Abstract
A 67-year-old female presented with unexplained abdominal pain. A contrast-enhanced computed tomography scan of the abdomen incidentally revealed a mass in the uncinate process of the pancreas. This mass was resected and based on histopathological findings, diagnosed as a solitary fibrous tumor (SFT) of the pancreas. A SFT is an extremely rare benign mesenchymal tumor that in 65% of cases affects the visceral pleura but can also affect extra-pleural sites. The intraoperative demarcation of pancreatic tumors, such as SFTs, can be challenging. In this report, the first clear intraoperative identification of a SFT of the pancreas in a human was shown using near-infrared fluorescence and methylene blue.
Keywords: Pancreatic solitary fibrous tumor, Near-infrared fluorescence, Image-guided surgery, Methylene blue
INTRODUCTION
Pancreatic tumors are most often adenocarcinomas, but can also be rare neuroendocrine tumors and even rarer benign mesenchymal tumors. Diagnostic workup of pancreatic tumors is typically performed using contrast-enhanced Doppler ultrasound (US), helical computed tomography (CT), enhanced magnetic resonance imaging, and endoscopic US. However, during pancreatic surgery, tumor localization and assessment of the extent of disease is presently made using visual inspection and palpation, and in some cases, intraoperative US. Inadequate intraoperative tumor identification can lead to recurrent disease and/or the need for re-exploration.
Near-infrared (NIR) fluorescence imaging is a promising technique to facilitate intraoperative, real time, visual identification of tumors[1,2], which uses tumor-specific fluorescent contrast agents[3-6]. Although several targeted NIR fluorophores have shown promise in preclinical model systems, there are no tumor-specific contrast agents presently available for clinical use. In fact, the only two NIR fluorescent contrast agents available clinically, and approved for other indications, are the 700 nm fluorophore methylene blue (MB) and the 800 nm fluorophore indocyanine green (ICG); both agents are classified as non-specific with respect to targeting. MB and ICG have been clinically used for decades. MB is used in parathyroid surgery[7] and for the treatment of septic shock[8]. ICG is used to assess the clearance capacity of the liver[9] and for angiography[10] and has been used off-label for NIR fluorescence sentinel lymph node mapping[11].
Although the enhanced permeability and retention effect can potentially be used to accumulate non-targeted contrast agents in tumors[12,13], a recent clinical study by our group, using intravenously injected ICG, suggested that this effect did not result in improved identification of adenocarcinoma of the pancreas[14]. In a different preclinical study by our group, it appeared that intravenous injection of MB into transgenic mice resulted in high sensitivity detection of insulinomas and a pancreatic neuroendocrine tumor[15]. The optimal dose of MB in that study was 1 to 2 mg/kg. Based on these results, a clinical study was initiated in which patients with a suspected neuroendocrine tumor of the pancreas were administered 1.0 mg/kg MB intraoperatively and the pancreas was imaged using an optimized NIR fluorescence imaging system.
CASE REPORT
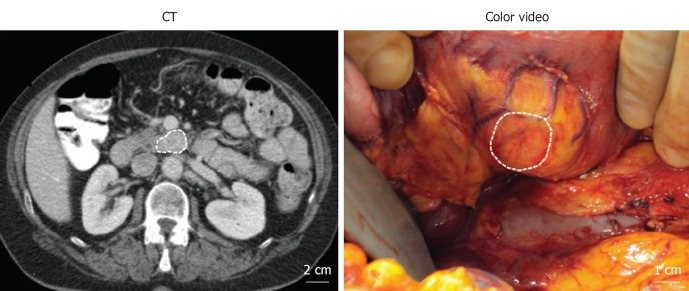
A 67-year-old female presented with unexplained abdominal pain. The patient did not have jaundice or other symptoms of obstruction. A contrast-enhanced CT scan of the abdomen incidentally revealed a mass of 1.6 cm × 2.8 cm in the uncinate process of the pancreas (Figure 1; dashed outline). The mass showed hyperattenuation during the arterial phase of contrast administration. Static total body and SPECT/low-dose CT scans using In-111-Octreotide showed a normal distribution in the body and no uptake in the pancreatic mass (data not shown). Moreover, no hormonal hypersecretion was observed. Family history of multiple endocrine neoplasia syndrome was negative. A non-functioning neuroendocrine tumor was suspected and the patient was scheduled for resection. During surgery, directly after exposure of the uncinate process, NIR fluorescence imaging of the pancreatic mass using MB was performed. Afterwards, the lesion was enucleated (Figure 1), while sparing the pancreatic duct, and processed for histology. No surgical complications or adverse events were reported and the patient was released from the hospital 7 d postoperatively.
