Abstract
Recent predictions of growth in human populations and food supply suggest that there will be a need to substantially increase food production in the near future. One possible approach to meeting this demand, at least in part, is the control of pests and diseases, which currently cause a 30–40% loss in available crop production. In recent years, strategies for controlling pests and diseases have tended to focus on short-term, single-technology interventions, particularly chemical pesticides. This model frequently applies even where so-called integrated pest management strategies are used because in reality, these often are dominated by single technologies (e.g., biocontrol, host plant resistance, or biopesticides) that are used as replacements for chemicals. Very little attention is given to the interaction or compatibility of the different technologies used. Unfortunately, evidence suggests that such approaches rarely yield satisfactory results and are unlikely to provide sustainable pest control solutions for the future. Drawing on two case histories, this paper demonstrates that by increasing our basic understanding of how individual pest control technologies act and interact, new opportunities for improving pest control can be revealed. This approach stresses the need to break away from the existing single-technology, pesticide-dominated paradigm and to adopt a more ecological approach built around a fundamental understanding of population biology at the local farm level and the true integration of renewable technologies such as host plant resistance and natural biological control, which are available to even the most resource-poor farmers.
The continuing growth of the human population will, for the foreseeable future, require that we find new ways to increase food production. Reducing losses caused by pests and diseases is one possible approach. Current estimates put global losses caused by pests (insects, nematodes, diseases, and weeds) at US$ 300 billion annually (1), which equals around 30–40% of potential global food, fiber, and feed production (1) with substantially higher proportions in particular developing countries. Moreover, these estimates generally concern losses to crop production only. If losses caused by postharvest pests and diseases are added, then figures approaching 60–70% may be more typical for the developing world (2).
Over the past 50 years, application of chemical pesticides has come to be the dominant form of pest control in developed, and increasingly in developing, countries (1). Future approaches to reducing the damage caused by pests are, however, likely to be very different from those that predominate today. Indeed, while pests have been a chronic problem in agriculture since its beginning, many of the serious pest problems in the developing world today are the direct consequence of actions taken to improve crop production (3). These pest problems associated with agricultural intensification particularly apply to insects. In recent decades, the dependence on chemical insecticides has led in some crop systems to a high frequency of insecticide resistance—now recorded in more than 500 insect species worldwide (4)—pest resurgence, acute and chronic health problems, environmental pollution, and uneconomic crop production. All of these problems are particularly severe in developing countries, where pesticide use is poorly regulated and farmers often lack appropriate information or training. For many of these farmers, pesticide use is becoming a rising and unreliable component of the cost of crop production.
In this environment, the concept of integrated pest management (IPM) is becoming more and more popular among farmers, researchers, and policy makers. In IPM, a range of methods are used for pest control. IPM seeks to minimize reliance on pesticides by emphasizing the contribution of other control methods, including biological control, host-plant resistance breeding, and cultural techniques. Further, because IPM places less emphasis on expensive pesticides and more on renewable technologies available to the resource-poor farmer, such as biological control and host plant resistance, it is more possible for these farmers to share the benefits of this approach.
All of this sounds very encouraging and suggests a clear role for IPM in crop production in the future. In practice, however, IPM means many different things to many different people (5), and the way it is actually conducted in the majority of crop systems today still places emphasis on single technologies such as the use of pesticides, biocontrol, or host plant resistance and rarely considers the interactions among them (6). The consequence of this single technology focus and the search for “magic bullets” is that pest control is often only partly effective and, because of problems such as environmental damage and development of resistance, the strategies themselves are rarely sustainable. Furthermore, the implementation of these technologies traditionally has followed a top-down approach, which is contrary to the concepts of user-orientation and empowerment that now are considered central to the development of sustainable IPM (5).
The aim of this paper is to highlight how the adoption of a more ecological approach, in which the actions and interactions of the component technologies are fully understood within the context of the local agroecosystem, could lead to the development of more effective and sustainable “truly integrated” pest management. To illustrate this argument I review some recent ecological work from two different areas of pest management. The first concerns the population dynamic basis for integrating host plant resistance breeding and biological control. The second is the application of ecology to microbial control and the practical development of biopesticides. The linking theme between these studies is the use of ecological principles to improve prediction and interpretation of the action of individual technologies and through this, identifying new opportunities for IPM.
Integrating Biological Control and Host Plant Resistance for Control of Insect Pests
Current Status.
As alluded to above, although both biological control and host plant resistance are thought of today as key components of IPM, their development in recent years has taken little account of their potential for integration in the control of particular insect pests. This situation is unfortunate given that for many key pest species, neither plant resistance or biological control has provided us with a satisfactory, sustainable control solution (6).
