Abstract
BACKGROUND
The combination of granulocyte–colony-stimulating factor (G-CSF [filgrastim]) and dexamethasone (G-CSF/dex) is an effective granulocyte mobilization regimen, but the variables that affect donor neutrophil response and granulocyte collection yield are not well characterized.
STUDY DESIGN AND METHODS
A computerized database containing records of 1198 granulocyte collections from 137 unrelated volunteer apheresis donors during a 13-year period was retrospectively analyzed. Donors were categorized by age, sex, and cumulative number of granulocyte donations. Complete blood counts at baseline and after G-CSF/dex stimulation were recorded. The outcome variables include the pre-procedure absolute neutrophil count (preANC), which reflects G-CSF/dex stimulation, and the granulocyte product yield per liter processed (BagGranYield/L).
RESULTS
Higher baseline ANC and platelet (PLT) counts were significantly associated with higher preANC while a larger number of prior granulocytapheresis procedures was associated with lower preANC. Total filgrastim dose (used in weight-based dosing) did not significantly impact preANC or the granulocyte yield; weight-based dosing at 5 μg per kg and a uniform 480-μg dose produced equivalent preANC. PreANC and weight were the key determinants of granulocyte yield (BagGranYield/L).
CONCLUSION
Apheresis donors with higher baseline PLT counts and ANCs have higher ANCs after G-CSF/dex stimulation; donor age, weight, and sex do not have a significant impact. A uniform G-CSF dose of 480 μg is as effective as weight-based dosing at 5 μg per kg. Donor ANC monitoring should be considered after serial granulocytapheresis procedures.
Before the mid-1990s, granulocyte transfusion therapy was limited by collection techniques that yielded a cell dose that was often marginal. With the introduction of recombinant human granulocyte–colony-stimulating factor (G-CSF), it was discovered that a single subcutaneous administration of G-CSF in normal donors followed by leukapheresis 12 to 16 hours later harvested three times the number of functionally normal neutrophils compared with corticosteroid pretreatment;1 the addition of corticosteroids resulted in even higher donor neutrophil counts.2
Previous work by our group3,4 and others5,6 using sub-cutaneous G-CSF at 5 μg per kg 18 hours before and 8 mg of oral dexamethasone 12 hours before granulocytapheresis showed that the mean granulocyte yield ranges from 5.5 × 1010 to 7.2 × 1010. The donor's neutrophil count at the time of leukapheresis is a key determinant of the granulocyte yield with any stimulation regimen; it ranges from 5.6 × 109 per L with dexamethasone alone to 13.6 × 109 per L with G-CSF alone to 28.9 × 109 per L with combined G-CSF and dexamethasone (G-CSF/dex).3
The purpose of this study was to explore the donor and procedural characteristics that affect neutrophil response to G-CSF/dex and granulocyte collection yield. Donor demographic and laboratory variables and the specifics of G-CSF dosing are some of the key variables that we considered.
MATERIALS AND METHODS
Donors
A total of 137 unrelated volunteer apheresis donors were recruited to undergo G-CSF/dex stimulation for granulocyte collection between October 1994 and November 2007. All donors met standard criteria for allogeneic blood donation, had donated PLTs by apheresis in the past, and had signed consent for participation in the granulocyte program. They underwent a thorough history and physical examination by a transfusion medicine physician before the first granulocyte mobilization and were specifically questioned about diabetes, hypertension, and a history of angina or untreated coronary heart disease before each subsequent granulocyte mobilization. These donors contributed 1198 granulocyte concentrates during the study period. The minimum interval between successive granulocyte donations was generally 1 month; exceptions were made if the donor was an HLA match for an alloimmunized recipient. At each donation visit, donor demographic information including sex, age, height, and weight were collected. Body mass index (BMI) was calculated as
The donors received a single subcutaneous injection of filgrastim (Amgen, Thousand Oaks, CA) 12 to 18 hours before leukapheresis and took 8 mg of dexamethasone orally 12 hours before leukapheresis. The dose of G-CSF was 5 μg per kg until July 2005 and 480 μg as a flat dose thereafter. G-CSF was administered by the nursing staff of the apheresis center.
