Abstract
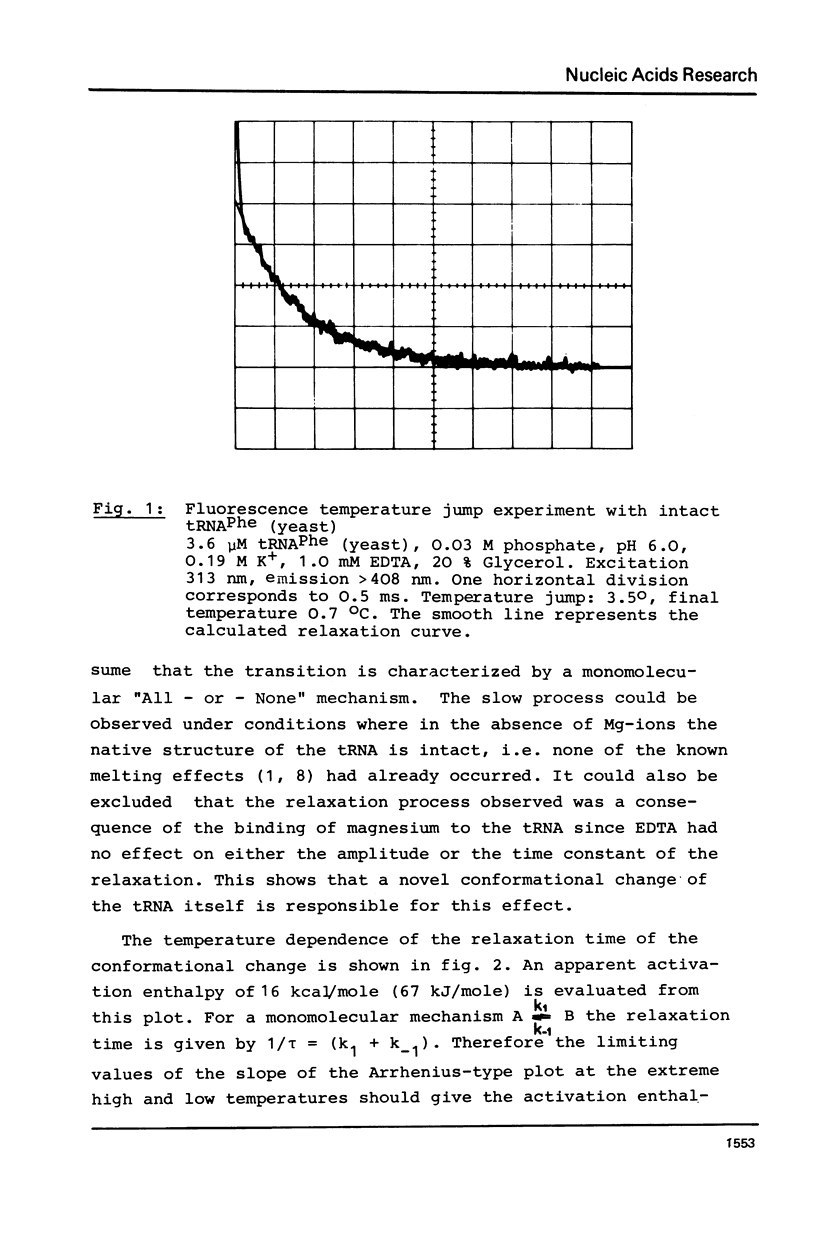
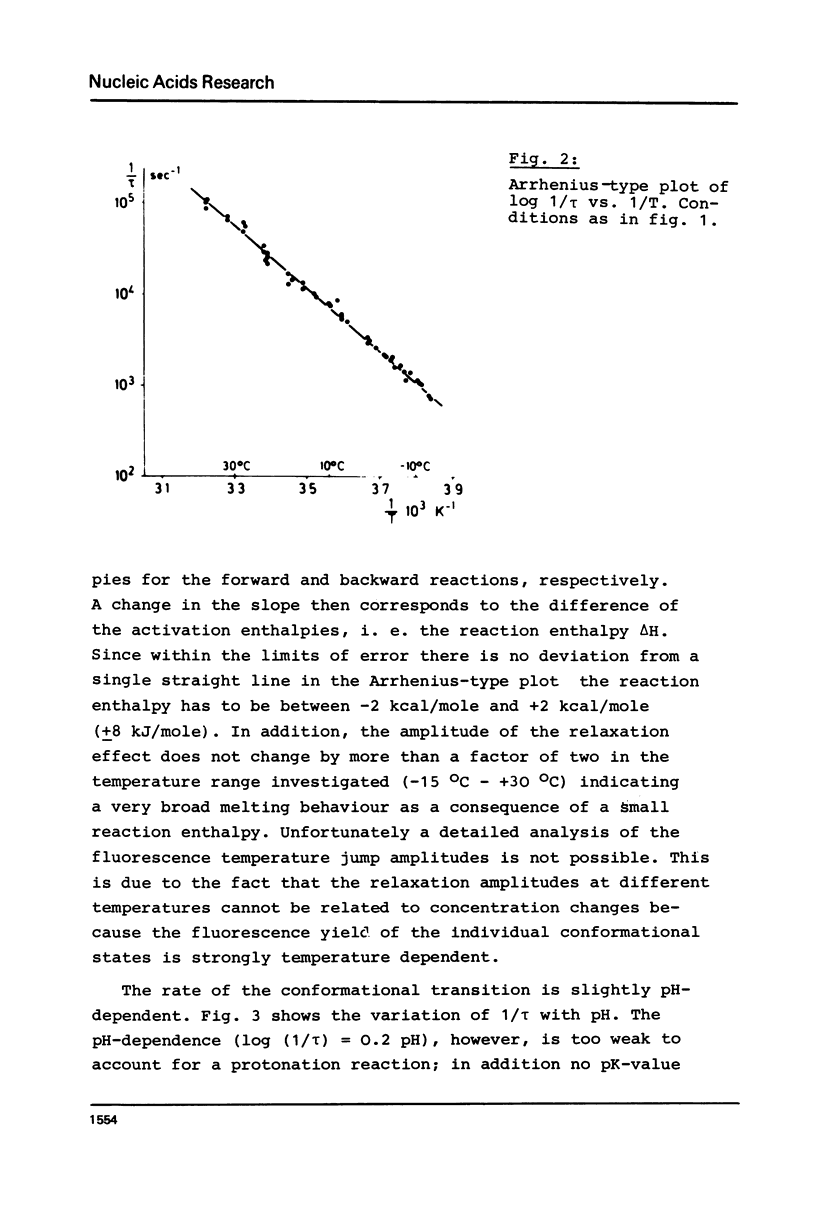
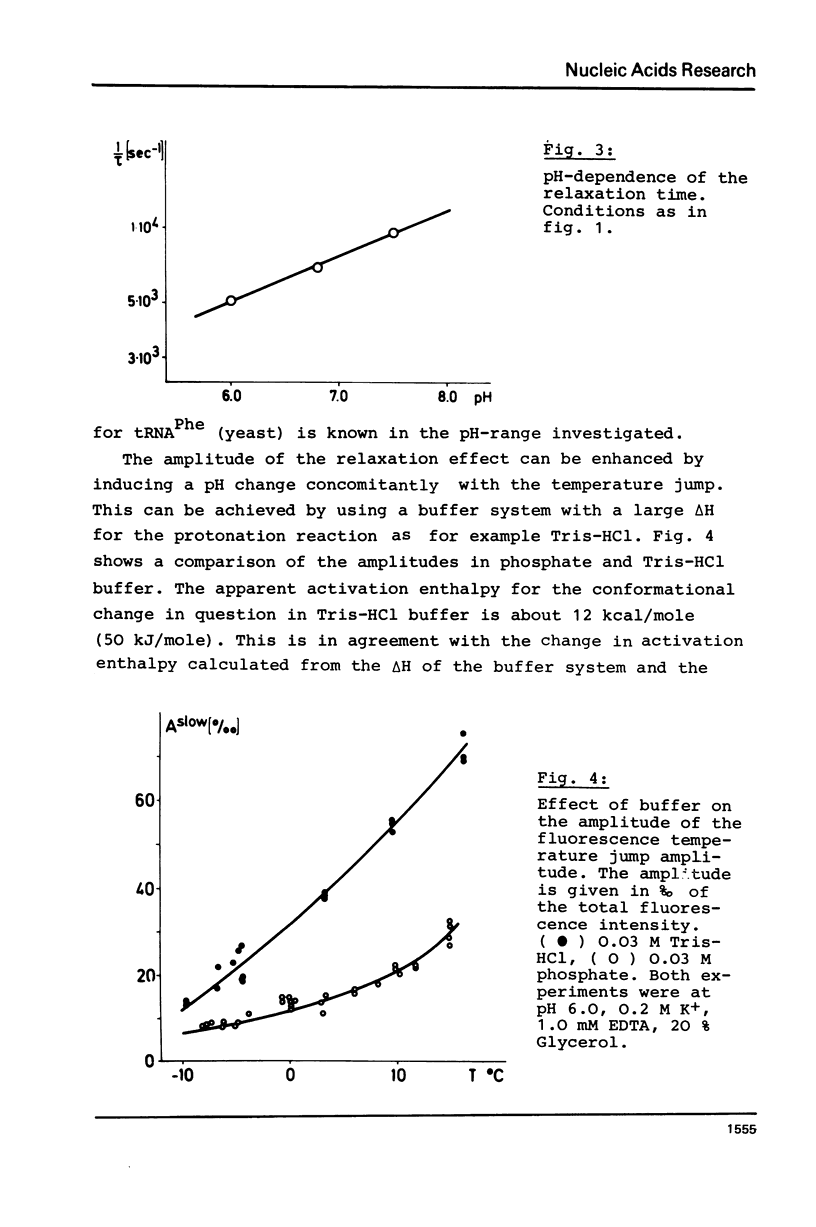
The temperature dependence of the fluorescence of the Y-base of tRNAPhe (yeast) was investigated kinetically by the temperature jump method. In the range between -15 degrees C and +30 degrees C A NOVEL CONFORMATIONAL TRANSITION OF THE TRNA could be characterized. This conformational change was found in the absence of any artificial label; it is a characteristic property of tRNAPhe in its native structure. This transition accounts for 30% of the total fluorescence change. Its activation enthalpy is 16 kcal/mole (67 kJ/mole), and the transition enthalpy is between -2 kcal/mole and +2 kcal/mole (+/-8 kJ/mole). A model is represented in which this transition can be explained by a a change in the stacking pattern of the anticodon loop. The experimental findings are discussed with respect to several hypotheses about the molecular mechanism of protein biosynthesis which postulate conformational rearrangements of the anticodon loop.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crick F. H., Brenner S., Klug A., Pieczenik G. A speculation on the origin of protein synthesis. Orig Life. 1976 Dec;7(4):389–397. doi: 10.1007/BF00927934. [DOI] [PubMed] [Google Scholar]