In a recent review, Waage and I (6) showed that the action of natural enemies can substantially modify the effectiveness and the durability of host plant resistance. It was found that partial resistance and partially effective biocontrol can be combined to give additive or synergistic reductions in pest density. Although neither strategy acting in isolation may be completely successful, opportunities exist for improving control through their combined, integrated use. However, the study also revealed that plant resistance and biocontrol can interact negatively with, under some conditions, plant resistance causing complete disruption of natural enemy activity. The exact nature of the interaction depends on the specific relationship between natural enemy and herbivore and the mode of action of the host plant resistance and its effects on herbivore life history. What this means in practice is that to fully explore these tritrophic (plant-herbivore-natural enemy) interactions and substantiate the effects and compatibility of host plant resistance and biocontrol, a fundamental understanding of population dynamics is required. This argument is illustrated below by using a simple population model. Here the focus is on the biological insights gained from the model so the model itself is described only verbally. Those readers interested in further details of the model and its derivations are referred to ref. 6. In addition, it should be noted that the model describes a basic scenario with an idealized crop, pest, and generalist natural enemies and does not itself attempt to encompass all the features of crop-pest-enemy interactions (such as local spatial dynamics and intergenerational dynamics), which may influence the ultimate outcome of the tritrophic interaction. Instead it provides a limiting case for a subset of possible interactions and importantly examines and interprets their effects in a population dynamic context.
Population Dynamics of Plant Resistance–Biocontrol Interactions.
The model explores how the population dynamic effects of the first (i.e., plant resistance traits) and third (i.e., natural enemies) trophic levels combine to determine pest population growth rate. The model system is an idealized annual crop attacked by a pest that builds up over the season. Population growth rate (r) of the pest is determined by the development time, the fecundity, and the mortality rate (of the juveniles and adults). It is assumed that plant resistance can affect any of these life history parameters independently. It also is assumed the pest is attacked by a series of natural enemies that are present or move into the crop but that do not reproduce in the crop (or that reproduction has no effect on natural enemy numbers in the current season). In this case the population dynamics of the natural enemy during the season need not be considered. Any effects of interactions in previous generations on pest and natural enemy numbers in the present generation are ignored. This assumption is justified when annual crops are rotated, or when pests and natural enemies disperse widely so that densities in any one place are not or are little affected by densities in the previous generation. Pests of annual crops that are attacked by birds, by natural enemies such as many spiders or beetles that may reproduce in the crop, but whose progeny would not that season consume pests, or by parasitoids whose progeny would not become adult until the next season, all would be described by this scenario.
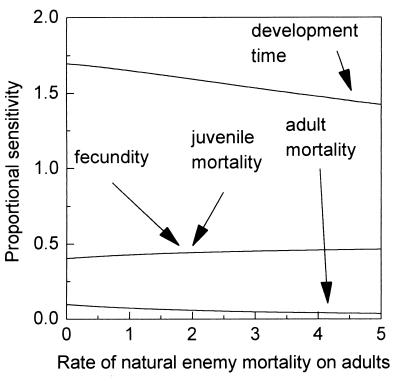
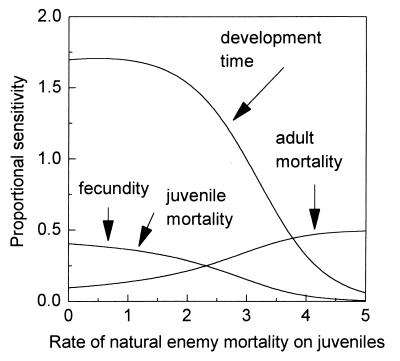
The model pest is an insect with a general life history similar to that of a sucking homopteran pest such as an aphid. The insect takes 2 weeks to develop from egg to adult and then lives for an additional 2 weeks. Juvenile mortality is responsible for the deaths of a little more than 60% of immatures. Throughout its adult life, the female produces 20 female eggs a day. A population with this natural history will grow at a rate r = 1.60 per week. This parameter set is used as a basis for exploring the consequences of the different plant resistance-biocontrol interactions. The natural enemies are assumed to act as an additional mortality factor influencing either juvenile or adult mortality. As stated above, the plant resistance potentially can influence pest fecundity, juvenile or adult mortality, or development time. The aim is to identify the change in the rate of pest increase (r) that results from increased resistance, and how such changes are influenced by the presence of natural enemies. To do this, a means of comparing the effect of plant resistance acting on different components of the pest’s life history is required. Here, the proportional sensitivity of the intrinsic rate of increase is used. This measure is of the change in r that results from a small percentage change in the value of a parameter. Its exact meaning becomes clearer in Figs. 1 and 2, which examine the proportional sensitivity of r to changes in the life history parameters (basically the relative impact or value of plant resistance) in the presence of natural enemies acting on either adult or juveniles stages of the pest, respectively.
Figure 1.
Proportional sensitivities of pest population growth rate for different pest life history parameters combined with natural enemy mortality acting on the adult pests. Positive slopes indicate synergy between host plant resistance acting on a particular life history parameter and the natural enemy activity. Negative slopes indicate subadditive interactions. Further details of the model and assumptions are given in the text and in ref. 6. Reproduced with permission from ref. 6.
Figure 2.
Proportional sensitivities of pest population growth rate for different pest life history parameters combined with natural enemy mortality acting on the juvenile pest stages. Other details are as in Fig. 1 and the text. Reproduced with permission from ref. 6.