Granulocyte collection
Granulocyte concentrates were collected with a blood cell separator (CS3000 Plus, Fenwal, Deerfield, IL) using a granulocyte separation chamber and an interface offset of 45. Seven liters of whole blood was targeted to be processed with trisodium citrate anticoagulant (Citra Anticoagulants, Braintree, MA) at a ratio of 1:10–1:13; whole blood flow rates ranged from 50 to 55 mL per minute. The actual volume processed (VolProc) was recorded for each procedure. Six percent hydroxyethyl starch (HES; Hespan, Braun Medical, Irvine, CA) was used as a red blood cell (RBC)-sedimenting agent (500 mL HES to 30 mL trisodium citrate).
Laboratory testing
Complete blood counts (CBCs) were performed on the donors on the day of granulocyte collection: the preprocedure white blood cell (WBC) count with an automated differential generates the preprocedure absolute neutrophil count (preANC); postprocedure CBC/ANC (postANC) was obtained to calculate collection efficiency. Although CBCs were not performed immediately before G-CSF administration, the donor's most recent unstimulated CBC, drawn just before a plateletpheresis donation within the prior 12 months, was recorded for each granulocyte collection procedure; these baseline variables included WBC count, ANC, PLT count, and hemoglobin level.
CBCs were performed on the granulocyte concentrates; the granulocyte concentrate dose (“BagGranYield”) was obtained from the BagANC multiplied by the volume of the component. Granulocyte collection efficiency (GCE, %) was calculated according to the formula
where
Outcome variables
The two outcome variables studied were the donor's preANC, reflecting the effects of G-CSF/dex stimulation, and the BagGranYield per liter processed. Collections where the minimal processing volume of 5 L was not reached due to donor discomfort or venous access issues were not evaluated for the BagGranYield per L analysis.We postulated that the preANC would be the major determinant of granulocyte yield, but that other variables might play a role as well.
Data analysis
Standard data analysis and graphics were performed with a spreadsheet application (Excel, Microsoft Corp., Seattle, WA). Data are provided as mean ± standard deviation (SD) unless otherwise noted. Comparisons of continuous variables between groups were performed with a two-tailed, nonpaired t test. Multivariate analyses were performed using stepwise forward logistic regression, based on variables that reached significance in univariate analysis (JMP, SAS Institute, Cary, NC).
RESULTS
Donor demographics and adverse events
A total of 137 donors underwent 1198 leukapheresis collections after G-CSF/dex stimulation, a mean of 8.7 ± 8.6 collections per donor (median, 5; range, 1–41). The mean interdonation interval was 198 days (median, 74; range, 14–3312), for the donors who donated granulocytes more than once. Approximately 12 percent of procedures (146/1198) were performed within 30 days of another granulocytapheresis, generally in support of an HLA-alloimmunized recipient; the minimum acceptable interval was 14 days. Since many of the granulocyte donors also donated PLTs, the cytapheresis interval (between any two cytapheresis procedures) was also calculated: the mean cytapheresis interval was 67 days. Ninety-six percent of the donors were white. There were almost twice as many males as females (90 vs. 47). For the male donors, the age was 52.04 ± 9.51 years (range, 27–77), and the weight was 89.01 ± 12.77 kg. For the female donors, the age was 47.63 ± 9.27 years (range, 20–68), and the weight was 74.64 ± 13.75 kg. The male donors contributed to 883 collections (mean, 9.8 collections per donor), while the female donors contributed to 315 collections (mean, 6.7 collections per donor).
There was one serious adverse event: a 62-year-old male who had undergone granulocyte collection eight times in the prior 4 years (interdonation intervals always greater than 30 days) experienced chest pain during the procedure and had electrocardiographic changes consistent with myocardial ischemia. In retrospect, he had been experiencing new-onset angina with exertion but did not mention this in the donor interview. He was documented to have a non-ST elevation myocardial infarction. He had a family history of coronary artery disease. He underwent coronary revascularization and has been doing well since.
