Abstract
BACKGROUND
Transfusion of granulocytapheresis concentrates can be limited by the volume of incompatible donor red blood cells (RBCs) in the component. Efficient reduction of RBCs in granulocyte units would result in safe transfusion of RBC-incompatible units.
STUDY DESIGN AND METHODS
Granulocyte concentrates were collected by continuous-flow apheresis from granulocyte–colony-stimulating factor (G-CSF) and dexamethasone-stimulated volunteer donors, with 6% hydroxyethyl starch (HES) added continuously during apheresis as a RBC sedimenting agent to enhance granulocyte collection efficiency. After collection, the component was placed in a plasma extractor for 4 hours. A sharp line of demarcation between the starch-sedimented RBCs and the granulocyte-rich supernatant developed, and the supernatant was transferred to a sterilely docked transfer pack. RBC reduction and white blood cell recovery were determined.
RESULTS
Gravity sedimentation was performed on 165 granulocyte concentrates. Mean sedimentation time was 267 minutes (range, 150–440 min). RBC depletion was 92% (range, 71%–99%) with mean residual RBC content of 3.2 ± 1.4 mL. Twelve percent of components contained less than 2 mL of RBCs. Mean granulocyte and platelet (PLT) recoveries were 80 and 81%, respectively. There were no transfusion reactions or signs of hemolysis after transfusion of 66 RBC-incompatible granulocyte concentrates (RBC volume, 1.6–8.2 mL). The remaining concentrates were used for topical or intrapleural applications.
CONCLUSIONS
RBCs were significantly reduced and granulocytes and PLTs effectively retained in G-CSF/ steroid–mobilized granulocyte components collected with HES and processed by gravity sedimentation. This procedure allows safe transfusion of RBC-incompatible sedimented granulocyte units and may be used to expand the pool of available granulocyte donors for specific recipients.
Granulocyte transfusions can be an effective adjunctive therapy in patients with profound neutropenia or granulocyte dysfunction who have progressive, life-threatening infections despite appropriate antimicrobial therapy. Several retrospective observational studies have demonstrated or suggested stability or improvement in recalcitrant infections in severely neutropenic patients after a course of granulocyte transfusions.1–12 However, recruitment of community donors for granulocyte collection is challenging. To collect a maximally therapeutic component with a high cell content, donors should be stimulated with a combination of granulocyte–colony-stimulating factor (G-CSF) and dexamethasone.13–15 The synergistic effect of these two agents substantially increases the number of circulating granulocytes available for harvest by apheresis. The donor pool is further limited to red blood cell (RBC)-compatible donors since there is a significant volume of RBCs in granulocyte units. These constraints make it difficult to sustain a granulocyte transfusion program.
In 1989, we first described the use of ex vivo gravity sedimentation to deplete RBCs in granulocyte concentrates.16 The components were transfused to a child with chronic granulomatous disease, pneumonia, and osteomyelitis, whose course was complicated by alloimmunization to multiple RBC antigens. G-CSF was not administered to donors in that study. The practice of depleting RBCs from granulocyte concentrates continues to be common in our center and expands the donor population from which patients are able to receive this therapy. We now describe the efficacy and feasibility of starch sedimentation to deplete incompatible RBCs in granulocyte components collected after G-CSF and dexamethasone stimulation of the donor.
MATERIALS AND METHODS
Donors
Granulocyte donors gave informed consent for participation in an institutional review board–approved research protocol. The donors were recruited from our platelet-pheresis donor pool and met all allogeneic donor screening and testing criteria as defined by the Food and Drug Administration (FDA) and the AABB. A quantity of 480 μg of G-CSF (Filgrastim, Amgen, Thousand Oaks, CA) was administered by a single subcutaneous injection 12 to 20 hours before starting the collection, and 8 mg of dexamethasone was taken orally 12 hours before the start of the granulocytapheresis procedure, which began at approximately 8:00 AM.