The first point to notice in both figures is that in the absence of any natural enemies, the relative effect of a proportional change in the different life history parameters (i.e., the base-line proportional sensitivity at the intercept of the y axis) varies considerably. With the basic parameter set, by far the greatest reduction in pest growth rate is achieved by increasing the pest development time; a decrease in fecundity or an increase in juvenile mortality have the same influence while an increase in adult mortality is the least effective way to reduce the pest growth rate. The generality of this conclusion is examined analytically in ref. 6. The results of this analysis reveal that the population dynamic effects of plant resistance depend not only on which specific life history parameters the resistance influences but also on the general life history of the pest. Thus, in slowly growing populations, increasing development time or increasing juvenile mortality have roughly similar effects on reducing r. As population growth rate increases, however, the value of increasing development time markedly increases. The relative advantages of manipulating adult mortality increases as fecundity declines, as the product of juvenile mortality and development time gets larger, and as the population growth rate increases. Finally, the relative value of decreasing fecundity depends on the parameter values for juvenile mortality and development time. In sum, not all components of resistance have equivalent effects on pest suppression; the effects of individual mechanisms depend on the life history of the pest.
Considering now the combined effects of plant breeding and biocontrol, Fig. 1 shows the proportional sensitivities of intrinsic growth rate with respect to the four pest life history parameters plotted as a function of the natural enemy mortality experienced by the adult pest (i.e., biocontrol acts only on the adult stages). In this figure (and Fig. 2), a decrease in proportional sensitivity (a negative slope) as natural enemy mortality increases indicates a subadditive interaction because the interaction of the two effects is less than the sum and the relative impact of changing the parameter will have fallen. Conversely, an increase in proportional sensitivity (a positive slope) as mortality by natural enemies increases indicates superadditivity or synergy such that the interaction of the two effects is greater than the sum of their individual actions. It can be seen in Fig. 1 that fecundity and juvenile mortality show a superadditive effect, the other two parameters a subadditive effect. However, the slope of the lines are rather shallow indicating that, at least for our particular pest life history, the effects of resistance and natural enemy mortality on adult pests are at least roughly additive.
Moving on to natural enemy mortality acting on the juvenile pest, it can be seen that the effect on r of a change in fecundity or juvenile mortality is subadditive over a range of different levels of juvenile mortality because of natural enemies (Fig. 2), which is the reverse of the effect shown in Fig. 1. With respect to the interaction between development rate and natural enemy mortality on juveniles it can be seen that for rates of natural enemy mortality of between 0 and 1 the proportional sensitivity of growth to development time is approximately constant (in fact it is slightly superadditive in the range 0 to 0.5) but is subadditive beyond. This effect may seem counterintuitive in the light of some studies that propose that slowing development time should increase the chances of being killed by a natural enemy (7, 8). However, although increasing the developmental period may well increase mortality by natural enemies to a certain extent, the main value is in fact to slow down the compounded growth rate of the pest population. If the natural enemy already has reduced the pest’s growth rate to a low level, the proportional benefit of an increase in development may be relatively small. Finally, natural enemy mortality affecting juveniles and adult mortality combine superadditively to reduce pest growth rate, which again is the reverse of Fig. 1. Even if severe juvenile mortality through natural enemies has reduced immature survival to very low levels, a further reduction in pest numbers can be achieved by an increase in adult mortality.
Again, the generality of these and the previous conclusions has been investigated analytically (6). This analysis reveals that within the constraints of the model and its assumptions, the interaction between natural enemy mortality of adult pests and resistance effects on fecundity or juvenile mortality always will be superadditive, whereas that with adult mortality always will be subadditive. When natural enemies act on the juvenile pest stages, the outcome of these equivalent interactions is reversed. The interaction of plant quality affecting development time and either adult or juvenile natural enemy mortality can be both superadditive or subadditive depending on specific conditions.
Implications for IPM.
The models presented here provide a theoretical framework for quantifying the population dynamic aspects of certain plant resistance-biological control interactions. The results show that the effectiveness of individual mechanisms in suppressing pest population growth rate depends critically on pest life history. They also show that biological control and host plant resistance can be compatible and can combine additively or synergistically to improve pest control. However, even with the simplest representation of biological control (i.e., natural enemies providing an additional density independent mortality) subadditive and complex interactions are also possible. What is important to note in this context is that current resistance screening and evaluation procedures do not examine the direct or indirect effects of resistance on the third trophic level (6) and hence do not account for the action of natural enemies or how this action may vary across time and space. This means that positive interactions between plant resistance and biological control are not identified and that potentially useful partially resistant varieties that, in combination with natural enemies, could provide satisfactory control, frequently are rejected. It also means that the effects of infochemicals or direct physical interactions between the host plant and the natural enemy are ignored. Worse, without examining the third trophic level it is possible that wide-scale deployment of resistant varieties that actively interfere with natural enemies could occur. This practice could not only reduce the benefits gained from resistance breeding but also could have long-term consequences for the persistence of certain key natural enemy species.
Overall, a central conclusion of this study is that population dynamics provides a common language for plant breeders and biocontrol practitioners with the power to reveal how the different technologies, acting perhaps on different stages and different aspects of pest population growth, may interact in determining the trajectory of pest populations and hence damage to crops. Presently, most information on the relationship of biological control and host plant resistance is conjectural, speculative, or based only on limited observation (9). Population dynamic thinking, aided by simple models, has the potential to help us to generate the right questions and design the right experiments for better understanding the interactions of host plant resistance and biological control.