Donor blood counts and granulocyte concentrate yields
The donors' blood counts are shown in Table 1. No donor was deferred permanently for persistently low PLT counts or absolute lymphocyte counts. The mean granulocyte concentrate yield (BagGranYield) was 7.26 × 1010 ± 1.98 × 1010 (range, 1.64 × 1010–14.92 × 1010); there was no sex difference (data not shown). The BagGranYield per liter processed was 1.10 × 1010 ± 0.30 × 1010 (range, 0.32 × 1010–2.15 × 1010) cells per liter processed. The VolProc was 6.61 ± 0.40 L (range, 2.26–7.34). The granulocyte collection efficiency was 55.8 ± 9.3 percent.
TABLE 1.
Granulocyte donors' baseline and preapheresis PLT count and preANC
| Characteristic | All donations* (×109/L) | Range (×109/L) | Females* (×109/L) | Males* (×109/L) | Sex comparison (p value) |
|---|---|---|---|---|---|
| Baseline ANC (n = 1038) | 3.320 (1.300) | 1.060–13.630 | 3.450 (1.280) | 3.220 (1.320) | 0.009 |
| Baseline PLT count (n = 985) | 237,000 (61,000) | 128,000–513,000 | 253,000 (62,000) | 224,000 (61,000) | <0.001 |
| PreANC (n = 1178) | 27.660 (7.033) | 11.370–53.190 | 29.370 (7.668) | 27.050 (6.686) | <0.001 |
| Preapheresis PLT count (n = 1184) | 255,000 (67,000) | 127,000–574,000 | 277,000 (64,000) | 247,000 (66,000) | <0.001 |
Data are reported as mean (SD).
We compared donor blood counts and granulocyte yields for the two G-CSF dosing regimens: dosing by weight (956 procedures) and a uniform dose (242 procedures). The mean and median filgrastim doses in the weight-based group were 427 and 450 μg, respectively (range 300–660 μg), leading us to choose 480 μg as the uniform dose for safe, economical, and convenient dosing. There was no significant difference in the donor preANC between the two groups (27.71 × 109/L vs. 27.46 × 109/L) or in the BagGranYield per L processed (both 1.10 × 1010). Within the weight-based dosing group, there was no significant association between total filgrastim dose and preANC (data not shown).
Association of preprocedure ANC and baseline PLT count, baseline ANC, age, and number of prior granulocyte donations
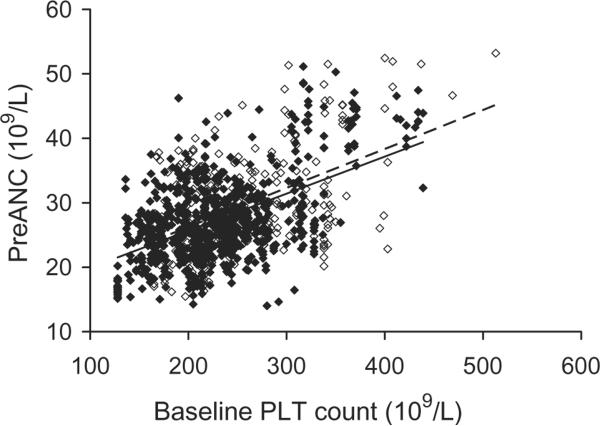
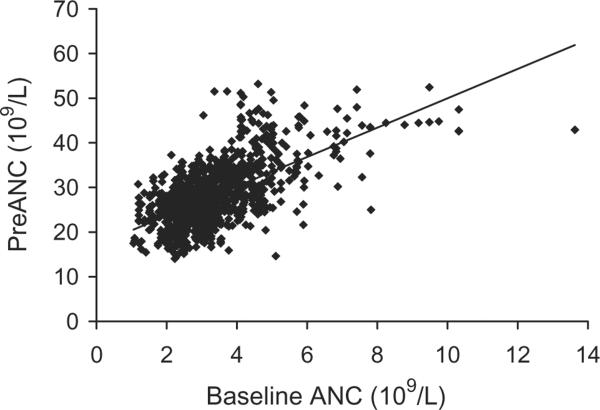
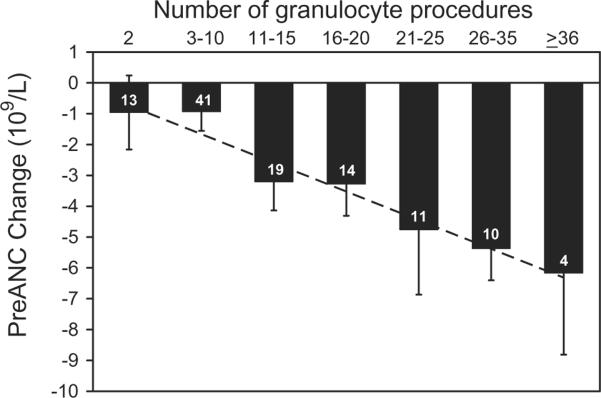
