Abstract
Fas-associated death domain (FADD) is a common adaptor molecule which plays an important role in transduction of death receptor mediated apoptosis. The FADD provides DED motif for binding to both procaspase-8 and cFLIP molecules which executes death receptor mediated apoptosis. Dysregulated expression of FADD and cFLIP may contribute to inhibition of apoptosis and promote cell survival in cancer. Moreover elevated intracellular level of cFLIP competitively excludes the binding of procaspase-8 to the death effector domain (DED) of FADD at the DISC to block the activation of death receptor signaling required for apoptosis. Increasing evidence shows that defects in FADD protein expression are associated with progression of malignancies and resistance to apoptosis. Therefore, improved expression and function of FADD may provide new paradigms for regulation of cell proliferation and survival in cancer. In the present study, we have examined the potential of FADD in induction of apoptosis by overexpression of FADD in HEK 293T cells and validated further its consequences on the expression of pro and anti-apoptotic proteins besides initiation of death receptor mediated signaling. We have found deficient expression of FADD and elevated expression of cFLIPL in HEK 293T cells. Our results demonstrate that over expression of FADD attenuates the expression of anti-apoptotic protein cFLIP and activates the cascade of extrinsic caspases to execution of apoptosis in HEK 293T cells.
Keywords: Apoptosis, Fas associated death domain (FADD), cFLIP, Death receptor mediated apoptosis
Introduction
Apoptosis is a programmed mechanism of cell death that is essential for proper embryonic development and tissue homeostasis. Apoptosis is mainly triggered by activation of caspases through complex signaling, which include death receptor (extrinsic) and mitochondrial dependent (intrinsic) (Taylor et al. 2008; Vaux et al. 1994). The death receptor mediated apoptosis is initiated by binding of death inducing signals to their cognate receptors at the cell surface which trigger the signals for activation of initiator and effector caspases for cell death (Holler et al. 2003). The death receptor mediated apoptosis is mainly contributed by group of receptors like CD95, TRAIL-R1 and TRAIL-R2 which belongs to the tumor necrosis factor-1 (TNF-1) receptor superfamily-1. These receptors have a characteristic death domain (DD) at its cytoplasmic tail which homophilically interacts with cytosolic DD containing Fas-associated death domain protein (FADD) adaptor molecule that is essential for transducing the apoptotic signals (Holler et al. 2003; Schulze-Osthoff et al. 1998).
Several reports highlight that this multiple functional protein FADD is associated with apoptotic and non-apoptotic functions including cell proliferation, cell cycle progression, tumor development, inflammation, innate immunity and autophagy (Beisner et al. 2003; Chinnaiyan et al. 1996; Osborn et al. 2010; Tourneur et al. 2005; Yeh et al. 1998; Zhang et al. 1998). The function of FADD is dictated by its localization and state of phopshorylation. The first role ascribed for FADD was to transmit apoptotic signals through its interaction with death receptors expressed at the cell membrane, hence it has been speculated that FADD is exclusively localized in the cytoplasm. However, recent reports demonstrate that FADD protein also possesses nuclear localization and export signals (Bell et al. 2008; Gomez-Angelats and Cidlowski 2003). The phosphorylated form of FADD has been found in the nucleus and implicated in cell-cycle regulation, although the mechanism of which is not yet clear. Aberrant regulation of FADD is associated with cancer and inflammatory disorders (Screaton et al. 2003). Earlier reports suggest that defects in FADD protein expression are corroborated with tumor progression in both mice and humans (Tourneur and Chiocchia 2010; Tourneur et al. 2003). Thus FADD is crucial for consequent cell death and survival. FADD contains two distinct domains, C-terminal death domain (DD) and N-terminal death effector domain (DED), which provides docking site for homophilic interaction, oligomerization and autocatalytic processing to activation of downstream apoptotic signals. The DD of FADD interacts with DD of the death receptors and DED allows to recruit DEDs carrying proteins like pro-caspase-8/10, which in turn initiates the formation of a death inducing signaling complex (DISC) (Tourneur et al. 2004). The initiation of DISC formation facilitates autocatalytic processing of caspases 8/10 and releases active enzyme into the cytoplasm to cleave and activate effector caspases such as caspase-3 and caspase-7, leading to a cascade of events in apoptotic cell death (Chinnaiyan et al. 1995; Peter and Krammer 2003).
The death receptor mediated apoptosis is effectively regulated by anti-apoptotic protein cFLIP (cellular fas-associated death domain-like interleukin-1-β-converting enzyme-inhibitory protein) which is structurally similar to procaspase-8 and -10 but lacks cysteine residue for autocatalytic activity (Algeciras-Schimnich et al. 2002; Irmler et al. 1997; Krueger et al. 2001). Upon recruitment with FADD into the DISC, the cFLIP protein competitively inhibits the binding and activation of procaspase-8 and hinders apoptosis when expressed at a high level. Dysregulation of cFLIP expression is a consistent feature in autoimmune diseases and several cancer types (Bagnoli et al. 2009; Matsuda et al. 2008; Rogers et al. 2007; Safa et al. 2008). In contrast, cFLIP exists in two more prominent forms as a long (cFLIPL) or as a short variant (cFLIPS/cFLIPR). cFLIPL contains two death effector domains (DED) and one inactive caspase like domain, whereas cFLIPS lacks the entire caspase like domain but has two DEDs (Longley et al. 2006). While all forms of cFLIP are believed to function as anti-apoptotic proteins that compete with procaspase-8 for recruitment into the DISC via their DEDs when expressed at high levels, however low level of cFLIP promotes activation of caspase-8 (Bagnoli et al. 2010; Ryu et al. 2001). Recent report suggests that downregulation of pro-apoptotic molecules like Fas and caspase-8 or upregulation of antiapoptotic molecules like FLICE inhibitory protein block the death signal received via death receptors (Yu et al. 2009). The growing body of evidence suggests that elevated expression of cFLIP and low level of FADD may lead to greater resistance to apoptosis (Shirley and Micheau 2010). However, despite the significant advances in understanding the death receptor mediated apoptosis, regulation of FADD and cFLIP remains underexplored. Therefore, an insight about regulated expression of cFLIP and FADD for the induction of apoptosis is important. In the present study we have found deficient expression of FADD and elevated expression of cFLIPL in HEK 293T cells. Moreover, we have investigated the consequences of FADD mediated signaling for execution of apoptosis in HEK 293T cells. These studies demonstrate that attenuation in the expression of cFLIP constitutes an important stratagem used by FADD for orchestrating the activation of the extrinsic pathway of apoptosis.