Ecology of Host–Pathogen Interactions and Microbial Pest Control
In response to the problems of intensification and chemical pesticide use touched on in the Introduction, there is today a growing interest in the potential of pathogens for use in biological control, particularly as biorational pesticides (10, 11). However, in spite of much evidence suggesting a role for parasites in the regulation of host abundance, our understanding of the impact of infectious diseases and the role they play in the population dynamics of their hosts remains poor (11, 12). An important applied consequence of this limited understanding is that efforts to use diseases for pest control often meet with failure. This certainly holds for the use of biopesticides, which generally are characterized by their poor and erratic performance under field conditions (13). Consequently, biopesticides and microbial agents are viewed by industry and crop protection specialists with considerable skepticism, which is reflected in the marketplace with biopesticides representing <1% of the global market for agrochemical crop protection (13). Moreover, the way microbial organisms are used as biopesticides tends to emphasize aspects of their biology that mimic conventional chemical pesticides (i.e., simply using the capacity of pathogens to kill the target host) and overlooks many other biological attributes that may have important population dynamic implications (5, 10, 11). This paradigm holds for the development of bioinsecticides and bioherbicides. If microbial control agents and biopesticides are to attain anything like their full potential, moving beyond the status of novelty products suitable only for limited niche markets and playing a major role in sustainable crop protection in the future, a far greater and more detailed understanding of host-parasite biology and disease dynamics is required.
This argument is further illustrated by some recent ecological studies conducted as part of an ongoing biocontrol program developing a biopesticide, based on a fungal entomopathogen, for control of locusts and grasshoppers in Africa. The program, LUBILOSA, has made considerable progress in the development of an effective and reliable biopesticide, and many successful field tests have been conducted against a range of locust and grasshopper hosts under a variety of ecological conditions (see ref. 14 for a recent review of the program). The pathogen itself acts like a chemical pesticide through direct contact and on the whole, the development, testing, and registration of the biopesticide have tended to follow what could be considered a traditional chemical pesticide model. However, a number of ecological studies have shown that the pathogen has a range of additional biological characteristics quite unlike a chemical, which contribute to its overall performance and which need to be considered in its evaluation and ultimate use.
The Importance of Horizontal Transmission.
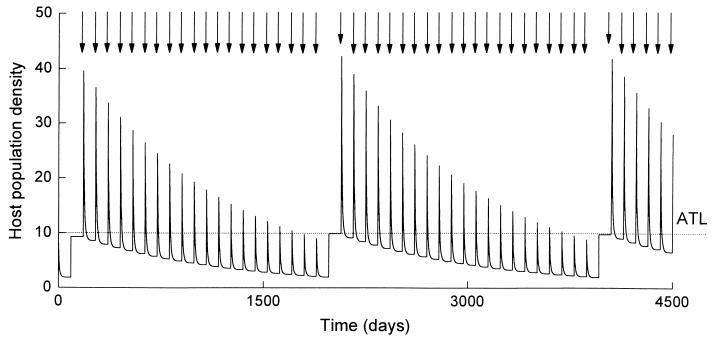
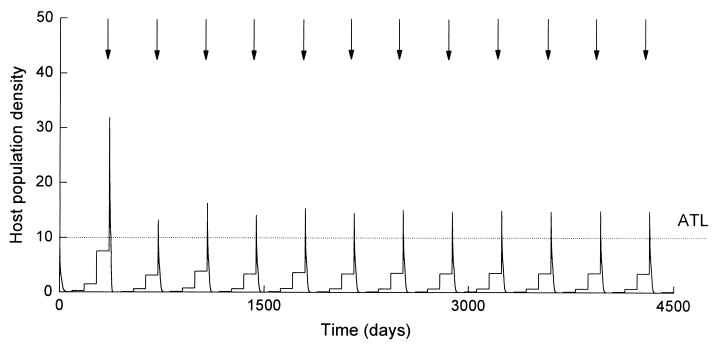
Studies after spray applications have identified that under certain conditions hosts infected by the biopesticide can go on to produce new spores and infect further hosts through horizontal transmission (11, 15, 16). The dynamics of this process are governed by the factors that regulate natural host-pathogen interactions because infections result from natural pathogen delivery mechanisms. These include a number of biotic factors relating to the specific life history and behavioral traits of the host and the pathogen, as well as a range of abiotic factors that have a fundamental influence on the physical and biochemical processes involved in the host-pathogen interaction (refs. 11, 15, and 16; S. Blanford and M.B.T., unpublished work). These processes have very few chemical analogues and consequently, quantifying the impact of horizontal transmission in the field is not straightforward and certainly not amenable to standard protocols adopted from short-term chemical pesticide evaluation studies. In the LUBILOSA program, therefore, evaluation of the horizontal transmission has been aided by the development of some simple population models, built around empirical data describing the different biological processes governing pathogen cycling. Full details of these models can be found in refs. 11, 15, and 16. In brief, the models, combined with field observations, have demonstrated that contact with spores from infected cadavers can provide an important source of infection by both prolonging the effects and increasing the impact of single spray applications. This is illustrated in Figs. 3 and 4, which show model simulations of grasshopper populations under two control scenarios.
Figure 3.
Output of a simple host-pathogen model showing predicted change in grasshopper population density through time after applications of a biopesticide. In this example, the biopesticide acts like a chemical through direct contact and residual pickup of spores only, with no horizontal transmission. The system is seasonal, with a season duration of 90 days and one grasshopper generation per season (each peak, therefore, represents the grasshopper density at the start of a new generation). Spraying occurs just once per season and only if the host density exceeds the spray action threshold (ATL) of 10 m−2. Each spray event is indicated with an arrow. Full details of the model and its assumptions are given in ref. 11.