- Fuller W., Hodgson A. Conformation of the anticodon loop intRNA. Nature. 1967 Aug 19;215(5103):817–821. doi: 10.1038/215817a0. [DOI] [PubMed] [Google Scholar]
- Kallenbach N. R., Berman H. M. RNA structure. Q Rev Biophys. 1977 May;10(2):138–236. doi: 10.1017/s0033583500000202. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Quigley G. J., Suddath F. L., McPherson A., Sneden D., Kim J. J., Weinzierl J., Rich A. Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain. Science. 1973 Jan 19;179(4070):285–288. doi: 10.1126/science.179.4070.285. [DOI] [PubMed] [Google Scholar]
- Krauss G., Römer R., Riesner D., Maass G. Thermodynamics and kinetics of the interaction of phenylalanine-specific tRNA from yeast with its cognate synthetase as studied by the flourescence of the Y-base. FEBS Lett. 1973 Feb 15;30(1):6–10. doi: 10.1016/0014-5793(73)80606-4. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Aminoacyl-tRNA binding at the recognition site is the first step of the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 1977 May;74(5):1903–1907. doi: 10.1073/pnas.74.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A., von der Haar F., Cramer F. Spectroscopic properties of oligonucleotides excised from the anticodon region of phenylalanine tRNA from yeast. Biopolymers. 1973;12(1):27–43. doi: 10.1002/bip.1973.360120104. [DOI] [PubMed] [Google Scholar]