Materials and method
Reagents
Molecular biology grade reagents and antibodies were obtained from commercial sources as indicated. Poly-L lysine, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), cycloheximide, 4′, 6-diamidino-2-phenylindole-dihydrochloride (DAPI), protease inhibitor cocktail, Trypan blue and BCA protein estimation kit were purchased from Sigma-Aldrich (St. Louis, MO, USA). CD 95L was purchased from Prospec (Israel). Annexin-V FITC Apoptosis detection kit and Caspase-8/FLICE Fluorometric assay kit were purchased from BioVision, (Mountain View, California). Protein molecular weight marker and Penicillin, streptomycin, neomycin (PSN) antibiotic cocktail were purchased from Invitrogen (UK). Dulbecco’s Modified Eagle’s Medium (DMEM), Dulbecco’s phosphate buffer saline (DPBS) and fetal bovine serum (FBS) were purchased from Lonza (Switzerland). PVDF membrane was obtained from Bio-Rad (Philadelphia, USA). Reagents for enhanced chemiluminescence were purchased from Biological Industries (Israel). Rabbit polyclonal antibodies against FADD (ab55399), cFLIP (ab8421), pro-caspase 8 (ab52183) and β-actin (ab8227) were purchased from Abcam (Cambridge, UK). Monoclonal anti-caspase-3 (9662) was obtained from Cell Signaling (Danvers, MA). Goat anti-rabbit immunoglobulin conjugated to HRP (horseradish peroxidase) secondary antibody was purchased from GE Healthcare (Amersham). All other chemicals of analytical grade were used and purchased from Merck (Darmstadt, Germany).
Cell culture
Human embryonic kidney 293T (HEK 293T) cell line used in this study was obtained from ATCC (Manassas, VA, USA). The HEK 293T cells were grown in DMEM containing L-Glutamine (2 mmol/L) supplemented with 10 % fetal bovine serum. In addition, an antibiotic cocktail consisting of penicillin (5 mg/ml), streptomycin (5 mg/ml) and neomycin (10 mg/ml) (GIBCO, Invitrogen, UK) was added in culture media. The cells were cultured in a CO2 containing 95 % O2 and 5 % CO2 at 37 °C. Exponentially growing cells were used for all the experiments.
Transfection and expression of YFP-FADD in HEK 293T cells
The full length FADD cloned in pEYFP-C2 was a kind gift from Dr. Andrew Thorburn, University of Colorado Denver, Aurora, USA. The plasmid was isolated by miniprep using plasmid isolation kit (Qiagen, USA) according to manufacturer’s protocol. Further, HEK 293T cells were transfected by calcium phosphate transfection method (Song and Lahiri 1995). In brief, 3.5 × 105 cells were seeded in 6-well plates and incubated for 16-24 h. Media were changed 1 h prior to transfection and fresh media added to each wells. The reaction mixture containing 2 μg of DNA, 15 μl of Calcium Chloride, 150 μl of 2X HEPES buffer in a final volume of 300 μl was added to each well and incubated for 8 h in a CO2 incubator (NuAire, Plymouth, USA) supplied with 5 % CO2 and 95 % O2 at 37 °C. Culture media was aspirated from the wells after every 24 h and replaced with fresh medium. Transfection efficiency was examined under a fluorescence microscope (DP71, Olympus, Japan) at different time intervals. Furthermore, cytoplasmic or nuclear localization of YFP-FADD has been determined by 4′, 6-diamidino-2-phenylindole (DAPI) staining. The cells were stained with DAPI (1 μg/ml in PBS) for 15 min in dark and observed under fluorescence microscope. Further expression of FADD in HEK 293T cells was confirmed by western blot analysis.
Analyses of cell death
To examine the commencement of cell death in FADD overexpressed HEK 293T cells, trypan blue exclusion assay and propidium iodide exclusion assay were carried out at 24–96 h in different time intervals. In brief, transfected cells at various time intervals were harvested, washed once with Dulbecco’s Phosphate Buffered Saline (DPBS) and resuspended in 100 μl of DPBS containing 0.4 % trypan blue and incubated for 15 min in the dark at 37 °C. The cell suspension (10 μl) was placed on Neubauer’s slide of hemocytometer and live and dead cells were counted under a light microscope (Olympus, Japan). The percentage of cell death was calculated (% cell death = Number of dead cells/Total number of cells x100). In addition, consequence of cell death in FADD overexpressed HEK 293T cells was monitored by morphological analysis and propidium iodide (PI) exclusion assay followed by their examination under the fluorescence microscope (DP, 71, Olympus, Japan). In brief, 5 × 105 cells were grown onto poly L-lysine treated coverslip in 6 well plates containing DMEM culture media. At given time periods cover slips were removed from the wells and adherent cells on cover slip were washed once with DPBS and thereafter, cells were incubated in mixture of 100 μl of binding buffer along with 1 μl of propidium iodide (20 μg/ml) followed by manufacturer’s instructions (AnnexinV-FITC apoptosis detection kit, Biovision, USA). Apoptotic nuclei stained with propidium iodide and were examined under fluorescent microscope. More than 100 cells from random fields were examined to assess cell death. All the images were analyzed by image analysis software (Image-Pro MC 6.1, Bethesda, MD, USA).
Stimulation of CD 95L and Cycloheximide (CHX) on FADD overexpressed HEK 293T cells
The death receptor mediated cell death was examined in FADD overexpressed HEK 293T cells in the presence of CD 95L and CHX individually and in combination. The cells were grown on poly L-lysine treated cover slips as mentioned earlier. Cells were treated with CD 95L (200 ng/ml) and CHX (5 μg/ml) after 48 h of FADD transfection. Induction of cell death at different intervals was examined by using propidium iodide (PI) and trypan blue exclusion assay as described earlier. In addition CHX treated HEK 293T cells were stained with Annexin-V FITC and propidium iodide staining where as YFP-FADD transfected cells were stained with propidium iodide only.
Western blotting for detection of apoptosis regulatory proteins
To determine the impact of over expression of FADD induced apoptosis in HEK 293T cells, the expression of cell death regulatory proteins including, FADD, cFLIP, procasapse 8 and caspsase 3 were examined by western blot analysis. The FADD overexpressed HEK 293T cells at 48 h were treated with CD 95L (1–4 h) and CHX (2–8 h) in individual and in combination (1–4 h) for different time intervals. In brief, incubated cells were harvested at mentioned time point and washed twice in DPBS. Cells were resuspended in lysis buffer (50 mM TrisCl, pH 8; 150 mM NaCl, 1 mM MgCl2, 150 mM CaCl2, 1 mM PMSF, 1 mM Na-Vandate, 0.05 % Nonidet P-40) containing protease inhibitor cocktail on ice for 30 min followed by vortexing and centrifugation at 8,000 rpm (5415R, Eppendorf, Germany) for 10 min at 4 °C. The resulting supernatant was collected and protein concentration was determined using a BCA protein estimation kit (Sigma Aldrich, USA) according to manufacturer’s protocol. The equal amount of protein (30 μg) from each sample was fractionated on 12 % SDS-PAGE and transferred to PVDF membrane by wet electro-blotting method at 4 °C. The membrane was blocked with 5 % nonfat milk in Tris buffered saline containing 0.05 % Tween 20 (TBST) for 3 h at room temperature followed by incubation with primary antibody of anti-FADD (1:500), anti-cFLIP (1:500), anti-procaspase 8 (1:500), anti-caspase-3 (1:1000) and anti-β-actin (1:500) for overnight at 4 °C. After washing with TBST, the membrane was probed with horseradish peroxidase conjugated secondary antibody. Expression of immune reactive protein was detected by using EZ-ECL kit according to the instruction manual and developed in Kodak X-Omat blue film (NEN Life Sciences, Inc., Boston, MA) in the dark.