Figure 4.
Predicted change in grasshopper population density through time after applications of a biopesticide when a horizontal transmission component is added. Spray frequency has fallen to just once in every 4–5 generations, and peak population densities are uniform compared with Fig. 3. Other details are as in Fig. 3 and ref. 11.
In Fig. 3 it is assumed that the biopesticide acts just through direct contact and residual pickup of spores, with no horizontal transmission. Spraying is restricted to once a season after egg hatch, and then only if the host density exceeds the spray action threshold of 10 hosts m−2. In combination, direct hit and contact with the spray residue result in approximately 80% mortality, causing the population to crash dramatically after spraying. However, in spite of this impact, given the pest reproductive rate (in this case a finite rate of increase = 5) the biopesticide fails to provide any long-term control and host densities recover to above the spray threshold nearly every season. Thus, under these model assumptions (which are based on real field data) the biopesticide acts just like a chemical pesticide inducing a standard level of density-independent mortality.
The addition of horizontal transmission (described in the model again by empirically derived data) changes this picture dramatically (Fig. 4). The reason is that the biopesticide now has a density-dependent, “numerical response” component to its action. Although pickup of spores from the initial spray application kills the majority of grasshoppers, horizontal transmission from infected cadavers can still act to clear up those remaining and reduce the host population to very low levels. This process contributes to better overall control by restricting population peaks and reducing spray frequencies.
This result is important whenever the choice of pathogen strain or formulation involves a tradeoff between direct and residual kill rates and the potential for secondary cycling. For example, a tradeoff between virulence and pathogen reproduction (i.e., spore production) has been noted for a number of fungal isolates (unpublished data). Similar relationships also have been identified for certain viruses (18). Although high virulence may be a desirable trait (virulence is often the principle criterion for isolate selection), selecting isolates on the basis of this factor alone may have unforeseen consequences for the population dynamics of the host-pathogen interaction and overall pest control. Furthermore, these studies reveal that under realistic conditions when direct hit and residual pickup have a high initial impact, the effect of horizontal transmission, although important, is very subtle and is not likely to be apparent until late in the season.
Behavioral Changes and Sublethal Effects.
Beyond horizontal transmission, other ecological studies have further identified some intriguing problems in assessing and quantifying the impact of the biopesticide. For example, studies have revealed that infected hosts appear to be more vulnerable to predation than healthy hosts before death (ref. 19 and unpublished data). This phenomenon may have benefits in that speed of kill after application may effectively be increased through the pathogen’s interaction with other mortality factors, although the extent to which this mortality occurs will depend on the nature and abundance of natural enemies at the site of application. Moreover, given that the biopesticide is specific to locusts and grasshoppers, these important natural enemies are conserved, providing a “value added” component to the biopesticide’s activity, relative to broad-spectrum chemical pesticide alternatives, which can remove this natural back ground biocontrol (at least temporarily). However, heavy predation also means that potential sources of inoculum for horizontal transmission are lost. Interestingly, this loss is balanced to some extent by the fact that infected cadavers are avoided by scavengers and persist far longer in the field than uninfected cadavers (unpublished data). Overall, the situation is complex but it is becoming clear that studies of interactions between infection and predation are relevant to understanding both short-term patterns of disease incidence and longer-term disease dynamics.
In addition, several studies indicate that the pest status of infected insects is markedly reduced soon after application of the pathogen because of a rapid reduction in feeding. This result has been shown in laboratory and field settings for a range of species and pathogen doses (19–22). Thus it appears that the overall impact of the biopesticide is determined by more than just mortality rate (which can be slow under field conditions; see below) and that even infections with very low levels of pathogen still may have important consequences for control.
Finally, studies have identified that the role of environmental temperature and host thermal biology are central for interpreting patterns of mortality observed following spray applications.
Many grasshoppers and locusts actively thermoregulate to maintain their body temperatures around a preferred set point during the day. This set point can be significantly different from ambient and can be maintained for a number of hours given the right environmental conditions. For example, studies during large-scale field trials in west Africa revealed that the preferred body temperature of the Senegalese grasshopper, Oedaleus senegalensis, (one of the most important grasshopper pests in the Sahel) is 39°C (23). This temperature is high enough to cause significant decline in growth of the pathogen inside infected insects. Moreover, this species was found to adopt a “behavioral fever” response to infection whereby internal body temperatures were elevated higher still to a new set point around 42°C. At this temperature all fungal growth is completely inhibited. However, this new set point is only achievable during the day. During the evening, night, and morning when active thermoregulation is not possible, the body temperature of these grasshoppers is close to ambient (22–32°C), providing a window for pathogen growth. Thus, the effect of the fever is to prolong the disease incubation period, allowing insects to survive longer than expected in the field. However, because, in field trials, insects are exposed to relatively high doses of pathogen, total mortality appears not to be affected. That said, additional follow-up studies in the laboratory suggest that thermoregulation and behavioral fever may enhance host resistance to lower, more natural doses of pathogen and restrict disease spread. Indeed, studies on a range of species infected with different pathogens suggest that host thermal biology and environmental temperature (rather than the widely assumed effects of environmental moisture and humidity) may be key factors in understanding natural disease dynamics and explaining seasonal variation in disease prevalence in the field (S. Blanford and M.B.T., unpublished work). This work has shown that key parameters such as pathogenicity, the latent period of infection, and host recovery rate all can vary dramatically across time and space because of thermal biology of the host and changes in environmental temperature. Such effects have not been thoroughly explored in any previous investigations (neither practically in the development of biopesticides or in the wealth of modeling studies that guide established theory) but have major qualitative and quantitative implications for disease dynamics in insects and possibly ectotherms in general.