Figure 1.
Presurgical and intraoperative visualization of the pancreatic mass.
Intraoperative NIR fluorescence imaging system
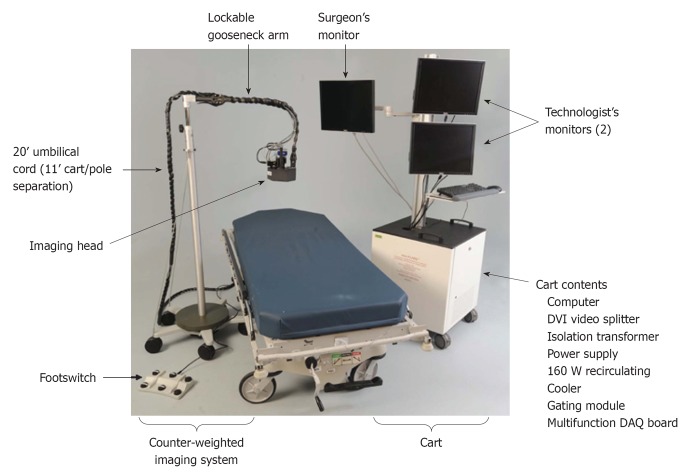
NIR fluorescence imaging was performed using the Mini-FLARE™ image-guided surgery system as described in detail previously (Figure 2)[16]. Briefly, the system consists of 2 wavelength-separated light sources: a “white” LED light source, generating 26 600 Lx of 400 to 650 nm light to illuminate the surgical field and an NIR LED light source, generating 7.7 mW/cm2 of 670 nm fluorescence excitation light. White light and NIR fluorescence images are acquired simultaneously and displayed in real time, using custom designed optics and software. A pseudo-colored (neon red) image of NIR fluorescence superimposed over the white light image is also displayed, to provide the NIR fluorescence signal in the proper anatomical context.
Figure 2.
Mini-FLARE imaging system.
Intraoperative NIR fluorescence imaging and fluorescence microscopy
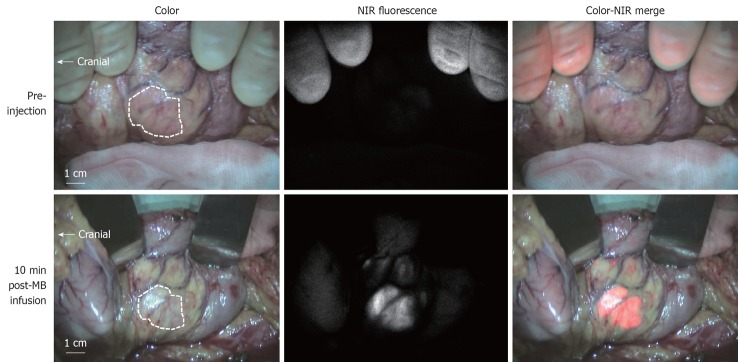
The current study was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. One patient with a suspected pancreatic neuroendocrine tumor was included and gave written informed consent. In this patient, a dose of 1.0 mg/kg MB (64 mg in 6.4 mL of water; 10 mg/mL final stock solution concentration) was infused intravenously over 5 min directly after exposure of the uncinate process. After the start of infusion, NIR fluorescence imaging of the pancreatic mass and the pancreas was performed approximately every 30 s for a period of 20 min. Ex vivo NIR fluorescence imaging of the sliced resection specimen was performed in the pathology department. NIR fluorescence imaging using the Mini-FLARE™ system enabled clear visualization of the pancreatic lesion (Figure 3). After an infusion time of 5 min, a tumor-to-background ratio of approximately 3 was reached and was stable for the next 15 min (Figure 4). No adverse effects occurred.