Determination of caspase 8 activity
Caspase 8 activity was determined in FADD transfected HEK 293T cells at 24–96 h. Similarly caspase 8 activity was determined in FADD overexpressed cells at 48 h treated with CD 95L (1–4 h) and CHX (2–8 h) in individually and in combination. The assay was performed using the Caspase-8/FLICE fluorometric assay kit according to the manufacturer’s instructions (Biovision, U.S.A.). In brief, HEK 293T cells were washed twice with the DPBS and spun at 6000 rpm (5415R, Eppendorf, Germany) for 10 min. The pellet was resuspended in 50 μl of chilled lysis buffer and incubated on ice for 10 min. 50 μl of lysate was mixed with equal volume of reaction buffer and thereafter substrate IETD-AFC was added in the reaction mixture. The incubation was done at 37 °C for 30 min. At the end of incubation, the liberated fluorescent group AFC emits a yellow-green fluorescence was determined on a SHIMADZU (Japan) spectrofluorimeter (Ex = 400 nm; Em = 505 nm). The results represent fold increase in relative fluorescence at different time points.
Total RNA extraction and real-time polymerase chain reaction (RT-PCR)
The expression of mRNA of cFLIPL, endogenous FADD and procaspase-8 was carried out in FADD transfected HEK 293T cells at 24–96 h. Total RNA was isolated with Trizol reagent (Invitrogen, UK) according to manufacturer’s protocol. The concentration of total RNA was determined by taking the absorbance at 280 nm under UV–vis spectrophotometer (PG Instrument, USA, T90). 1 μg of total RNA was reversely transcribed and synthesized to cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). A Real-time PCR was performed using the StepOne plus real-time PCR detection system (Applied Biosystems, Carlsbad, California). The reaction was conducted with 20 μl final reaction volume containing 4 μl of cDNA (230pmolar), 10 μl iQ™ SYBR® Green Supermix (Bio-Rad, USA), and 2 μl of (1 μM) each primer and remaining 2 μl of nuclease free water. All reactions were performed in MicroAmp fast optical 96-well PCR plates (Applied Biosystem) and sealed with optical adhesive covers (Applied Biosystem). 18SrRNA was used as the endogenous controls. Thermal cycler conditions were as follows: After denaturing at 95 °C for 10 min, PCR was performed for 40 cycles, each of which consisted of denaturing at 95 °C for 15 s, annealing/extending at 65 °C for 1 min. Afterwards, final PCR products were heated to 72 °C for 30 s and the expected size products were confirmed by melting curve analysis. These following sets of PCR primers were used. cFLIP Forward, 5′-TGGCCTCCCTCAAGTTCCT-3′ and reverse, 5′-TGGAATAACATCAAGGCATCCTT-3′, endogenous FADD Forward, 5′-GTGTGCGGGAGTCACTGAGA-3′ and reverse, 5′-GGGCCACTGTTGCGTTCT-3′; Procaspase 8 Forward, 5′-TGGCCTCCCTCAAGTTCCT-3′ and reverse, 5′-TGGGTTCTTGCTTCCTTTGC-3′ 18SrRNA Forward, 5′-AGAAACGGCTACCACATCCAA-3′and reverse, 5′ TGTCACTACCTCCCCGTGTCA-3. Each assay was normalized by using the difference in critical thresholds (CT) between target genes and 18SrRNA. The expression of mRNA of respective genes was compared with control (empty vector, pEYFP without FADD) transfected HEK 293T cells using the values of 2-ΔΔCT.
Statistical analysis
Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Duncan’s multiple range t-test using SigmaStat statistical analysis software. Values were expressed as mean ± S.E.M. from three independent experiments. Pairwise and multiple comparisons versus control group were performed to evaluate the significant difference between the different groups. Differences were considered statistically significant at P ≤ 0.05 & 0.001. * indicates P ≤ 0.05 and ** P ≤ 0.001.
Results
Overexpression of FADD initiates cell death
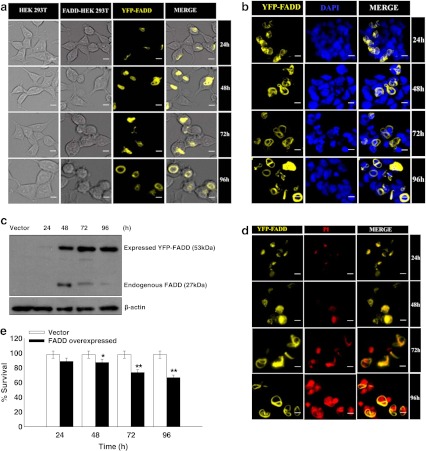
The potential role of FADD in apoptosis progression is manifested by its availability at the DISC in adequate amounts. However, its expression in tumors is inadequate allowing them to evade programmed cell death (Tourneur and Chiocchia 2010; Tourneur et al. 2004). Hence, initially we examined the functional localization and the expression of YFP-FADD in HEK 293T cells at 24, 48, 72 and 96 h post-transfection of FADD. All together, HEK 293T cells were counterstained with nuclear staining dye 4′ 6′-diamidino-2-phenylindole (DAPI) which is showing that YFP-FADD is constitutively expressed in the cytoplasm and after 48 h of post transfection tends to get localized in the periphery of the cell membrane. Interestingly, appearance of apoptotic features like formation of apoptotic bodies, cell shrinkage, and surface detachment were also noticed around the same time (Fig. 1a & b). Next, we confirmed the expression of FADD by western blot using FADD antibody. We found prominent expression of YFP-FADD (~53 kDa) in HEK 293T cells after 24 h of transfection. However, expression of FADD was absent in HEK 293T cells transfected with vector pEYFP only (pEYFP without FADD). Interestingly, expression of endogenous FADD appeared at 48 h post transfection but the expression was depleted at 72 and 96 h (Fig. 1c). This outcome suggests that FADD express in the cytosol under apoptotic stresses to initiate apoptosis. In addition, apoptotic cell death was confirmed by propidium iodide exclusion assay which showed that gradual increase of apoptotic nuclei in FADD overexpressed conditions in a time dependent manner (Fig. 1d). Moreover, trypan blue exclusion assay for cell viability also showed an increase in cell death when FADD is expressed (Fig. 1e). Thus these above results indicate that over expression of FADD has an immense potential for induction of cell death in HEK 293T cells.
Fig. 1.