Granulocytapheresis
Granulocyte units were collected by continuous-flow apheresis (CS-3000 Plus, Fenwal, Inc., Lake Zurich, IL) with use of a separation chamber and a collection chamber (Granulo and A-35, respectively, Fenwal). The device interface offset detector was set at 45 to optimize granulocyte yield.17 Trisodium citrate, 30 mL of a 46.7% solution (Tri-Citrasol, Cytosol Laboratories, Braintree, MA), was added to a 500-mL bag of 6% hydroxyethyl starch (HES; Hespan, B. Braun Medical Inc., Irvine, CA). The resulting citrate-containing HES solution was added continuously at a 1:13 ratio with whole blood to maintain anticoagulation and maximize granulocyte yield. The procedure ended when the bag of citrate-containing HES was completely infused, after approximately 6000 mL of whole blood had been processed. Blood counts (Cell-Dyn 4000, Abbott Laboratories, Abbott Park, IL) were performed on samples collected from the donors immediately before and after donation and from the granulocyte concentrates before and after gravity sedimentation.
RBC depletion procedure
After completion of the apheresis procedure, a transfer pack (Baxter, Deerfield, IL) was attached to the granulocyte component bag using a sterile coupling device (Terumo sterile tubing welder, Terumo Medical Corp., Elkton, MD). The unit was placed upright in a plasma expressor (Fenwal), with entry ports at the top, and left undisturbed for approximately 4 hours at room temperature, allowing the cells to sediment by gravity. A sharp line of demarcation between the starch-sedimented RBCs and the granulocyte-rich supernatant developed, and the supernatant was transferred to the transfer pack. Postsedimentation blood counts of the RBC-depleted, granulocyterich units were analyzed to determine the degree of RBC reduction and white blood cell (WBC) and platelet (PLT) recoveries.
Testing
FDA- and AABB-required transmissible-disease testing, ABO typing, Rh grouping, and antibody screening tests were performed on samples collected at the start of the apheresis procedure. Results of all testing were available on the same day as collection and were used for product release. Units were labeled, stored, and issued in accordance with FDA and AABB standards. All units released for transfusion were infused on the same day as collection.
Patients
Requests for granulocyte transfusions were assessed by Department of Transfusion Medicine physicians in consultation with the patients’ primary medical teams. Severely neutropenic patients had aplastic anemia or had recently undergone hematopoietic progenitor cell transplantation. Patients with granulocyte dysfunction had leukocyte adhesion deficiency-1, Job syndrome, or chronic granulomatous disease.
During the transfusions, patients were monitored for adverse effects in an identical manner whether they received ABO-compatible or -incompatible granulocyte components. Vital signs were taken before starting the infusion, at 15 minutes into the transfusion, and at the end of the transfusion. The components were administered at a flow rate of 1 to 2 mL/minute under direct nursing observation for the first 15 minutes, after which the rate was increased to 2 to 3 mL/minute, with completion of the transfusion in approximately 2 hours. Lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, and bilirubin were determined serially to monitor for posttransfusion hemolysis.
Routine compatibility testing was performed as required by the FDA and AABB for transfused granulocyte components. All units were crossmatch incompatible due to the presence of antibodies in the patients’ sera directed against RBCs in the granulocyte concentrates. When requests for a course of granulocyte therapy were first approved, the patients’ physicians were made aware of the possibility that RBC-compatible granulocytes might not be available and approved the use of crossmatch-incompatible, sedimented granulocytes. Compatibility testing was not required for topical or intrapleural application of granulocytes.18
In several cases involving small-weight recipients or recipients receiving topical or intrapleural granulocytes, the granulocyte components were divided into two or more aliquots. This facilitated the distribution of divided granulocyte units to more than one recipient if necessary or to the same recipient with multiple topical or intrapleural applications over a period of several hours.
Statistical analyses
Data for pre- and postsedimentation blood cell counts were analyzed by descriptive statistics (mean, range, standard deviation [SD]). When appropriate, paired t tests were utilized, with significance assigned at the p value of less than 0.05 level. Values are given as mean ± SD.
RESULTS
Gravity sedimentation was performed on 165 granulocytapheresis concentrates. These concentrates were collected over an 18-month period and used to support the clinical needs of 13 patients. Indications for granulocyte sedimentation are shown in Table 1.
TABLE 1.