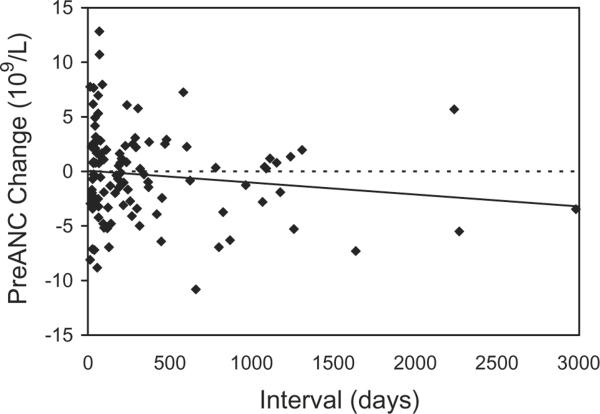
Donors with higher baseline PLT counts had significantly higher ANCs after G-CSF/dex stimulation (Fig. 1). This positive correlation was true for both male donors and female donors. Donors with higher baseline ANC also had higher preANC; for each increase in the baseline ANC of 1 × 109 per L, the preANC increased by approximately 3 × 109 per L (Fig. 2). Donor age was not significantly associated with preANC for the first procedure (median age, 47 years; range, 27–67 years). Repeat leukapheresis procedures were associated with lower baseline ANCs: we examined the change in baseline ANCs between the first and last granulocyte donations for 112 donors who donated more than once: the first baseline ANC was 3.650 × 109 ± 1.440 × 109 and L and the last baseline ANC was 3.160 × 109 ± 0.997 × 109 per L (p < 0.001). We examined the change in preANC (between the preANC for the first procedure and the preANC for the last procedure) grouping donors by donation frequency: for donors who underwent 2 to 10 granulocyte collections (n = 54), the preANC decrease was on the order of 1 × 109 per L; for donors who underwent more than 20 granulocyte collections (n = 25), the preANC decrease was on the order of 6 × 109 per L (Fig. 3). Since age may confound the effect of multiple donations, we examined the change in preANC for the first two donations over time (Fig. 4); there was a modest trend toward a lower preANC over time reflecting the pure effect of age, although it did not reach significance.
Fig. 1.
Association of baseline PLT count with preANC after G-CSF/dex stimulation. (◇) Female: R2 = 0.2260, p < 0.0001; (◆) male: R2 = 0.2682, p < 0.0001.
Fig. 2.
Association of baseline ANC with preANC after G-CSF/dex stimulation. R2 = 0.3726, p < 0.0001.
Fig. 3.
Effect of donation frequency on the mean change in preANC from the first to the last granulocyte donation, in the 112 donors who donated granulocytes more than once. Numbers in the bars represent the number of donors in each category of donation frequency.
Fig. 4.
The effect of interdonation interval on the change in preANC for the first two granulocyte donations, in the 112 donors who donated granulocytes more than once.