Implications for Microbial Control and IPM.
Although the work presented here derives from one specific biocontrol program, there are a number of insights relevant to development of insect pathogens as biocontrol agents in general. First, because of the biological nature of the active ingredient, a biopesticide may have a “numerical response” component to its action. As stated above, this possibility rarely has been considered in the development of biopesticides, with current commercial development following a chemical pesticide model and emphasizing the “functional response” components only (11). Clearly, however, such properties can be important and could provide new opportunities for using pathogens in biological pest control and have significant consequences for the economics of biopesticide use. This aspect is seen in the combined model where although in absolute terms, secondary cycling of the pathogen appears to have little impact, its effect on reducing the frequency of spray applications is most pronounced. This thinking also extends to sublethal effects such as reduction in feeding and increased susceptibility to natural enemies. All of these features combine to define the impact, competitiveness and utility of a biopesticide product and must be known.
Beyond this, the work on temperature and thermal biology has major implications for use of microbial agents in insect pest control and for managing disease dynamics in natural populations. For example, basic screening bioassays for selecting pathogens for use in biological control usually are conducted under constant laboratory conditions far removed from conditions in the field (24). However, given that virulence and host recovery appear highly temperature sensitive, it is clear that this approach can lead to erroneous conclusions and the selection of inappropriate isolates. This conclusion is supported by a number of studies that report initial positive results in the laboratory only to find performance in the field highly variable with environmental factors, and in particular temperature, appearing to play an important role (25, 26). Understanding the effect of temperature and/or the role of host thermal behavior on the host-pathogen interaction could make a major contribution to improving this situation. This conclusion extends to assessing the risks of biopesticides against nontarget species and determining the ultimate fate of pathogens in the environment. Finally, natural epizootics frequently are linked with periods of rainfall and high relative humidity (27, 28). However, such conditions are also likely to lower ambient temperatures and increase cloud cover. Thus, in addition to any direct effect on disease transmission, rain episodes are likely to alter the relative susceptibility of hosts to pathogens through effects on body temperature. For mobile species such as locusts and grasshoppers, suboptimal temperatures also would reduce movement, possibly increasing opportunities for contact between hosts at a time when susceptibility to disease is increased. Understanding such complex interplay between humidity, temperature, and physiological and behavioral changes in susceptibility could reveal exciting new opportunities for using pathogens via strategies of conservation, augmentation, and classical introduction. Such approaches have virtually been ignored to date.
Conclusions
In this paper I have tried to demonstrate the value of adopting a more rigorous ecological approach to understanding the impact of pest control technologies. The need for this approach derives from the fact that the single solution, “magic bullet” approach has undermined our basic understanding of how individual components actually work and has oriented IPM toward quick fixes, often underpinned by commercial incentives. Developing truly integrated pest management that addresses the problems of sustainable agriculture will require that we break away from this model. The downside is that pest control may be more complicated. At the very least, it will be “knowledge intensive,” requiring a greater input of appropriate ecological research in the development stage and with more emphasis placed on long-term solutions, even if the practical outputs are inherently simple. That said, it must be remembered that the complexity of nature also makes it difficult to apply conventional area-wide prescriptions successfully across all systems. Equally, compensating for a lack of understanding with excessive reliance on single technologies and short-term solutions does not make sense; experience of the last 30 years has shown the greater the apparent success in achieving pest or disease control in the short term, the greater the likelihood of a serious breakdown in the long run (29). Moreover, and perhaps most importantly, the upside of this increased effort is that it could provide access to some of the substantial amounts of yield currently lost to pests and diseases. Stressing the theme of the symposium and the question of time, an important dimension here is that this yield is available now, if we can manage these biotic constraints. To this end, many IPM tools are available now (e.g., partially resistant germplasm, partially effective indigenous natural enemies, and potentially effective but unreliable microbial agents) and do not require further technological breakthroughs to be useful. The immediate challenge lies in the genuine application of ecological research (i.e., truly dealing with applied problems and not merely paying them lip service) to understand how these components interact and identify more effective and sustainable integrated strategies for their use.