Overexpression of FADD mediated apoptosis in HEK 293T cells: HEK 293T cells were transfected with FADD-pEYFP plasmid. a Cellular localization and morphological analyses of pEYFP-FADD transfected HEK 293T cells at 24–96 h. Left panel is showing empty vector (pEYFP without FADD) transfected HEK 293T cells as a control. Yellow fluorescence is showing expression of FADD in HEK293T cells localize to periphery of cell membrane. Morphology of cells was observed under bright field microscope. Figure shown are representative of more than 150 cells analyzed from random fields, scale bar represents 2 μm. b Localization of YFP-FADD was monitored by DAPI (1 μg/ml) staining on FADD transfected HEK 293T cells at 24–96 h of post transfection. Images showing YFP-FADD and DAPI stained nuclei were co-localized in a common panel. The data shown are representative of more than 150 cells analyzed of random fields. Scale bar represents 2 μm. c Cytosolic expression of YFP-FADD (~ 53 kDa) and endogenous FADD (~27 kDa) was analyzed by western-blot for the designated time points, empty vector (pEYFP without FADD) transfected HEK 293T cells were taken as control and β actin was used as a loading control. d Apoptotic cell death in FADD transfected HEK 293T cells at 24–96 h were examined with propidium iodide staining shown in red observed under fluorescent microscope. YFP-FADD and PI stained nuclei were merged in a common panel. The figure shown is representative of more than 100 cells analyzed of random fields. Scale bar represents 2 μm. e Quantitative measurement of cell death was carried out by trypan blue assay at the 24–96 h. Empty vector (pEYFP without FADD) transfected HEK 293T cells were taken as control. Error bars represent mean ± SEM from three independent experiments. P value indicates *P ≤ 0.05 and **P ≤ 0.001.
Overexpression of FADD attenuates expression of cFLIP and activates cascades of extrinsic caspases
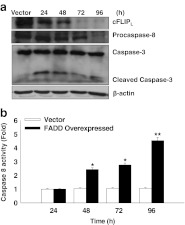
We next investigated the expression of the cell death regulatory protein cFLIPL and extrinsic caspases in FADD transfected HEK 293T cells at different time intervals. The role of cFLIP in death receptor mediated signaling pathway has been studied extensively (Beisner et al. 2003; Osborn et al. 2010; Scaffidi et al. 2000; Yeh et al. 1998; Zhang et al. 2001). The earlier reports suggest that elevated expression of cFLIP blocks the activation of pro-caspase-8 which is necessary for the execution of apoptosis (Golks et al. 2006; Shirley and Micheau 2010). Therefore, we examined the expression of cFLIPL and pro-caspase-8 and caspase-3 in FADD over expressed conditions. We found that overexpression of FADD in HEK 293T cells lead to a progressive loss in the cFLIPL expression from 48–96 h compared to control (HEK 293T cells transfected to an empty vector pEYFP) (Fig. 2a). Next we examined the strength of caspase activation for apoptosis. Our results showed that reduction in the level of procaspase-8 was time dependent, which indicates processing and activation of procaspase-8 on availability of FADD expression (Fig 2a). The activity of major initiator caspase-8 was found to significantly increase in FADD over expressed conditions for 24–96 h compared to the controls (vector transfected cells) (Fig. 2b), which may activate downstream effector caspases 3. To confirm this we examined the activation of caspase-3 at different time points by western blotting. We found subsequent activation of caspase 3 by cleavage of its subunits at 24 h onwards (Fig. 2a). The results obtained strongly indicate that overexpression of FADD attenuates expression of anti-apoptotic protein cFLIPL which results in the subsequent activation of cascade of extrinsic caspases to execution of apoptosis in HEK 293T cells.
Fig. 2.
Over expression of FADD attenuates expression of cFLIP and activates cascades of extrinsic caspases. a Expression of cFLIPL procaspase-8 and caspase-3 in cytosolic fractions was examined by western blot analysis at 24–96 h post transfection of YFP-FADD in HEK 293T cells, empty vector (pEYFP without FADD) transfected HEK 293T cells of 96 h were taken as control. b Activity of caspase 8 in YFP-FADD transfected HEK 293T cells at 24–96 h were determined using IETD-AFC substrate. Empty vector (pEYFP without FADD) transfected cells were taken as control. Error bars represent mean ± SEM from three independent experiments. P value indicates *P ≤ 0.05 and **P ≤ 0.001.
Overexpression of FADD down regulates expression of cFLIP mRNA to execute apoptosis
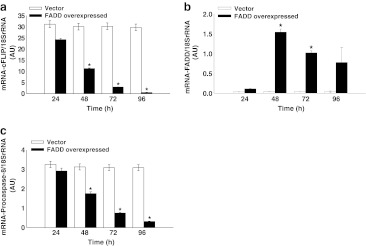
We examined the mRNA expression of cFLIPL, endogenous FADD and procaspase-8 in FADD overexpressed HEK 293T cells at different time points using real-time quantitative PCR (RT-qPCR). The data obtained illustrate that expression of cFLIPL mRNA is getting down regulated with progression of FADD expression in HEK 293T cells compared to control (empty vector transfected cells) from 24–96 h (Fig. 3a). Although, expression of endogenous FADD mRNA was found lower at 24 h but it was moderate at 48–96 h, however poor basal expression of FADD was observed in control (vector transfected HEK293T cells) (Fig. 3b). In addition, we further monitored the mRNA expression of procaspase-8 in FADD overexpressed HEK 293T cells and we found a significant down regulation of procaspase-8 in a time dependent manner compared to vector control (Fig. 3c). Thus, these results indicate that the availability of FADD has an eminent potential in regulation of anti-apoptotic protein cFLIP and execution of caspase dependent apoptosis.
Fig. 3.
Real-time quantitative PCR analyses for transcriptional expression of cFLIPL, FADD and procaspase-8. a Expression of cFLIPL mRNA at 24–96 h. b Expression of endogenous FADD mRNA at 24–96 h. c Expression of procaspase-8 mRNA at 24–96 h. Total RNA was isolated from YFP-FADD transfected and empty vector (pEYFP without FADD) transfected HEK 293T cells at 24–96 h and after the synthesis of cDNA, RT-qPCR was performed. The values were normalized by using the difference in critical thresholds (CT) between target gene and 18SrRNA (endogenous control). The expression of mRNA of respective genes in FADD transfected HEK 293T cells were compared with control (empty vector transfected HEK 293T cells) using the values of 2−ΔΔCT. Error bars represent mean ± SD from three independent experiments. P value indicates *P ≤ 0.05.