Figure 3.
Intraoperative near-infrared fluorescence imaging.
Figure 4.
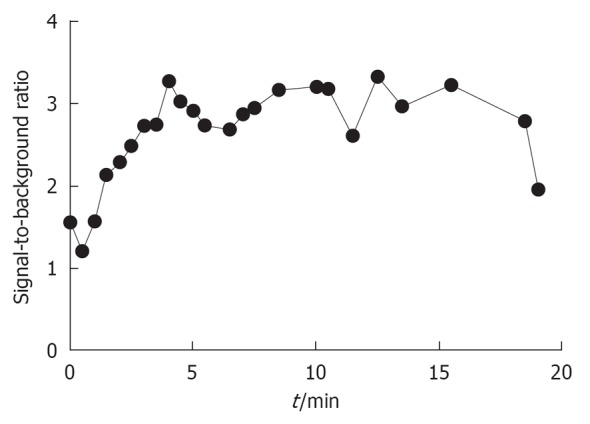
Signal-to-background ratio of the solitary fibrous tumor.
Histopathology
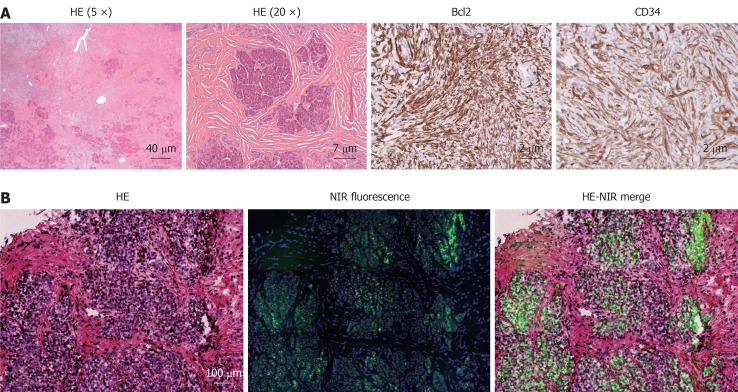
Part of the excised tissue was fixed in formalin and embedded in paraffin for hematoxylin and eosin (HE) and immunohistopathological staining, and part of the excised tissue was snap frozen for fluorescence microscopy. Macroscopically, a clearly defined well-circumscribed pale tan firm nodule was seen. Sections showed an unencapsulated, circumscribed tumor, consisting of spindle cell proliferation with hypercellular areas alternating with hypocellular foci (Figure 5A). The hypercellular areas were made up of spindle shaped cells without cytological atypia. There was no cellular pleomorphism, mitosis or necrosis. Furthermore, deposition of abundant hyalinized collagen was identified (sometimes keloid-like) and the stroma contained several vessels, a few of which were dilated and hemangiopericytic. Several normal pancreatic acinar elements are embedded within the mass. No islets of Langerhans were identified within the lesion. Additional immunohistochemical staining showed that the tumor cells were positive for CD34, CD99, and Bcl2 (Figure 5A). CD117 and b-catenin were negative (data not shown). Based on these histopathological findings the tumor was diagnosed as a solitary fibrous tumor (SFT) of the pancreas.
Figure 5.
Histopathological evaluation (A) and fluorescence microscopy (B).
Fluorescence microscopy
Snap frozen tissue was sectioned at 6 μm for fluorescence microscopy and additionally stained with a blue 4’,6-diamidino-2-phenylindole nuclear stain. Fluorescence microscopy was performed on a Zeiss LSM 700 Confocal Laser Scanning Microscope and a Zeiss HBO 100 Microscope Illuminating System (Jena, Germany) using a 633 nm laser for excitation and a 650 nm high pass emission filter. Subsequently, tissue sections were stained with HE and overlay images of NIR fluorescence were created. A fluorescence signal was located in remnants of the exocrine pancreatic tissue, encapsulated by the tumor tissue (Figure 5B).