Indications for sedimentation of granulocyte concentrates
| Reasons | Number (n = 165) |
|---|---|
| ABO incompatible | 61 |
| Non-ABO RBC antigen incompatible | 2 |
| ABO and Rh incompatible | 2 |
| Topical application to wounds in patient with leukocyte adhesion deficiency-1 | 89 |
| Intrapleural application in patient with Job syndrome | 1 |
| Volume reduction for pediatric patient | 1 |
| Divided units (topical application to leukocyte adhesion deficiency-1 patient and intrapleural application to Job syndrome patient) | 4 |
| Divided units (topical application to leukocyte adhesion deficiency-1 patient and transfusion to Job syndrome patient) | 5 (1 transfusion ABO incompatible) |
Granulocyte concentrates: content and collection efficiency
Granulocytes were collected at a whole blood flow rate of 50 to 60 mL/minute. Mean whole blood volume processed, excluding anticoagulant, was 6084 ± 289 mL (range, 4577–6767 mL). Mean product volume was 257 ± 8 mL (range, 225–315 mL; Table 2). Total WBC content per component was 8.0 × 1010 ± 2.2 × 1010 cells, with a differential of 90 ± 3% granulocytes, 6 ± 3% monocytes, and 4 ± 1.4% lymphocytes.
TABLE 2.
Effect of sedimentation on content of granulocyte components*
| Parameter | Before sedimentation | After sedimentation | Percent recovery |
|---|---|---|---|
| Volume (mL) | 257 ± 8.0 (225–315) | 177 ± 24 (75–220) | 69 ± 10 |
| Granulocytes (×1010) | 7.3 ± 2.0 (3.7–14.9) | 5.8 ± 2.0 (0.8–11.4) | 80 ± 15 |
| Monocytes (×109) | 4.2 ± 1.7 (1.1–8.7) | 3.2 ± 1.7 (0.1–8.3) | 75 ± 23 |
| Lymphocytes (×109) | 3.1 ± 1.1 (0.8–6.7) | 2.5 ± 0.9 (0.7–4.7) | 80 ± 19 |
| PLTs (×1011) | 4.0 ± 1.0 (2.2–7.8) | 3.2 ± 0.8 (1.5–5.6) | 81 ± 14 |
| RBCs (mL) | 40 ± 7.6 (25–76) | 3.2 ± 1.4 (1.0–11) | 8.3 ± 3.7 |
Data are reported as mean ± SD (range) or mean ± SD. Before sedimentation, granulocytapheresis components were composed of a mean of 90% granulocytes, 6% monocytes, and 4% lymphocytes. This differential was not significantly changed by sedimentation.
Granulocyte collection efficiency (%CE) was determined using the formula:
Mean granulocyte collection efficiency was 56.5 ± 8% (range, 32%–77%).
Sedimentation time
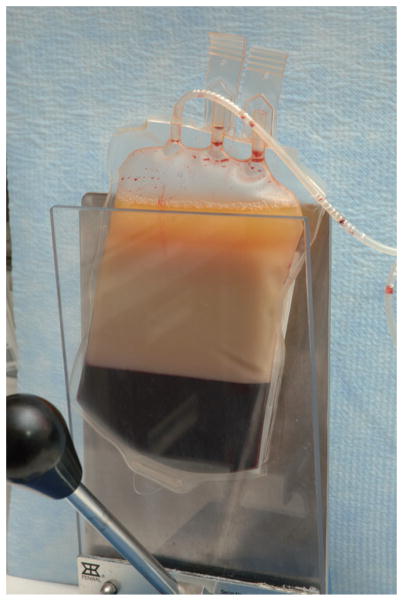
Units were allowed to sediment in an upright position in a plasma expressor for 267 ± 48 minutes (range, 150–440 min) for a sharp line of demarcation to form between the starch-sedimented RBCs and the granulocyte-rich supernatant (Fig. 1). Units that sedimented slowly were allowed to remain in the plasma expressor for a longer time to achieve optimum sedimentation. The granulocyte-rich supernatant was then transferred into the transfer pack.
Fig. 1.
Granulocyte concentrate undergoing gravity sedimentation in a plasma expressor.
