sensitivity density for the GrOx/Pt-black biosensor and other biosensors, together with other biosensing parameters (sensitivity, linear range and response time), is presented in Table.1. The sensitivity density of the GrOx/Pt-black biosensor was the highest among the cited works (Kohma et al. 2007; Kurita et al. 2002; Li et al. 2012; Periasamy et al. 2011; Zhang et al. 2005). The linear range was not as desirable as some works (e.g, (Periasamy et al. 2011)), which might be improved by further optimizing the construction procedure of the biosensor.
4. Conclusions
This work presented a novel aqueous media compatible approach for preparing GrOx/Pt-black nanocomposites that can be used as platforms for the design of glucose sensors with enhanced sensitivity compared to other electrochemical biosensing configurations. This approach avoided the common problems with applying graphene to biosensor construction, such as the use of polymers and hydrazine. Water based immobilization of GrOx was simple, while the electrodeposition of Pt-black following GrOx deposition entrapped GrOx and prevented it from dissolving back off. Surface expansion, improved electrocatalytic activities, and improved sensitivity towards measuring glucose due to GrOx/Pt-black have been demonstrated. These results showed the effectiveness of using drop-coated GrOx as a molecular template for the electrodeposition of Pt-black. Glucose microbiosensors based on GrOx/Pt-black exhibited a low detection limit of 1 μM, a linear response range of 1 μM – 2 mM, response time of ~4 s, and desirable selectivity and stability. The novel GrOx/Pt-black nanocomposite combining the advantages of water soluble GrOx and electrocatalytic Pt-black is an excellent material platform that can be extended towards the fabrication of other biosensors for versatile physiological sensing applications.
Supplementary Material
Table 1.
Summary for the major parameters of biosensors based on nanocomposites.
| Biosensor design | Sensitivity [pA mM−1] | Sensitivity Density [μA mM−1 cm−2] | Linear range [mM] | Response time [sec] | Reference |
|---|---|---|---|---|---|
| GrOx/Pt-black/GOx | (4.7±0.5) ×105 | 78.0±3.6 | 0.001–2 | 4 | This work |
| PtNP-CS/GOx | 1.03×106 | 8.2 | 0.001–4 | 5 | Li (2012) |
| Gelatin-MWCNT/GOx | - | 2.47 | 6.3–20.1 | - | Periasamy (2011) |
| Carbon fiber/Ru/GOx | 430 | 25.2 | up to 7 | 20 | Kohma (2007) |
| AuNP/GOx | - | 2.47 | 0.02–5.7 | 8 | Zhang (2005) |
| Carbon film/GOx | 4.2 ×104 | 21 | 0.005–5 | - | Kurita (2002) |
We developed GrOx/Pt black nanocomposite as a platform for biosensors.
GrOx acted as a nanoscale molecular template for the electrodeposition of Pt-black.
Effective surface area and electrocatalytic activity were significantly improved.
GrOx/Pt-black glucose microbiosensors exhibited an LOD of 1 μM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alwarappan S, Erdem A, Liu C, Li CZ. The Journal of Physical Chemistry C. 2009;113(20):8853–8857. [Google Scholar]
- Banks CE, Davies TJ, Wildgoose GG, Compton RG. Chem Commun. 2005;(7):829–841. doi: 10.1039/b413177k. [DOI] [PubMed] [Google Scholar]
- Chang G, Oyama M, Hirao K. Thin Solid Films. 2007;515(7–8):3311–3314. [Google Scholar]
- Chou TS, Worley FL, Luss D. Industrial & Engineering Chemistry Fundamentals. 1979;18(3):279–283. [Google Scholar]
- Claussen JC, Franklin AD, ul Haque A, Porterfield DM, Fisher TS. ACS Nano. 2009;3(1):37–44. doi: 10.1021/nn800682m. [DOI] [PubMed] [Google Scholar]
- Dreyer DR, Park S, Bielawski CW, Ruoff RS. Chem Soc Rev. 2009;39(1):228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- He H, Forster M, Klinowski J. The Journal of Physical Chemistry B. 1998a;102(23):4477–4482. [Google Scholar]
- He H, Klinowski J, Forster M, Lerf A. Chem Phys Lett. 1998b;287(1–2):53–56. [Google Scholar]
- House JL, Anderson EM, Ward WK. Journal of diabetes science and technology (Online) 2007;1(1):18. doi: 10.1177/193229680700100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummers WS, Offeman RE. J Am Chem Soc. 1958;80(6):1339–1339. [Google Scholar]
- Jaffe LF, Nuccitelli R. J Cell Biol. 1974;63(2):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung I, Dikin DA, Piner RD, Ruoff RS. Nano Lett. 2008;8(12):4283–4287. doi: 10.1021/nl8019938. [DOI] [PubMed] [Google Scholar]
- Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y. Biosens Bioelectron. 2009;25(4):901–905. doi: 10.1016/j.bios.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kissinger PT, William RH. J Chem Educ. 1983;60:702–706. [Google Scholar]