DISCUSSION
Despite the availability of many preoperative imaging modalities, intraoperative identification and demarcation of pancreatic tumors remains challenging. In this report, the first clear identification of a SFT of the pancreas in a human was shown using NIR fluorescence and MB. A SFT is an extremely rare benign mesenchymal tumor that affects the visceral pleura in 65% of cases but can also affect extra-pleural sites. Until now, 9 cases of a SFT affecting the pancreas have been reported[17-25]. In these cases, patients were asymptomatic or complained of abdominal pain.
Although the current study was initiated to assess the ability to detect neuroendocrine tumors intraoperatively using NIR fluorescence and MB, the unexpected findings in this patient with a SFT in the pancreas are encouraging. Although SFTs mimic neuroendocrine tumors radiologically, it remains to be seen whether MB will provide strong contrast in other non-adenocarcinoma pancreatic tumors. SFTs, like neuroendocrine tumors, typically show a hypervascular, hyperenhancing mass using contrast-enhanced CT, and because MB is a phenothiazine derivative that acts as a perfusion tracer, it remains likely that future clinical testing will confirm what has been seen preclinically in neuroendocrine tumors[15]. Our clinical study enrolling patients with a suspected neuroendocrine tumor remains open for recruitment. Moreover, NIR fluorescence imaging using MB could potentially be translated to patients with other diseases giving hypervascular lesions, such as hyperparathyroidism and angiodysplasia of the gut. A clinical trial in hyperparathyroidism patients using MB and NIR fluorescence is currently ongoing in our center.
In conclusion, the current study demonstrated the first NIR fluorescence imaging of a SFT in the pancreas in human using MB, a registered and approved pharmaceutical. Larger series will be needed to confirm this result.
ACKNOWLEDGMENTS
We thank Frans Prins (Department of Pathology) for his assistance with the fluorescence microscopy. We thank Lindsey Gendall for editing.
Footnotes
Supported by (in part) NIH grant R01-CA-115296; and the Dutch Cancer Society grant UL2010-4732; the Center for Translational Molecular Medicine, project MUSIS (grant 03O-202)
Peer reviewers: Vishalkumar G Shelat, PhD, Department of Surgery, Tan Tock Seng Hospital, 11, Jalan Tan Tock Seng 308433, Singapore; Simon R Bramhall, MD, FRCS, Liver Unit, Queen Elizabeth Hospital, Birmingham B15 2TH, United Kingdom
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Vahrmeijer AL, Frangioni JV. Seeing the invisible during surgery. Br J Surg. 2011;98:749–750. doi: 10.1002/bjs.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SB, Hassan M, Fisher R, Chertov O, Chernomordik V, Kramer-Marek G, Gandjbakhche A, Capala J. Affibody molecules for in vivo characterization of HER2-positive tumors by near-infrared imaging. Clin Cancer Res. 2008;14:3840–3849. doi: 10.1158/1078-0432.CCR-07-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutteman M, Mieog JS, van der Vorst JR, Dijkstra J, Kuppen PJ, van der Laan AM, Tanke HJ, Kaijzel EL, Que I, van de Velde CJ, et al. Intraoperative near-infrared fluorescence imaging of colorectal metastases targeting integrin α(v)β(3) expression in a syngeneic rat model. Eur J Surg Oncol. 2011;37:252–257. doi: 10.1016/j.ejso.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Que I, Dijkstra J, Kaijzel EL, Prins F, Löwik CW, Smit VT, et al. Image-guided tumor resection using real-time near-infrared fluorescence in a syngeneic rat model of primary breast cancer. Breast Cancer Res Treat. 2011;128:679–689. doi: 10.1007/s10549-010-1130-6. [DOI] [PubMed] [Google Scholar]