Regression analysis of factors affecting preprocedure ANC and granulocyte yield
In univariate logistic regression analysis of preANC, higher baseline PLT counts and baseline ANCs were associated with higher preANCs, while male sex, older age, shorter cytapheresis interval (which includes both PLT and granulocyte donations), higher total filgrastim dose (for donors dosed by weight), and a larger number of prior G-CSF–stimulated granulocyte donations were associated with lower preANC. The interval between granulocyte donations and the total number of cytapheresis donations (PLTs and granulocytes) were not associated with preANC. In the multivariate stepwise regression analysis (R2 = 0.495), higher baseline ANC and baseline PLT were significantly associated with higher preANC while a larger number of prior G-CSF–stimulated granulocytapheresis procedures and older age were associated with lower preANC; the effect of age was modest compared to the baseline ANC and PLT. Factors such as sex, filgrastim dose, and cytapheresis interval were no longer significantly associated with preANC, after adjustment for the other factors.
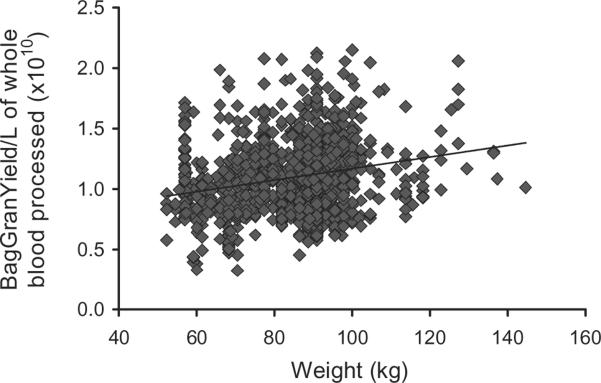
In univariate logistic regression analysis of BagGranYield per L processed, higher preANC, preprocedure PLT count, preprocedure hematocrit (Hct), baseline ANC, baseline PLT, baseline Hct, filgrastim dose, and higher weight or BMI were associated with higher BagGranYield per L processed. In the multivariate stepwise regression analysis (R2 = 0.744), preANC and weight were the key determinants of granulocyte yield. The association between donor weight and BagGranYield per L processed is shown in Fig. 5; the association is weak (r = 0.22) and possibly explained by the effects of a larger blood volume on cell separation characteristics. Filgrastim dose and the other hematologic variables were no longer significantly associated with the granulocyte yield.
Fig. 5.
Association between BagGranYield per L of whole blood processed and donor weight. R2 = 0.0514, p = 0.001.
DISCUSSION
We have shown in a large cohort of granulocyte collections in volunteer apheresis donors mobilized with G-CSF/dex that there is a striking correlation between the donors' baseline (unstimulated) neutrophil and PLT counts and the neutrophil count after G-CSF/dex stimulation. Baseline blood counts were not obtained immediately before G-CSF/dex administration; this would have been preferable, but was not practical in a routine donor setting. The strong association between the baseline PLT count and the poststimulation ANC was somewhat unexpected, but is consistent with findings from our group that baseline PLT count was a significant predictor of peak CD34 cell mobilization after G-CSF administration.7 There is evidence than thrombopoietin impacts the number of hematopoietic stem cells and progenitors of all myeloid lineages.8,9 This implies that a vigorous steady-state equilibrium for thrombopoiesis, as reflected in the baseline PLT count, may be a surrogate for the neutrophil response to G-CSF stimulation. The association between baseline PLT count and ANC response to G-CSF implies that apheresis donors with a high PLT count would be the optimal subjects to recruit for granulocyte donation. Although the baseline ANC also predicts the ANC after G-CSF/dex stimulation, it may not be a variable that is routinely monitored in plateletpheresis donors.
Our donors range widely in age, including many who were older than 55 years of age. Specifically, 35 percent (420/1198) of donations were made by donors age 55 or greater at the time of donation. There was a modest although not significant trend for a lower preANC with increasing age. Older donors who tolerate and respond well to the stimulation regimen constitute a valuable pool of donors for granulocytapheresis, which takes more time than plateletpheresis and is often scheduled on short notice. A recent study examined collections from 329 granulocyte donors (related and unrelated); this was a younger population overall (median age, 33 years), but age was also not found to be a significant predictor of granulocyte yield.10 The mobilization regimen and the granulocytapheresis procedure involving HES were well tolerated by both younger and older donors in our study. The one donor who experienced a myocardial infarction during the procedure had been experiencing unrecognized crescendo angina in the few weeks preceding the donation.