RBC depletion
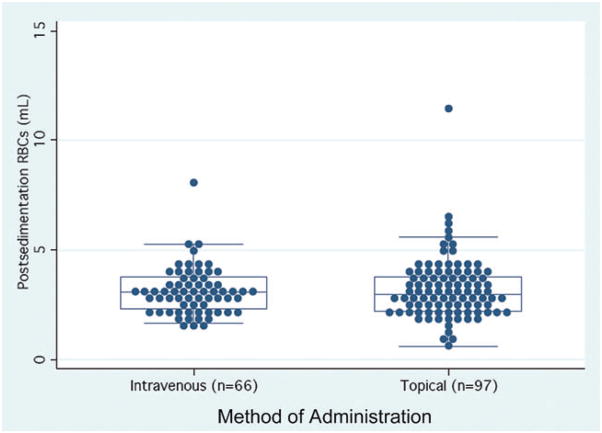
RBC volume in the granulocyte units immediately before sedimentation was 40.3 ± 7.6 mL (range, 24.7–75.6 mL; Table 2). After sedimentation, the RBC volume decreased to 3.2 ± 1.4 mL (range, 0.6–11.4 mL). The percentage of RBC depletion was 92 ± 4% (range, 71%–99%). Twelve percent (19/165) of all sedimented units contained less than 2 mL of RBCs. Among the RBC-incompatible granulocyte units that were transfused intravenously, the postsedimentation RBC content was 3.1 ± 1.1 mL (range, 1.6–8.2 mL), and 11% (7/66) contained less than 2 mL of RBCs (Fig. 2).
Fig. 2.
Distribution of residual RBC volumes after sedimentation of granulocyte units. The box in the center of each plot represents the middle 50% of the data (the 25% to 75% quartiles), with the median shown as the enclosed line in the center of the box. Seventy-five percent of all components contained less than 4.0 mL of RBCs.
Granulocyte recovery
The mean granulocyte count in the units before and after gravity sedimentation was 7.3 × 1010 ± 2.1 × 1010 (range, 3.7 × 1010–14.9 × 1010) and 5.8 × 1010 ± 2.0 × 1010 (range, 0.8 × 1010–11.4 × 1010) respectively (Table 2). Sedimentation resulted in a mean granulocyte recovery of 80 ± 15% (range, 20%–159%). One donor in the series had a poor response to G-CSF mobilization, which resulted in lower than expected granulocyte yields. The cause of the poor response was not known, and he was not recruited for further granulocyte donations.
PLT recovery
Mean PLT content in the units was 4.0 × 1011 ± 1.0 × 1011 (range, 2.2 × 1011–7.8 × 1011) and decreased to 3.2 × 1011 ± 0.8 × 1011 (range, 1.5 × 1011–5.6 × 1011) after sedimentation (Table 2). PLT recovery postsedimentation was 81 ± 14% (range, 42%–109%). Again, the donor with the lowest PLT yield was the same donor described above who had poor mobilization response to G-CSF.
Transfusion of RBC-incompatible sedimented granulocytes
Sixty-six units of RBC-incompatible sedimented granulocytes were transfused to 12 patients (one patient in the study received only topical granulocytes). Sixty-four transfusion events involved ABO incompatibility, two of which also involved D+ units into a D− subject who received Rh immune globulin. Two events involved E+ units into a recipient with an anti-E that was strongly reactive at anti-globulin phase of testing. No transfusion reactions were reported, and no patient had clinical or laboratory evidence of hemolysis after transfusion.
Analysis of granulocyte transfusions
Four patients with severe aplastic anemia received both sedimented and nonsedimented granulocyte concentrates in a nonrandomized fashion, depending on the blood group of available donors. The outcome of these transfusions was retrospectively analyzed and compared. Although the granulocyte content of the sedimented units was 20% less than that of the nonsedimented ones, post-transfusion absolute neutrophil count (ANC) increments were not significantly different among the sedimented versus nonsedimented components (Table 3). These transfusions were not derived from the same donors and did not have the same initial granulocyte content per unit. Many issues confounded patients’ responses to granulocyte transfusions including hemodynamic stability, degree and source of sepsis, presence or absence of fever, chemotherapeutic regimen, and hematopoietic progenitor cell transplant status, and thus this comparison cannot be used to imply that granulocyte dose was unrelated to granulocyte increment after transfusion. Rather, it demonstrates that the granulocytes remaining in the component after sedimentation appeared to survive and circulate as expected in vivo, in comparison with the behavior of nonsedimented components.
TABLE 3.