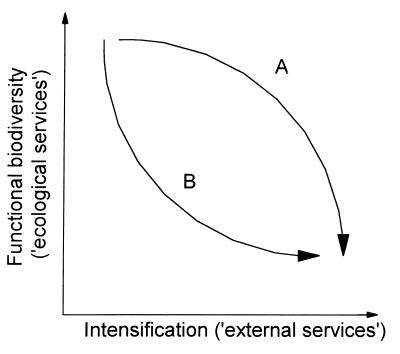
Putting these conclusions into a broader ecological context, we need a greater understanding of how different components in the agroecosystem function and how their “ecological services” can be maintained, while moving toward the goals of intensification. In essence, replacing chemicals and capital with locally available biological resources and knowledge. This problem is summarized visually in Fig. 5. Fig. 5 illustrates the general negative relationship or tradeoff between functional biodiversity, which provides biological services that contribute to system resilience, and intensification, which tends to strip away this diversity and replaces the biological services with external inputs to provide insurance against collapse. Moreover, from the figure it is clear that what is important is the exact nature of this negative relationship; in the context of sustainability and minimisation of external inputs, intensification model A is clearly better than model B. Overall, it is this tradeoff, our understanding of it and our ability to manage it that is fundamental to IPM, and one that places basic ecology at the heart of sustainable crop production. Boldly stated, the shortcomings of IPM over the last 30 years generally can be ascribed to a lack of such ecological thinking. This situation is unfortunate given that the types of manipulations and management practices necessary to maintain the functional components of the ecosystem themselves may be inherently simple. For example, studies in the Philippines have demonstrated that intercropping maize and peanuts can create a sufficiently complex and beneficial food web to keep maize stemborer in check (30). Similarly, the simple addition of narrow grass-covered banks to cereal fields in the United Kingdom has been shown to improve overwintering conditions for key predators of aphids and facilitate effective colonization of the fields in the spring (31).
Figure 5.
Representation of the general negative relationship between functional biodiversity and the contribution of ecological services to community stability, and agricultural intensification and its associated reliance on external inputs. For model A the system is managed in such a way that the ecological services are well maintained as the system is intensified (at least over moderate levels of intensification). Model A contrasts with model B in which intensification causes a sharp loss of functional diversity, resulting in a rapid increase in external inputs.
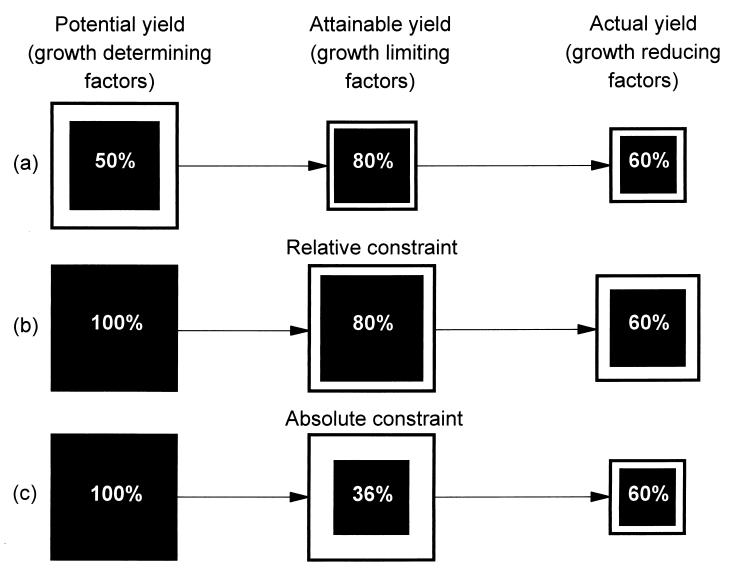
Finally, in any particular setting, the role of IPM needs to be considered in the context of the overall production system. To do this it is necessary to determine the role of different constraints in the production chain and define where the greatest gains per unit investment can be made. A conceptual framework for this approach is presented in Fig. 6. Here, factors influencing production in the field are grouped into three categories (a fourth could be added to account for postharvest losses). At the most basic level, productivity is determined by yield potential, which is defined by characteristics of the crop, temperature, and sunlight (17). Yield potential, therefore, describes the maximum possible yield under optimum growing conditions for a given crop. At the next level, attainable yield is determined by the real (and often more limited) availability of factors such as nutrients, water, and sunlight in the farmers’ field (17). Finally, actual yields obtained tend to be lower again because of the action of growth-reducing factors such as pests and diseases (17). In a perfect system, yield potential, attainable yield, and actual yield all would be 100%, with no losses along the production chain. A more typical scenario, however, might look like Fig. 6a where yield potential is 50% of the maximum, attainable yield is 80%, and actual yield is 60%. In this system it would appear that the greatest restriction to yield, and hence the priority for research input, lies with the growth defining factors affecting yield potential. However, in addition to identifying where in the production chain losses occur, it is necessary to consider the nature of the constraints and whether they are relative or absolute. With relative constraints, increases in yield are passed along the production chain. This process is demonstrated in Fig. 6b where improvements in yield potential translate to relative increases in attainable yield and actual yield, in spite of the percentage constraints at these points in the chain remaining the same. However, if one of the constraints is absolute rather than relative, a bottleneck is created that restricts the passage of yield gain along the chain. In Fig. 6c, where the constraint to attainable yield is assumed to be absolute, none of the gain in yield potential ultimately is attained. Under these circumstances, the research priority may switch to examining why the constraint is absolute, or to addressing factors below the absolute constraint where gains can still be realized. What is important to appreciate here is that the size, position, and nature (i.e., whether absolute or relative) of the constraints in any system will be highly location specific. Thus, a critical step in future efforts to increase productivity will be to move away from generalist, area-wide management prescriptions, toward local solutions developed in response to questions generated at the local level. Unfortunately, such an approach presents considerable challenges and even conflicts with existing research incentives and institutional structures (5). This conclusion identifies that whatever technological or methodological advances to improve productivity are identified, effective implementation will be possible only with appropriate economic and political support.
Figure 6.