A progressive decrease in preANC was seen after increasing numbers of granulocyte donations. We observed the same phenomenon in repeat PLT donors who were not specifically selected to donate on the basis of sustainably high PLT counts; they experienced a decline in PLT count with increasing numbers of plateletpheresis donations.11 However, the interdonation interval was longer for granulocyte donations than plateletpheresis donations. Our granulocyte donors could have donated PLTs on many occasions in between granulocyte donations, but there was no particular effect of successive plateletpheresis procedures on baseline (unstimulated) donor neutrophil counts in our study (data not shown). Computerized systems that track serial ANCs and automatically generate medical referral would be useful in a frequent granulocytapheresis program. Adoption of a preANC cutoff such as 15 × 109 per L could be considered to qualify the donor for future granulocytapheresis donations.
The effect of different filgrastim doses in granulocyte mobilization was reported by Liles and coworkers,12 who compared filgrastim 450 μg versus 600 μg, combined with 8 mg of dexamethasone. These two filgrastim doses produced equivalent ANC responses. Heuft and coworkers13 found a dose response for lenograstim (glycosylated G-CSF) up to 6 μg per kg. In our study, total filgrastim dose (used in weight-based dosing) did not significantly impact preANC or the granulocyte yield; there also was no change in preANC after a change from weight-based dosing to a uniform 480-μg dose; a vial-based flat dose is economical, convenient, and less prone to error. This finding was in contrast to our experience with a 5-day course of filgrastim in the mobilization of CD34+ peripheral blood stem cells, where total filgrastim dose was a significant predictor of peak blood CD34+ cell count.7 Granulocyte mobilization, unlike stem cell mobilization, does not require upstream responses in the marrow progenitor cell niche and perhaps as such is less dependent on filgrastim dose. Neutrophilia after single-dose G-CSF/dex is attributed to a shift of neutrophils from the marrow storage pool into the peripheral blood; it has been estimated that approximately 75 percent of the mature neutrophils in the marrow are mobilized with this stimulation regimen.14
Our study has several limitations. First, our apheresis donor population is mostly white, so we are not able to study the effects of ethnicity on granulocyte mobilization. Second, our donor population is older than other series and our findings may not be generalizable to younger adults. Third, this granulocytapheresis series reflects multiple donations from a relatively small group of community apheresis donors, so our data points are not independent observations. On the other hand, the effect of prior filgrastim-stimulated granulocyte collections on subsequent preANCs would not have been evident if we had confined our study to first-time granulocyte donors only. Finally, we did not look at the donors' baseline RBC sedimentation rate, which has been shown to correlate positively with granulocyte collection efficiency and collection yields in dexamethasone-stimulated donors.15
In conclusion, we have shown that apheresis donors with higher baseline PLT count and ANC have a higher ANC response to G-CSF and dexamethasone stimulation; donor age, weight, and sex do not have a significant impact on the ANC after stimulation. A uniform dose of 480 μg is as effective as weight-based dosing at 5 μg per kg. Donor ANC monitoring should be considered after serial granulocytapheresis procedures.
ABBREVIATIONS
- ANC(s)
absolute neutrophil count(s)
- BagGranYield
granulocyte product yield
- BMI
body mass index
- CBC(s)
complete blood count(s)
- G-CSF/dex
granulocyte–colony-stimulating factor and dexamethasone
- postANC
postprocedure absolute neutrophil count
- preANC
preprocedure absolute neutrophil count
- VolProc
volume processed.
Footnotes
The views expressed in this paper are those of the authors and are not to be construed as the official position of the United States Department of Health and Human Services.