Posttransfusion granulocyte count increments in four patients with severe aplastic anemia receiving granulocyte transfusions
| Patient | Nonsedimented granulocyte transfusions
|
Sedimented granulocyte transfusions
|
p value* | ||||
|---|---|---|---|---|---|---|---|
| Number | Component granulocyte count (×1010) | Posttransfusion ANC increment ± SD (/μL) | Number | Component granulocyte count after sedimentation (×1010) | Posttransfusion ANC increment ± SD (/μL) | ||
| 1 | 2 | 8.94 ± 0.1 | 1300 ± 283 | 6 | 5.98 ± 2.0 | 4881 ± 1925† | 0.05 |
| 6 | 10 | 6.93 ± 1.98 | 519 ± 301 | 3 | 6.74 ± 2.49 | 468 ± 143 | 0.79 |
| 5 | 3 | 6.59 ± 2.26 | 834 ± 2859 | 4 | 3.92 ± 1.05 | 997 ± 972 | 0.92 |
| 13 | 3 | 5.55 ± 1.16 | 549 ± 121 | 9 | 5.95 ± 1.52 | 666 ± 480 | 0.69 |
Comparison of posttransfusion ANC increments after transfusion of nonsedimented versus sedimented granulocytes.
The exceptionally robust increment in granulocyte counts following these transfusions was not due to autologous recovery or transplant engraftment.
DISCUSSION
Maintenance of a granulocytapheresis donor program presents many challenges for a blood center. To manufacture a component with greatest therapeutic efficacy, as assessed by the granulocyte content of the unit and the increment in count in the recipient after transfusion, stimulating agents must be given to the donor to increase the circulating granulocyte count before donation. The combination of a single oral dose of dexamethasone and a single subcutaneous injection of G-CSF, administered within 24 hours before donation, was first described in 199513 and has become the standard regimen used in programs that produce high-content granulocytapheresis concentrates. These programs depend on the availability of a dedicated and committed pool of community donors, supplemented by family members when eligible. Granulocyte donors are often called upon with only a few hours’ to a few days’ notice, must be willing to travel to the blood center to receive stimulatory agents, and are frequently required to be RBC compatible and HLA compatible with specific patient needs. These requirements can severely hinder the donor selection process.
To expand our choices of potential donors for granulocytapheresis donation, we used HES-assisted gravity sedimentation to deplete incompatible RBCs from these components. Using an entirely closed-system technique, the granulocyte-rich supernatant harvested after component sedimentation contained 80% of the initial granulocyte and 81% of the initial PLT content, with only 8% of the initial RBC content. A substantial granulocyte dose of 5.8 × 1010 cells was recovered after sedimentation, which resulted in posttransfusion granulocyte increments in neutropenic recipients that were indistinguishable from increments after transfusions of nonsedimented concentrates. The process of starch sedimentation was simple, inexpensive, and efficient, requiring materials readily available in a blood center. At our institution, granulocyte concentrates were not routinely released until the completion of same-day transmissible disease testing; therefore, sedimentation was performed while awaiting the results of infectious disease marker assays and did not delay release of the component. In other facilities, where infectious disease testing is done on samples drawn before donation, the approximately 4-hour period required for sedimentation may delay transfusion, but is unlikely to affect the potency of the component, as post-transfusion increments were not affected by the duration of sedimentation (data not shown).
This is the first study to describe starch sedimentation of granulocyte components manufactured by steroid and G-CSF stimulation of the donor. In 1989, we first described RBC reduction of granulocyte components by starch-assisted gravity sedimentation to support a 7-year-old, 17-kg child with chronic granulomatous disease and alloantibodies to c, E, and Jka antigens.16 A 1- to 2-hour sedimentation process reduced the volume of RBCs to 2.5 ± 1.0 mL with a mean granulocyte recovery of 70% in 37 non–G-CSF-mobilized units. In 1997, Wuest and Reich19 reported removal of ABO-incompatible RBC from 29 lymphocytapheresis and 11 nonstimulated granulocyte components, utilizing postcollection addition of HES and inverted sedimentation of the collection bag for 1 hour, with subsequent draining of the RBC from the sedimented component. The final components retained 96% of the original granulocytes and 6 mL of RBCs. Adkins and coworkers20 subjected 10 G-CSF–mobilized granulocyte concentrates to 1 hour of starch-assisted gravity sedimentation in a plasma expressor and documented an 80.8% recovery of granulocytes, an 81.4% recovery of PLTs, and a mean RBC content of 6.3 mL in the final component. In contrast, the longer sedimentation time of 4 hours in the present study of 165 granulocyte concentrates resulted in a lower volume of residual RBCs after sedimentation (mean, 3.2 ± 1.4 mL), with an equivalent recovery of granulocytes and PLTs (80 and 81%, respectively). It is not known if this modest loss of granulocytes by sedimentation to remove RBCs alters the clinical efficacy of the granulocyte product.