Conceptual framework for defining constraints to crop production along the production chain and identifying where the greatest gains per unit investment can be made. Shaded areas represent the amount of yield available at one point in the chain that can be passed onto the next. (a) A system with 50% loss to potential yield, 20% loss to attainable yield and 40% loss to actual yield. (b) The effect of relative constraints. (c) The effect of an absolute constraint (in this case attainable yield). Further details are provided in the text.
ABBREVIATION
- IPM
integrated pest management
References
- 1.Natural Resources Institute. A Synopsis of Integrated Pest Management in Developing Countries in the Tropics. Chatham, U.K.: Natural Resources Institute; 1992. [Google Scholar]
- 2.Kumar R. Insect Pest Control with Special Reference to African Agriculture. London: Edward Arnold; 1984. [Google Scholar]
- 3.Waage J K. In: Agriculture and Environmental Challenges: Proceedings of the Thirteenth Agricultural Sector Symposium. Srivastava J P, Alderman H, editors. Washington, DC: World Bank; 1993. pp. 119–134. [Google Scholar]
- 4.Georghiou G P. In: Managing Resistance to Agrochemicals. Green M B, LeBaron H M, Moberg W K, editors. Washington, DC: Am. Chem. Soc.; 1990. pp. 18–41. [Google Scholar]
- 5.Waage J K. Entomologia Sinica. 1998;5:257–271. [Google Scholar]
- 6.Thomas M B, Waage J K. Integration of Biological Control and Host Plant Resistance Breeding: A Scientific and Literature Review. Wageningen, The Netherlands: Technical Centre for Agricultural and Rural Cooperation of the European Union; 1996. [Google Scholar]
- 7.Price P W, Bouton C E, Gross P, McPheron B A, Thompson J N, Weis A J. Annu Rev Ecol Syst. 1980;11:41–65. [Google Scholar]
- 8.Price P W. In: Interactions of Plant Resistance and Parasitoids and Predators of Insects. Boethel D J, Eikenbary R D, editors. Chichester, U.K.: Wiley; 1986. pp. 11–30. [Google Scholar]
- 9.Herzog D C, Funderburk J E. In: Biological Control in Agricultural IPM Systems. Hoy M A, Herzog D C, editors. New York: Academic; 1985. pp. 67–88. [Google Scholar]
- 10.Lacey L A, Goettel M S. Entomophaga. 1995;40:3–27. [Google Scholar]
- 11.Thomas M B, Wood S N. Br Crop Protection Council Symp Proc. 1997;68:63–72. [Google Scholar]
- 12.Grenfell B T, Dobson A P, editors. Ecology of Infectious Diseases in Natural Populations. Cambridge: University Press; 1995. [Google Scholar]
- 13.Lisansky S. Br Crop Protection Council Symp Proc. 1997;68:3–10. [Google Scholar]
- 14.Bateman R P. Outlook Agric. 1997;26:13–18. [Google Scholar]
- 15.Thomas M B, Wood S N, Lomer C J. Proc R Soc London Ser B. 1995;259:265–270. [Google Scholar]
- 16.Wood S N, Thomas M B. Proc R Soc London Ser B. 1996;263:673–680. doi: 10.1098/rspb.1996.0101. [DOI] [PubMed] [Google Scholar]
- 17.Rabbinge R. In: Crop Protection and Sustainable Agriculture. Chadwick D J, Marsh J, editors. Chichester, U.K.: Wiley; 1993. pp. 2–29. [Google Scholar]
- 18.Hails R S. Br Crop Protection Council Symp Proc. 1997;68:53–62. [Google Scholar]
- 19.Thomas M B, Blanford S, Gbongboui C, Lomer C J. Entomol Exp Appl. 1998;87:93–102. [Google Scholar]
- 20.Moore D, Reed M, Le Patourel G, Abraham Y J, Prior C. J Invertebr Pathol. 1992;60:304–307. [Google Scholar]
- 21.Seyoum E, Moore D, Charnley A K. J Appl Entomol. 1994;118:310–315. [Google Scholar]
- 22.Thomas M B, Blanford S, Lomer C J. Biocontr Sci Technol. 1997;7:327–334. [Google Scholar]
- 23.Blanford S, Thomas M B, Langewald J. Ecol Entomol. 1998;23:9–14. [Google Scholar]
- 24.Thomas M B, Jenkins N E. Mycol Res. 1997;101:1469–1474. [Google Scholar]
- 25.Samways M J, Grech N M. Agric Ecosyst Environ. 1986;15:231–239. [Google Scholar]
- 26.Inglis G D, Johnson D L, Goettel M S. Biol Control. 1996;7:131–139. [Google Scholar]
- 27.Hajek A E, St. Leger R J. Annu Rev Entomol. 1994;39:239–322. [Google Scholar]
- 28.Carruthers R I, Ramos M E, Larkin T S, Hostetter D L, Soper R S. Mem Entomol Soc Can. 1997;171:329–353. [Google Scholar]
- 29.Conway G. The Doubly Green Revolution: Food for All in the 21st Century. London: Penguin; 1997. [Google Scholar]
- 30.Altieri M A. Agroecology: the Science of Sustainable Agriculture. 2nd Ed. London: Intermediate Technology; 1995. [Google Scholar]
- 31.Thomas M B, Wratten S D, Sotherton N W. J Appl Ecol. 1991;28:906–917. [Google Scholar]