- Kohma T, Oyamatsu D, Kuwabata S. Electrochem Commun. 2007;9(5):1012–1016. [Google Scholar]
- Kurita R, Hayashi K, Fan X, Yamamoto K, Kato T, Niwa O. Sensors and Actuators B: Chemical. 2002;87(2):296–303. [Google Scholar]
- Li J, Yuan R, Chai Y, Che X, Li W. Bioprocess Biosyst Eng. 2012:1–7. doi: 10.1007/s00449-012-0693-5. [DOI] [PubMed] [Google Scholar]
- Liu Z, Suenaga K, Harris PJF, Iijima S. Phys Rev Lett. 2009;102(1):15501. doi: 10.1103/PhysRevLett.102.015501. [DOI] [PubMed] [Google Scholar]
- Lu LM, Li HB, Qu F, Zhang XB, Shen GL, Yu RQ. Biosens Bioelectron. 2011;26(8):3500–3504. doi: 10.1016/j.bios.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Makino K, Maruo S, Morita Y, Takeuchi T. Biotechnol Bioeng. 1988;31(6):617–619. doi: 10.1002/bit.260310615. [DOI] [PubMed] [Google Scholar]
- Mauko L, Ogorevc B, Pihlar B. Electroanalysis. 2009;21(23):2535–2541. [Google Scholar]
- McLamore ES, Diggs A, Calvo Marzal P, Shi J, Blakeslee JJ, Peer WA, Murphy AS, Porterfield DM. The Plant Journal. 2010a;63(6):1004–1016. doi: 10.1111/j.1365-313X.2010.04300.x. [DOI] [PubMed] [Google Scholar]
- McLamore ES, Mohanty S, Shi J, Claussen J, Jedlicka SS, Rickus JL, Porterfield DM. J Neurosci Methods. 2010b;189(1):14–22. doi: 10.1016/j.jneumeth.2010.03.001. [DOI] [PubMed] [Google Scholar]
- McLamore ES, Porterfield DM, Banks MK. Biotechnol Bioeng. 2009:791–799. doi: 10.1002/bit.22128. [DOI] [PubMed] [Google Scholar]
- McLamore ES, Shi J, Jaroch D, Claussen JC, Uchida A, Jiang Y, Zhang W, Donkin SS, Banks MK, Buhman KK, Teegarden D, Rickus JL, Porterfield DM. Biosens Bioelectron. 2011;26(5):2237–2245. doi: 10.1016/j.bios.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell LD, Maloy JT. Anal Chem. 1975;47(2):299–307. [Google Scholar]
- Periasamy AP, Chang YJ, Chen SM. Bioelectrochemistry. 2011;80(2):114–120. doi: 10.1016/j.bioelechem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Pumera M, Scipioni R, Iwai H, Ohno T, Miyahara Y, Boero M. Chemistry – A European Journal. 2009;15(41):10851–10856. doi: 10.1002/chem.200900399. [DOI] [PubMed] [Google Scholar]
- Rourke JP, Pandey PA, Moore JJ, Bates M, Kinloch IA, Young RJ, Wilson NR. Angewandte Chemie International Edition. 2011;50(14):3173–3177. doi: 10.1002/anie.201007520. [DOI] [PubMed] [Google Scholar]
- Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y. Electroanalysis. 22(10):1027–1036. [Google Scholar]
- Shi J, Cha T, Claussen J, Diggs A, Choi JH, Porterfield DM. Analyst. 2011a;136:4916–4924. doi: 10.1039/c1an15179g. [DOI] [PubMed] [Google Scholar]
- Shi J, Claussen J, McLamore ES, Jaroch D, Haque A, Diggs A, Calvo Marzal P, Rickus J, Porterfield DM. Nanotechnology. 2011b;22(35):355502. doi: 10.1088/0957-4484/22/35/355502. [DOI] [PubMed] [Google Scholar]
- Shi J, McLamore E, Jaroch D, Claussen J, Rickus J, Porterfield DM. Anal Biochem. 2011;411:185–193. doi: 10.1016/j.ab.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Porterfield DM. Surface Modification Approaches for Electrochemical Biosensors. In: Andrea P, editor. Biosensors for Health, Environment and Biosecurity / Book 1. Intech; Vienna: 2011c. [Google Scholar]
- Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen SBT, Ruoff RS. Nature. 2006;442(7100):282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen SBT, Ruoff RS. Carbon. 2007;45(7):1558–1565. [Google Scholar]
- Sun CL, Lee HH, Yang JM, Wu CC. Biosens Bioelectron. 2011;26(8):3450–3455. doi: 10.1016/j.bios.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Sun TP, Shieh HL, Ching CTS, Yao YD, Huang SH, Liu CM, Liu WH, Chen CY. Int J Nanomedicine. 2010;5:343. [PMC free article] [PubMed] [Google Scholar]
- Van Benschoten JJ. J Chem Educ. 1983;60(9):772–776. [Google Scholar]
- Wang J. J Pharm Biomed Anal. 1999;19(1–2):1–2. doi: 10.1016/s0731-7085(98)00134-4. [DOI] [PubMed] [Google Scholar]
- Wang J. Electroanalysis. 2005;17(1):7–14. [Google Scholar]
- Wu H, Wang J, Kang X, Wang C, Wang D, Liu J, Aksay IA, Lin Y. Talanta. 2009;80(1):403–406. doi: 10.1016/j.talanta.2009.06.054. [DOI] [PubMed] [Google Scholar]
- Xu F, Sun Y, Zhang Y, Shi Y, Wen Z, Li Z. Electrochem Commun. 2011;13(10):1131–1134. [Google Scholar]
- Yang X, Zhu J, Qiu L, Li D. Adv Mater (Weinheim, Ger) 23(25):2833–2838. doi: 10.1002/adma.201100261. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Li DW, Karpuzov D, Long YT. Int J Electrochem Sci. 2011;6:819–829. [Google Scholar]
- Zhang S, Wang N, Yu H, Niu Y, Sun C. Bioelectrochemistry. 2005;67(1):15–22. doi: 10.1016/j.bioelechem.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.