AABB standards require that apheresis granulocytes be ABO-compatible with recipient plasma and undergo crossmatch unless they contain less than 2 mL of RBC.21 As only 12% of our sedimented components contained less than 2 mL of RBC, and as the cellular content of the final product was not known at the time of crossmatch, it was necessary to crossmatch all components undergoing sedimentation, and all were found to be incompatible. Among 165 sedimented components, the largest residual RBC volume was 11.4 mL (8.2 mL in a transfused component), yet there were no untoward clinical effects and no laboratory evidence of hemolysis associated with the 66 ABO-incompatible components that were administered intravenously. Prior studies of indium-labeled donor granulocytes showed that ABO incompatibility did not alter the in vivo recovery, survival, or migration of the transfused cells.22 Consistent with these studies, our analysis of four patients receiving both sedimented and nonsedimented granulocytes showed no significant differences in count increments or clinical outcomes associated with the two products. This lack of adverse clinical sequelae is consistent with the experience in recipients of ABO-incompatible marrow or peripheral blood stem cell grafts, where transfusion of 10 to 60 mL of ABO-incompatible RBCs is generally well tolerated. We are not aware of alternative methods, approved for use in transfusable products, that can routinely reduce the RBC volume of a granulocyte concentrate to less than 2 mL. Several RBC lysing solutions are available for in vitro processing of WBC preparations, but these solutions are not approved for in vivo use, and the components would have to be washed after processing, which might result in substantial granulocyte loss. All primary care physicians in our study were informed that their patients would be receiving ABO-incompatible granulocyte components and had assessed the potential life-saving benefits of the transfusions as substantially greater than the potential for adverse effects due to incompatible RBCs.
Gravity sedimentation of granulocytapheresis concentrates to reduce the content of incompatible RBCs makes it possible to maintain a granulocyte transfusion program that can support several patients concurrently. Although RBC-compatible donors should be chosen whenever possible, the ability to select donors who are ABO or minor RBC antigen incompatible with recipients significantly expands the pool of potential donors. This is the largest study to date to demonstrate the efficacy, safety, and practicality of gravity sedimentation of granulocytes with HES to deplete RBCs while retaining high neutrophil content, particularly after steroid and G-CSF stimulation of the donor. Processing of granulocyte components in this manner should contribute to the more widespread use of these complex products, with the caveat that such transfusions should always be accompanied by careful clinical monitoring.
Acknowledgments
We acknowledge the expertise of the donor room staff and the transfusion service laboratory staff of the NIH Department of Transfusion Medicine and the generosity and commitment of our granulocytapheresis donors.
ABBREVIATION
- ANC
absolute neutrophil count
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interests to disclose.
References
- 1.Hester JP, Dignani MC, Anaissie EJ, Kantarjian HM, O’Brien S, Freireich EJ. Collection and transfusion of granulocyte concentrates from donors primed with granulocyte stimulating factor and response of myelosuppressed patients with established infections. J Clin Apheresis. 1995;10:188–93. doi: 10.1002/jca.2920100406. [DOI] [PubMed] [Google Scholar]
- 2.Price TH, Bowden RA, Boeckh M, Bux J, Nelson K, Liles WC, Dale DD. Phase I/II trial of neutrophil transfusions from donors stimulated with G-CSF and dexamethasone for treatment of patients with infections in hematopoietic stem cell transplantation. Blood. 2000;96:3302–9. [PubMed] [Google Scholar]
- 3.Adkins DR, Goodnough LT, Shenoy S, Brown R, Moellering J, Khoury H, Vij R, DiPersio J. Effect of leukocyte compatibility on neutrophil increment after transfusion of granulocyte colony-stimulating factor-mobilized prophylactic granulocyte transfusions and on clinical outcomes after stem cell transplantation. Blood. 2000;95:3605–12. [PubMed] [Google Scholar]