- 7.Han N, Bumpous JM, Goldstein RE, Fleming MM, Flynn MB. Intra-operative parathyroid identification using methylene blue in parathyroid surgery. Am Surg. 2007;73:820–823. [PubMed] [Google Scholar]
- 8.Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010;30:702–715. doi: 10.1592/phco.30.7.702. [DOI] [PubMed] [Google Scholar]
- 9.Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:380–384. doi: 10.1007/s00534-009-0229-9. [DOI] [PubMed] [Google Scholar]
- 10.Hassenstein A, Meyer CH. Clinical use and research applications of Heidelberg retinal angiography and spectral-domain optical coherence tomography - a review. Clin Experiment Ophthalmol. 2009;37:130–143. doi: 10.1111/j.1442-9071.2009.02017.x. [DOI] [PubMed] [Google Scholar]
- 11.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagen A, Grosenick D, Macdonald R, Rinneberg H, Burock S, Warnick P, Poellinger A, Schlag PM. Late-fluorescence mammography assesses tumor capillary permeability and differentiates malignant from benign lesions. Opt Express. 2009;17:17016–17033. doi: 10.1364/OE.17.017016. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 14.Hutteman M, van der Vorst JR, Mieog JS, Bonsing BA, Hartgrink HH, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Near-infrared fluorescence imaging in patients undergoing pancreaticoduodenectomy. Eur Surg Res. 2011;47:90–97. doi: 10.1159/000329411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer JH, Choi HS, Gibbs-Strauss SL, Ashitate Y, Colson YL, Frangioni JV. Intraoperative localization of insulinoma and normal pancreas using invisible near-infrared fluorescent light. Ann Surg Oncol. 2010;17:1094–1100. doi: 10.1245/s10434-009-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugawara Y, Sakai S, Aono S, Takahashi T, Inoue T, Ohta K, Tanada M, Teramoto N. Solitary fibrous tumor of the pancreas. Jpn J Radiol. 2010;28:479–482. doi: 10.1007/s11604-010-0453-x. [DOI] [PubMed] [Google Scholar]
- 18.Chetty R, Jain R, Serra S. Solitary fibrous tumor of the pancreas. Ann Diagn Pathol. 2009;13:339–343. doi: 10.1016/j.anndiagpath.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Ishiwatari H, Hayashi T, Yoshida M, Kuroiwa G, Sato Y, Kobune M, Takimoto R, Kimura Y, Hasegawa T, Hirata K, et al. [A case of solitary fibrous tumor of the pancreas] Nihon Shokakibyo Gakkai Zasshi. 2009;106:1078–1085. [PubMed] [Google Scholar]
- 20.Kwon HJ, Byun JH, Kang J, Park SH, Lee MG. Solitary fibrous tumor of the pancreas: imaging findings. Korean J Radiol. 2008;9 Suppl:S48–S51. doi: 10.3348/kjr.2008.9.s.s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan VD, Wayne JD, Rao MS, Zynger DL. Solitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literature. JOP. 2008;9:526–530. [PubMed] [Google Scholar]
- 22.Miyamoto H, Molena DA, Schoeniger LO. Solitary fibrous tumor of the pancreas: a case report. Int J Surg Pathol. 2007;15:311–314. doi: 10.1177/1066896907302419. [DOI] [PubMed] [Google Scholar]
- 23.Gardini A, Dubini A, Saragoni L, Padovani F, Garcea D. [Benign solitary fibrous tumor of the pancreas: a rare location of extra-pleural fibrous tumor. Single case report and review of the literature] Pathologica. 2007;99:15–18. [PubMed] [Google Scholar]
- 24.Chatti K, Nouira K, Ben Reguigua M, Bedioui H, Oueslati S, Laabidi B, Alaya M, Ben Abdallah N. [Solitary fibrous tumor of the pancreas. A case report] Gastroenterol Clin Biol. 2006;30:317–319. doi: 10.1016/s0399-8320(06)73174-8. [DOI] [PubMed] [Google Scholar]
- 25.Lüttges J, Mentzel T, Hübner G, Klöppel G. Solitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumours. Virchows Arch. 1999;435:37–42. doi: 10.1007/s004280050392. [DOI] [PubMed] [Google Scholar]