CD 95L stimulation on FADD overexpressed cells promote extrinsic apoptosis by attenuating the level of cFLIP
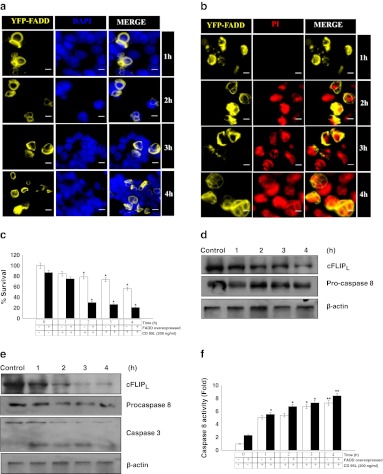
As CD 95L quickly stimulates the DISC formation through the adaptor molecule FADD to activate death receptor mediated apoptosis. Therefore, we extended our study to gain insight into the response of CD 95L stimulation on FADD overexpressed HEK 293T cells. Taken together, earlier data shows that the expression and localization of FADD in HEK 293T cells were prominent at 48 h of post transfection. Therefore, experiments were performed after 48 h of FADD transfected HEK 293T cells for 1–4 h. Our results show that CD 95L stimulation for 1–4 h quickly initiates cell death within 2 h (Fig. 4a, b & c). Although it has recently been reported that cFLIP expression inhibits CD 95L or TRAIL induced non-apoptotic signaling (Kavuri et al. 2011). Hence, we monitored the apoptotic events and expression of cFLIP upon stimulation with CD 95L on HEK 293T cells and YFP-FADD transfected HEK 293T cells. We found that the levels of cFLIPL and apoptotic events in HEK 293T were moderately reduced at 1–4 h upon stimulation with CD 95L (Fig. 4d). In contrast, FADD over-expressed cells stimulated with CD 95L showed a significant reduction in the levels of cFLIPL which leads to activation of procaspase-8 and downstream effector caspase 3 for execution of cell death (Fig. 4e). In addition, we measured the activity of capsase 8 upon CD 95L treatment to HEK 293T cells for the given time duration 1–4 h. The results show that activation of caspase 8 was noticed 1 h onward stimulated with CD 95L compared to the control (Fig. 4f). Indeed, FADD overexpressed cells stimulated with CD 95L showed profound activation of caspase 8 for 1–4 h (Fig. 4f). These obtained results suggest that CD 95L in the presence of FADD promotes apoptosis in a striking manner through the death receptor pathway.
Fig. 4.
Cell death and expression of cell death regulatory proteins in FADD overexpressed HEK 293T cells treated with CD 95L. a Localization of YFP-FADD upon CD 95L treatment (200 ng/ml) was monitored by DAPI (1 μg/ml) counterstaining on 48 h post FADD transfected HEK 293T cells for 1–4 h. Images showing YFP-FADD and DAPI stained nuclei were co-localized in a common panel. Figures shown are representative of more than 150 cells analyzed of random fields. Scale bar represents 2 μm. b Propidium iodide staining for detection of apoptotic cell death upon stimulation of CD 95L (200 ng/ml) on 48 h post FADD transfected HEK 293T cells for 1–4 h. Nuclei shown in red display apoptotic cell death. Figures shown are representative of more than 150 cells analyzed of random fields. Scale bar represents 2 μm. c Quantitative measurement of cell death upon stimulation of CD 95L on HEK 293T cells and 48 h post FADD transfected HEK 293T cells were carried out by trypan blue assay. Control represents 48 h of incubated HEK 293T cells and 48 h of FADD transfected HEK 293T cells without treatment of CD 95L. Error bars represent mean ± SEM from three independent experiments. d Expression of cFLIPL and procapsase 8 proteins in cytosolic fraction of HEK 293T cell stimulated with CD 95L for 1–4 h was examined by western blot analyses. CD 95L untreated cells incubated for 48 h in culture media were taken as a control and β actin was used as a loading control. e Expression of cFLIPL,procapsase 8 and procaspase 3 proteins in cytosolic fraction of 48 h post FADD transfected HEK 293T cell stimulated with CD 95L for 1–4 h was examined by western blot analyses. Without CD 95L treated 48 h post FADD transfected cells were taken as control and β-actin was used as a loading control. f Activity assay of caspase 8 was determined in HEK 293T cells and post 48 h FADD transfected HEK 293T cells upon stimulation with CD 95L for mentioned time points using IETD-AFC as a substrate. Control represents 48 h of incubated HEK 293T cells and 48 h post FADD transfected HEK 293T cells without treatment of CD 95L. Error bars represent mean ± SEM from three independent experiments. Error bars represent mean ± SEM from three independent experiments. The P value indicates *P ≤ 0.05 and **P ≤ 0.001.
Combination of Cyclohexamide and CD 95L in FADD over expressed cells rapidly downregulates cFLIP and augments apoptosis
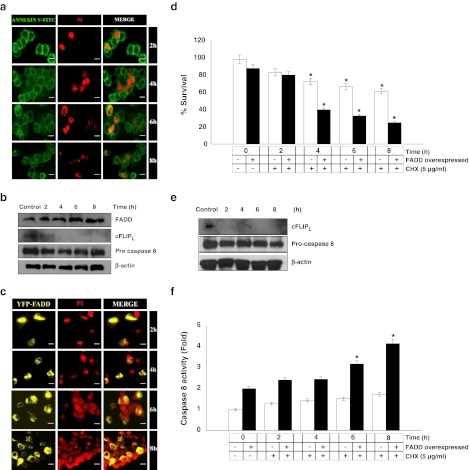
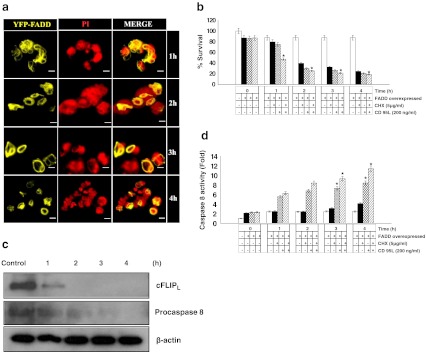
Cycloheximide (CHX) is a potent protein synthesis inhibitor and it is also known to sensitize CD95 mediated apoptosis by regulating the expression of cFLIP (Fulda et al. 2000; Wajant et al. 2000). However, under the FADD overexpressed conditions the effect of CHX treatment and in the added presence of CD 95L is not known. To address this issue FADD overexpressed HEK 293T cells were subjected to treatments with CHX and CD 95L individually and in combination for different time durations and cell death was analyzed with concurrent re-evaluation of extrinsic apoptotic signaling. Consistent with the earlier reports, CHX significantly inhibits the expression of cFLIPL but did not affect the expression of procaspase 8 and FADD [39]. In the present study we found that after CHX treatment, cFLIPL protein completely disappeared compared to the untreated cells, but no changes were observed in the protein level of FADD and procaspase-8 (Fig 5a & b). This outcome suggests that cFLIP expression was highly sensitive towards CHX treatment without affecting that of pro-apoptotic proteins. To assess further the effect of CHX treatment on FADD overexpressed HEK 293T cells, we analyzed the expression of pro-apoptotic proteins and commencement of apoptosis. Cell viability and propidium iodide exclusion assay showed major cell death in FADD overexpressing cells as compared to CHX alone. Interestingly, protein expression data showed that expression of cFLIPL was diminished with a concurrent decrease of procaspase-8 and an increase of caspase-8 activity (Fig. 5c–f). These results suggest that a reduction in the cFLIP protein expression by CHX treatment alleviates the dominant anti-apoptotic effect of cFLIP at DISC and facilitates the FADD mediated extrinsic apoptotic death. Finally, we examined the execution of cell death upon CD 95L treatment in the presence of sub-lethal concentration of CHX which leads to a gain in sensitivity to early induction of apoptosis (Fig. 6a & b). Moreover, protein expression data showed that level of cFLIPL rapidly decreased after 1 h of treatment with CHX and CD 95L with a concomitant reduction in the level of procaspase-8 at 4 h (Fig. 6c). Similarly, the activity of caspase-8 found increased when FADD overexpressed cells were treated with CHX and CD 95L together (Fig. 6d). Thus, our results indicate that FADD overexpressed cells in the presence CD 95L and CHX underwent increased apoptotic signaling in a striking manner by suppressing the expression of cFLIPL.