REFERENCES
- 1.Caspar C, Seger R, Burger J, Gmur J. Effective stimulation of donors for granulocyte transfusions with recombinant methionyl granulocyte colony-stimulating factor. Blood. 1993;81:2866–71. [PubMed] [Google Scholar]
- 2.Liles WC, Huang JE, Llewellyn C, SenGupta D, Price TH, Dale DC. A comparative trial of granulocyte-colony-stimulating factor and dexamethasone, separately and in combination, for the mobilization of neutrophils in the peripheral blood of normal volunteers. Transfusion. 1997;37:182–7. doi: 10.1046/j.1537-2995.1997.37297203521.x. [DOI] [PubMed] [Google Scholar]
- 3.Stroncek DF, Yau YY, Oblitas J, Leitman SF. Administration of G-CSF plus dexamethasone produces greater granulocyte concentrate yields while causing no more donor toxicity than G-CSF alone. Transfusion. 2001;41:1037–44. doi: 10.1046/j.1537-2995.2001.41081037.x. [DOI] [PubMed] [Google Scholar]
- 4.Leitman SF, Yu M, Lekstrom J. Pair-controlled study of G-CSF and dexamethasone for granulocytapheresis donors. Transfusion. 1995;35:53S. [Google Scholar]
- 5.Safdar A, Hanna HA, Boktour M, Kontoyiannis DP, Hachem R, Lichtiger B, Freireich EJ, Raad I. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia. Cancer. 2004;101:2859–65. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- 6.Kerr JP, Liakopolou E, Brown J, Cornish JM, Fleming DE, Massey E, Oakhill A, Pamphilon DH, Robinson SP, Totem A, Valencia A, Marks DI. The use of stimulated granulocyte transfusions to prevent recurrence of past severe infections after allogeneic stem cell transplantation. Br J Haematol. 2003;123:114–8. doi: 10.1046/j.1365-2141.2003.04583.x. [DOI] [PubMed] [Google Scholar]
- 7.Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett AJ, Fowler DH, Bishop MR, Kang EM, Malech HL, Dunbar CE, Khuu HM, Wesley R, Yau YY, Bolan CD. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112:2092–100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushansky K, Fox N, Lin NL, Liles WC. Lineage-specific growth factors can compensate for stem and progenitor cell deficiencies at the postprogenitor cell level: an analysis of doubly TPO- and G-CSF receptor-deficient mice. Blood. 2002;99:3573–8. doi: 10.1182/blood.v99.10.3573. [DOI] [PubMed] [Google Scholar]
- 9.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–45. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 10.Ofran Y, Avivi I, Oren I, Zuckerman T, Bonstein L, Rowe JM, Dann EJ. Granulocyte transfusions for neutropenic patients with life-threatening infections: a single center experience in 47 patients, who received 348 granulocyte transfusions. Vox Sang. 2007;93:363–9. doi: 10.1111/j.1423-0410.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus EF, Browning J, Normal J, Oblitas J, Leitman SF. Sustained decreases in platelet count associated with multiple, regular plateletpheresis donations. Transfusion. 2001;41:756–61. doi: 10.1046/j.1537-2995.2001.41060756.x. [DOI] [PubMed] [Google Scholar]
- 12.Liles WC, Rodger E, Dale DC. Combined administration of G-CSF and dexamethasone for the mobilization of granulocytes in normal donors: optimization of dosing. Transfusion. 2000;40:642–4. doi: 10.1046/j.1537-2995.2000.40060642.x. [DOI] [PubMed] [Google Scholar]
- 13.Heuft HG, Goudeva L, Pulver N, Grigull L, Schwella N, Blasczyk R. A dose-response analysis of lenograstim plus dexamethasone for neutrophil mobilization and collection. Transfusion. 2005;45:604–12. doi: 10.1111/j.0041-1132.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- 14.Dale DC, Liles WC, Llewellyn C, Rodger E, Price TH. Neutrophil transfusions: kinetics and functions of neutrophils mobilized with G-CSF and dexamethasone. Transfusion. 1998;38:713–21. doi: 10.1046/j.1537-2995.1998.38898375509.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Klein HG. The effect of donor red cell sedimentation rate on efficiency of granulocyte collection by cen trifugal leukapheresis. Transfusion. 1995;35:384–8. doi: 10.1046/j.1537-2995.1995.35595259147.x. [DOI] [PubMed] [Google Scholar]