- Olson T., Fournier M. J., Langley K. H., Ford N. C., Jr Detection of a major conformational change in transfer ribonucleic acid by laser light scattering. J Mol Biol. 1976 Apr 5;102(2):193–203. doi: 10.1016/s0022-2836(76)80048-4. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Gassen H. G. Codon-dependent rearrangement of the tertiary structure of tRNAPhe from yeast. FEBS Lett. 1977 Jun 15;78(2):267–270. doi: 10.1016/0014-5793(77)80320-7. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Menzel H. M., Gassen H. G. Codon-dependent rearrangement of the three-dimensional structure of phenylalanine tRNA, exposing the T-psi-C-G sequence for binding to the 50S ribosomal subunit. Biochemistry. 1976 Jun 1;15(11):2484–2490. doi: 10.1021/bi00656a035. [DOI] [PubMed] [Google Scholar]
- Thach S. S., Thach R. E. Translocation of messenger RNA and "accommodation" of fMet-tRNA. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1791–1795. doi: 10.1073/pnas.68.8.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanke C., Römer R., Maass G. Tertiary structure of tRNAPhe (yeast): kinetics and electrostatic repulsion. Eur J Biochem. 1975 Jul 1;55(2):439–444. doi: 10.1111/j.1432-1033.1975.tb02180.x. [DOI] [PubMed] [Google Scholar]
- Woese C. Molecular mechanics of translation: a reciprocating ratchet mechanism. Nature. 1970 May 30;226(5248):817–820. doi: 10.1038/226817a0. [DOI] [PubMed] [Google Scholar]