Fig. 5.
Cell death and expression of cell death regulatory proteins in FADD overexpressed HEK 293T cells treated with CHX. a Cycloheximde dependent apoptotic cell death was monitored by Annexin V-FITC/PI staining. HEK 293T cells were subjected to CHX treatment (5 μg/ml) for 2–8 h and stained with Annexin V-FITC and PI. Nuclei shown in red are indicating early induction of cell death. Figures shown are representative of more than 200 cells analyzed of random field. Scale bar represents 2 μm. b Expression of FADD, cFLIPL, and procapsase 8 proteins in the cytosolic fraction of HEK 293T cell stimulated with CHX for 2–8 h was examined by western blot analyses. Untreated HEK 293T cells were taken as an control and β-actin was used as a loading control. c–f 48 h post FADD transfected HEK 293T cells were treated with CHX (5 μg/ml) for 2–8 h and cell death, expression of apoptosis regulatory proteins and activity of caspase 8 were examined. c Propidium iodide staining of 48 h post FADD transfected HEK 293T cells treated with CHX (5 μg/ml) for 2–8 h. Nuclei shown in red display apoptotic cell death. Figures shown are representative of more than 150 cells analyzed from random fields. Scale bar represents 2 μm. d Quantitative measurement of cell death upon treatment of CHX on HEK 293T cells and 48 h post FADD transfected HEK 293T cells was carried out by trypan blue assay. Control represents 48 h of incubated HEK 293T cells and 48 h of FADD transfected HEK 293T cells without treatment of CHX. Error bars represent mean ± SEM from three independent experiments. e Expression of cFLIPL, and procapsase 8 proteins in cytosolic fraction of 48 h post FADD transfected HEK 293T cell stimulated with CHX for 2–8 h was examined by western blot analyses. Without CHX treated 48 h post FADD transfected HEK 293T cells were taken as a control and β-actin was used as a loading control. f Caspase 8 activity was determined in untransfected HEK 293T cells and post 48 h FADD transfected HEK 293T cells upon stimulation with CHX for mentioned time points using IETD-AFC as a substrate. Control represents 48 h of incubated HEK 293T cells and 48 h post FADD transfected HEK 293T cells without treatment of CHX. Error bars represent mean ± SEM from three independent experiments. Error bars represent mean ± SEM from three independent experiments. The P value indicates *P ≤ 0.05
Fig. 6.
Cell death and expression of cell death regulatory proteins in FADD overexpressed HEK 293T cells upon combined treatment of CD 95L and Cycloheximide (CHX). 48 h post FADD transfected HEK 293T cells were subjected to combine treatment of CD 95L (200 ng/ml) and CHX (5 μg/ml) for 1–4 h. a Cells death analysis by Propidium iodide exclusion assay. Nuclei shown in red display apoptotic cell death. Figures shown are representative of more than 150 cells analyzed from random fields. Scale bar represents 2 μm. b Quantitative measurement of cell death were carried out by trypan blue assay. Control represents 48 h of incubated HEK 293T cells and 48 h post FADD transfected HEK 293T cells without treatment of CHX and CD 95L. Error bars represent mean ± SEM from three independent experiments. c Expression of cFLIPL, and procapsase 8 proteins in cytosolic fraction of 48 h post FADD transfected HEK 293T cells treated with CHX and CD 95L were examined by western blot analyses at given time points. Untreated 48 h post FADD transfected HEK 293T cells were taken as controls and β actin was used as a loading control. d Caspase-8 activity in presence of CD 95L and CHX in individual and in combination on FADD overexpressed HEK 293T cells was determined using IETD-AFC as a substrate. Control represents 48 h of incubated HEK 293T cells and 48 h of FADD transfected HEK 293T cells without treatment of CHX and CD 95L. Error bars represent mean ± SEM from three independent experiments. P value indicates *P ≤ 0.05
Discussion
FADD is the central adaptor molecule which is essential for all the death receptor mediated pathways for the initiation of apoptosis. However, an interestingly it is realized that the regulated expression of FADD is involved in embryonic development, cell survival, proliferation, cell cycle progression, inflammation, innate immunity, necrosis, and autophagy (Beisner et al. 2003; Chinnaiyan et al. 1996; Osborn et al. 2010; Tourneur et al. 2005; Yeh et al. 1998; Zhang et al. 1998). As a consequence, a defect in the FADD molecule can contribute to the development of pathological conditions, particularly cancer. The absence of FADD protein expression enhances cell proliferation in the mouse. Also an under-expression of FADD as a prognostic factor for poor response to chemotherapy in humans has been highlighted earlier (Tourneur et al. 2005). Hence, it is important to understand how the expression of FADD regulates apoptosis. We have found deficient expression of FADD and elevated expression of cFLIPL in HEK 293T cells. However upon overexpression of FADD promotes cell death in HEK 293T cells. Earlier reports have shown that the DED region of FADD recruits it to the cytoplasmic side of the cell membrane and rapidly recruits the procaspase-8 to initiate cell death (Peter and Krammer 2003; Scaffidi et al. 2000; Schulze-Osthoff et al. 1998). Thus the function of FADD depends upon its localization and expression. Earlier reports have shown that while FADD possesses a strong nuclear localization and nuclear export signal. It functions within cytoplasm upon treatment with apoptotic stimuli (Foger et al. 2009). In the present study we found that overexpression of FADD in HEK 293T cells showed a cytosolic localization and expression which has been noticed from 48 to 96 h of post transfection. However, expression of endogenous FADD in cytosolic fraction was noticed at 48 h onwards but tends to decrease at 72 and 96 h. This indicates that protein expression of endogenous FADD appears in the cytosol with induction of apoptotic stresses and decreases with progression of cell death. Whereas, overexpressed FADD accumulates predominantly in the cytosol and commences the extrinsic signaling for induction of apoptosis.
Although, death receptor mediated apoptosis is negatively regulated by apoptosis regulatory protein cFLIP (Krueger et al. 2001). cFLIP acts by modulating the activity of pro-caspase-8 and inhibits death receptor (DR) mediated apoptosis. In DR signaling procaspase-8 and cFLIPL are both known to oligomerize at DED domain of FADD but because of the high affinity of cFLIPL for DISC. It excludes procaspase-8 from DISC which interferes with efficient DISC formation when the concentration of cFLIP are high (Irmler et al. 1997; Safa et al. 2008). An earlier study demonstrates that cFLIPL has a high affinity for the DISC and competes with procaspase-8 for recruitment to the death receptor complex and blocks the activation of procaspase-8 (Scaffidi et al. 2000). Thus, cFLIP is an inhibitory molecule for procaspase 8 and thereby promotes cell survival by impeding apoptosis. Among the different forms of cFLIP(L/S/R), cFLIPL has been described to play both a pro and anti-apoptotic role depending upon its level of expression (Golks et al. 2005). Thus, low concentration in the cells, cFLIPL permits procaspase-8 activation at the DISC, but at higher concentration it inhibits death receptor mediated apoptosis (Chang et al. 2002; Yu et al. 2009). Hence, we sought to determine whether overexpression of FADD can attenuate expression of cFLIPL. Interestingly our results show that increased expression and peripheral localization of FADD in HEK 293T cells initiates cell death by activation of procaspase-8 which in turn leads to activation of caspase 8 and downstream effector capsase 3. More interestingly, increased expression of FADD markedly attenuates the expression of cFLIPL compared to vector transfected HEK 293T cells. Thus, these findings clearly demonstrate that availability of FADD in sufficient amount is able to initiate apoptosis by providing more FADD for the activation of pro-caspase-8 at DISC and facilitated activation of caspase-8 and downstream effector caspase 3 by counteracting the action of cFLIPL. Therefore, the level of FADD and cFLIP might be a critical factor in determining the balance between apoptotic and pro-survival signaling. Moreover, expression of mRNA of cFLIPL, endogenous FADD and procaspase-8 were examined in FADD transfected HEK293T cells and vector transfected HEK293T cells. We noticed the expression of endogenous FADD mRNA was lower at 24 h but moderate at 48 which tend to decline at 72 and 96 h. Here, our data shows expression of protein and mRNA of endogenous FADD is comparable. The possible expiation for this might be an expression of endogenous FADD is associated with apoptotic stresses and viability of the cells. The percentage of cell death is increased to an expression of FADD in HEK 293T cells. Interestingly, we found that down-regulation of cFLIPL and a concomitant declined expression of procaspase-8 mRNA when FADD is overexpressed. In fact, FADD appears with the induction of apoptotic stress and depleted with progression of cell death strongly suggests that the presence of FADD in an adequate amount can attenuates cFLIPL to facilitate activation of procaspase-8 which in turn activates downstream effector caspases for execution of extrinsic signaling of cell death.
It is widely reckoned that the death receptor mediated apoptosis is initiated by ligand engagement to cell surface death receptors such as CD95 (APO-1/Fas) which promote the formation of DISC (Krammer 1999; Lavrik et al. 2008). It has been shown that high expression of cFLIPL blocks procaspase-8 activation at the DISC and provides for the resistance to CD95 mediated apoptosis (Scaffidi et al. 1999). A growing body of evidence suggests that CD95 is frequently down regulated during cancer progression rendering cells resistant to apoptosis in cancer (Debatin and Krammer 2004). Moreover, CD95 is not only a potent inducer of apoptosis, but it is also implicated in the survival pathways (Peter et al. 2005). Thus a growing body of evidence suggests that CD95 has a dual role which triggers survival by activation of NF-kB pathway at low concentration and triggers cell death by activating the DISC formation and an extrinsic cascade of caspases at higher concentration (Golks et al. 2006; Lavrik et al. 2007). In the light of the current studies, unavailability of FADD may also be one of the possible reasons for the inhibition of death receptor mediated apoptosis. Several lines of evidences demonstrate that CD 95L stimulates phosphorylation of ser194 residue which sets in motion its export from the nucleus promoting its association with the activated death receptors at the plasma membrane to initiate the activation of caspase 8 (Scaffidi et al. 2000). The extent of apoptosis on FADD overexpressed HEK 293T cells were tested with stimulation of CD 95L. It is noted that HEK 293T cells stimulated with CD 95L moderately activate procaspase-8 processing by attenuating the protein expression cFLIPL. However, in HEK 293T cells during FADD overexpressed conditions activation of procaspase-8, caspase-8 and capsase-3 was found elevated with a concomitant attenuated expression of cFLIPL. The above findings suggest that insufficient availability of FADD and elevated expression of cFLIPL may account for the resistance of death receptor mediated pathway of apoptosis.
Although, it has been reported that cycloheximide (CHX), a metabolic inhibitor of protein synthesis down regulates both forms of cFLIP (cFLIPL and cFLIPS) and also downregulates the proteins downstream of FADD but does not appear to have any impact on the synthesis of caspases (Chang et al. 2002). The earlier evidence suggests that CHX sensitize cells to CD95-mediated apoptosis by down-regulating the expression of cFLIP in a caspase dependent manner (Fulda et al. 2000). Similarly, our findings are in accord with earlier reports wherein a decrease in the expression of cFLIPL was noticed upon CHX treatment in HEK 293T cells without any appreciable changes in expression of procaspase-8. In addition, CHX treatment exerts no impact on FADD expression or its interaction at the cell membrane. Moreover, moderate increase in activity of caspase-8 was recorded. However, observation for effect of CHX on FADD overexpressing HEK 293T cells governs a rapid depletion in the expression of cFLIPL with a concomitant increase in the caspase-8 activity and cell death. Recent reports suggest that a reduction in the level of cFLIPL was noted at DISC upon CHX treatment to CD95L stimulated cells and thereby enhancing recruitment and activation of caspase-8. (Brumatti et al. 2008; Fischer-Posovszky et al. 2011). In our study we observed that depletion of cFLIP, cell death and activation of caspase 8 was prompt with stimulation of CD95L and CHX in FADD expressed HEK 293T cells compared to individual treatments of CD95 and CHX. Thus, verifying the effects of sub-lethal doses of CHX and CD 95L in the presence of FADD provide us new insights of these molecules and their robust effect in execution of cell death in HEK 293T cells.
In conclusion, a dysregulated expression of FADD and cFLIP may compromise death receptor mediated apoptosis and promote cell survival in cancer. Thus a life or death decision is, to a significant extent, is determined by the ratio between the two crucial proteins—a proapoptotic (FADD) and an antiapoptotic protein (cFLIP) in each cell. Therefore, it is important to develop some approaches for regulation of apoptosis in pathological situations such as cancer. Our study thus reveals a novel facet of the modulation of apoptosis wherein a regulated expression of FADD attenuates the expression of cFLIP and activates the cascade of extrinsic caspases for execution of apoptosis in HEK 293T cells. This study indicates that FADD has a potent role in transducing the death receptor mediated apoptosis in a regulated manner. Considering these findings, further study is needed to unravel the molecular mechanism of FADD and cFLIP mediated signaling on cell death and survival, respectively, which may open the way towards a novel therapeutic approach.
Acknowledgements
We are thankful to Dr. Andrew Thorburn, University of Colorado Denver, Aurora for providing the construct of full length FADD and Puri Foundation for education and research in India for providing existing facility and supports. This work was supported by grants from the Department of Biotechnology, Ministry of Science & Technology, Government of India under Rapid Grant for Young Investigator Scheme 2011 (BT/PR15083/GBD/27/306/2011) and J. C. Bose fellowship of Department of Science & Technology to AS.
Conflicts of interest
No potential conflicts of interest were disclosed.
Glossary
- DED
Death Effector Domain
- DISC
Death-Inducing Signaling Complex
- cFLIP
Cellular fas-associated death domain-like interleukin-1-β- converting enzyme-inhibitory protein
- CHX
Cycloheximide
- CD 95L
CD 95 ligand
Contributor Information
Avadhesha Surolia, Email: surolia@mbu.iisc.ernet.in.
Chandramani Pathak, Email: cmpathak@iiar.res.in.
References
- Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME. Molecular ordering of the initial signaling events of CD95. Mol Cell Biol. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli M, Ambrogi F, Pilotti S, Alberti P, Ditto A, Barbareschi M, Galligioni E, Biganzoli E, Canevari S, Mezzanzanica D. c-FLIPL expression defines two ovarian cancer patient subsets and is a prognostic factor of adverse outcome. Endocr Relat Canc. 2009;16:443–453. doi: 10.1677/ERC-08-0218. [DOI] [PubMed] [Google Scholar]
- Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–213. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Beisner DR, Chu IH, Arechiga AF, Hedrick SM, Walsh CM. The requirements for Fas-associated death domain signaling in mature T cell activation and survival. J Immunol. 2003;171:247–256. doi: 10.4049/jimmunol.171.1.247. [DOI] [PubMed] [Google Scholar]
- Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumatti G, Yon M, Castro FA, Bueno-da-Silva AE, Jacysyn JF, Brunner T, Amarante-Mendes GP. Conversion of CD95 (Fas) Type II into Type I signaling by sub-lethal doses of cycloheximide. Exp Cell Res. 2008;314:554–563. doi: 10.1016/j.yexcr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Tepper CG, Seldin MF, O’Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Keuper M, Nagel S, Hesse D, Schurmann A, Debatin KM, Strauss G, Wabitsch M. Downregulation of FLIP by cycloheximide sensitizes human fat cells to CD95-induced apoptosis. Exp Cell Res. 2011;317:2200–2209. doi: 10.1016/j.yexcr.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Foger N, Bulfone-Paus S, Chan AC, Lee KH. Subcellular compartmentalization of FADD as a new level of regulation in death receptor signaling. FEBS J. 2009;276:4256–4265. doi: 10.1111/j.1742-4658.2009.07134.x. [DOI] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 2000;60:3947–3956. [PubMed] [Google Scholar]
- Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006;203:1295–1305. doi: 10.1084/jem.20051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Angelats M, Cidlowski JA. Molecular evidence for the nuclear localization of FADD. Cell Death Differ. 2003;10:791–797. doi: 10.1038/sj.cdd.4401237. [DOI] [PubMed] [Google Scholar]
- Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, Deperthes D, Calderara S, Schulthess T, Engel J, Schneider P, Tschopp J. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Kavuri SM, Geserick P, Berg D, Dimitrova DP, Feoktistova M, Siegmund D, Gollnick H, Neumann M, Wajant H, Leverkus M. Cellular FLICE-inhibitory protein (cFLIP) isoforms block CD95- and TRAIL death receptor-induced gene induction irrespective of processing of caspase-8 or cFLIP in the death-inducing signaling complex. J Biol Chem. 2011;286:16631–16646. doi: 10.1074/jbc.M110.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/S0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Riess D, Bentele M, Eils R, Krammer PH. Analysis of CD95 threshold signaling: triggering of CD95 (FAS/APO-1) at low concentrations primarily results in survival signaling. J Biol Chem. 2007;282:13664–13671. doi: 10.1074/jbc.M700434200. [DOI] [PubMed] [Google Scholar]
- Lavrik IN, Mock T, Golks A, Hoffmann JC, Baumann S, Krammer PH. CD95 stimulation results in the formation of a novel death effector domain protein-containing complex. J Biol Chem. 2008;283:26401–26408. doi: 10.1074/jbc.M800823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, Johnston PG. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–848. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- Matsuda F, Inoue N, Goto Y, Maeda A, Cheng Y, Sakamaki K, Manabe N. cFLIP regulates death receptor-mediated apoptosis in an ovarian granulosa cell line by inhibiting procaspase-8 cleavage. J Reprod Dev. 2008;54:314–320. doi: 10.1262/jrd.20051. [DOI] [PubMed] [Google Scholar]
- Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A. 2010;107:13034–13039. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Peter ME, Legembre P, Barnhart BC. Does CD95 have tumor promoting activities? Biochim Biophys Acta. 2005;1755:25–36. doi: 10.1016/j.bbcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rogers KM, Thomas M, Galligan L, Wilson TR, Allen WL, Sakai H, Johnston PG, Longley DB. Cellular FLICE-inhibitory protein regulates chemotherapy-induced apoptosis in breast cancer cells. Mol Cancer Ther. 2007;6:1544–1551. doi: 10.1158/1535-7163.MCT-06-0673. [DOI] [PubMed] [Google Scholar]
- Ryu BK, Lee MG, Chi SG, Kim YW, Park JH. Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol. 2001;194:15–19. doi: 10.1002/path.835. [DOI] [PubMed] [Google Scholar]
- Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8:37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Volkland J, Blomberg I, Hoffmann I, Krammer PH, Peter ME. Phosphorylation of FADD/MORT1 at serine 194 and association with a 70-kDa cell cycle-regulated protein kinase. J Immunol. 2000;164:1236–1242. doi: 10.4049/jimmunol.164.3.1236. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem/FEBS. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Kiessling S, Sansom OJ, Millar CB, Maddison K, Bird A, Clarke AR, Frisch SM. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: a potential link between genome surveillance and apoptosis. Proc Natl Acad Sci U S A. 2003;100:5211–5216. doi: 10.1073/pnas.0431215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley S, Micheau O (2010) Targeting c-FLIP in cancer. Cancer Lett. doi:10.1016/j.canlet.2010.10.009 [DOI] [PubMed]
- Song W, Lahiri DK. Efficient transfection of DNA by mixing cells in suspension with calcium phosphate. Nucleic Acids Res. 1995;23:3609–3611. doi: 10.1093/nar/23.17.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260–269. doi: 10.1016/j.it.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Mistou S, Michiels FM, Devauchelle V, Renia L, Feunteun J, Chiocchia G. Loss of FADD protein expression results in a biased Fas-signaling pathway and correlates with the development of tumoral status in thyroid follicular cells. Oncogene. 2003;22:2795–2804. doi: 10.1038/sj.onc.1206399. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Delluc S, Levy V, Valensi F, Radford-Weiss I, Legrand O, Vargaftig J, Boix C, Macintyre EA, Varet B, Chiocchia G, Buzyn A. Absence or low expression of fas-associated protein with death domain in acute myeloid leukemia cells predicts resistance to chemotherapy and poor outcome. Cancer Res. 2004;64:8101–8108. doi: 10.1158/0008-5472.CAN-04-2361. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Buzyn A, Chiocchia G. FADD adaptor in cancer. Med Immunol. 2005;4:1. doi: 10.1186/1476-9433-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Haecker G, Strasser A. An evolutionary perspective on apoptosis. Cell. 1994;76:777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, Grell M, Smith C, Scheurich P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV, Mak TW. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Yu JW, Jeffrey PD, Shi Y. Mechanism of procaspase-8 activation by c-FLIPL. Proc Natl Acad Sci U S A. 2009;106:8169–8174. doi: 10.1073/pnas.0812453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kabra NH, Cado D, Kang C, Winoto A. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J Biol Chem. 2001;276:29815–29818. doi: 10.1074/jbc.M103838200. [DOI] [PubMed] [Google Scholar]