- 4.Lee JJ, Chung IJ, Park MR, Hwang TJ, Ryang DW, Kim HJ. Clinical efficacy of granulocyte transfusion therapy in patients with neutropenia-related infections. Leuk. 2001;15:203–7. doi: 10.1038/sj.leu.2402007. [DOI] [PubMed] [Google Scholar]
- 5.Illerhaus G, Wirth K, Dwenger A, Waller CF, Garbe A, Brass V, Lang H, Lange W. Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Ann Hematol. 2002;81:273–81. doi: 10.1007/s00277-002-0439-6. [DOI] [PubMed] [Google Scholar]
- 6.Rutella S, Pierelli L, Sica S, Serafini R, Chiusolo P, Paladini U, Leone F, Zini G, D’Onofrio G, Leone G, Piccirillo N. Efficacy of granulocyte transfusions for neutropenia-related infections: retrospective analysis of predictive factors. Cytotherapy. 2003;5:19–30. doi: 10.1080/14653240310000047. [DOI] [PubMed] [Google Scholar]
- 7.Safdar A, Hanna HA, Kontoyiannis BP, Hachem R, Lichtiger B, Freireich EJ, Raad II. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia: retrospective case-control analysis of 491 episodes of Candida species bloodstream infections. Cancer. 2004;101:2859–65. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- 8.Ofran Y, Avivi I, Oliven A, Oren I, Zuckerman T, Bonstein L, Rowe JM, Dann EJ. Granulocyte transfusions for neutropenic patients with life-threatening infections: a single centre experience in 47 patients, who received 348 granulocyte transfusions. Vox Sang. 2007;93:363–9. doi: 10.1111/j.1423-0410.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 9.Bux J, Cassens U, Dielschneider T, Duchscherer M, Edel E, Eichler H, Haas C, Moog R, Peschke H, Peters C, Ryzenkov I, Schlenke P, Ullrich H, Wiesneth M. Tolerance of granulocyte donors towards granulocyte colony-stimulating factor stimulation and of patients towards granulocyte transfusions: results of a multicentre study. Vox Sang. 2003;85:322–5. doi: 10.1111/j.0042-9007.2003.00373.x. [DOI] [PubMed] [Google Scholar]
- 10.Peters C. Granulocyte transfusions in neutropenic patients: beneficial effects proven? Vox Sang. 2009;96:275–83. doi: 10.1111/j.1423-0410.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 11.Vamvakas EC, Pineda AA. Meta-analysis of clinical studies of the efficacy of granulocyte transfusions in the treatment of bacterial sepsis. J Clin Apheresis. 1996;11:1–9. doi: 10.1002/(SICI)1098-1101(1996)11:1<1::AID-JCA1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Vamvakas EC, Pineda AA. Determinants of the efficacy of prophylactic granulocyte transfusion: a meta-analysis. J Clin Apheresis. 1997;12:74–81. doi: 10.1002/(sici)1098-1101(1997)12:2<74::aid-jca4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Leitman SF, Yu M, Lekstrom J. Pair-controlled study of granulocyte colony-stimulating factor (G-CSF) plus dexamethasone for granulocytapheresis donors. Transfusion. 1995;35:53s. [Google Scholar]
- 14.Liles WC, Rodger E, Dale DC. Combined administration of G-CSF and dexamethasone for the mobilization of granulocytes in normal donors: optimization of dosing. Transfusion. 2000;40:642–4. doi: 10.1046/j.1537-2995.2000.40060642.x. [DOI] [PubMed] [Google Scholar]
- 15.Stroncek DF, Yau YY, Oblitas J, Leitman SF. Administration of G-CSF plus dexamethasone produces greater granulocyte concentrate yields while causing no more donor toxicity than G-CSF alone. Transfusion. 2001;41:1037–44. doi: 10.1046/j.1537-2995.2001.41081037.x. [DOI] [PubMed] [Google Scholar]
- 16.DePalma L, Leitman SF, Carter CS, Gallin JI. Granulocyte transfusion therapy in a child with chronic granulomatous disease and multiple red cell autoantibodies. Transfusion. 1989;29:421–3. doi: 10.1046/j.1537-2995.1989.29589284143.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Leitman SF, Klein HG. A controlled comparison of the efficacy of hetastarch and pentastarch in granulocyte collection by centrifugal leukapheresis. Blood. 1995;86:4662–6. [PubMed] [Google Scholar]
- 18.Bryant BJ, Khuu HM, Uzel G, Holland SM, Leitman SF. Topical granulocyte concentrates for chronic wound healing in a patient with leukocyte adhesion deficiency-1. Transfusion. 2007;47:16a. [Google Scholar]
- 19.Wuest DL, Reich LM. Removal of ABO-incompatible red cells from lymphocytapheresis and granulocytapheresis components before transfusion. Transfusion. 1997;37:144–9. doi: 10.1046/j.1537-2995.1997.37297203516.x. [DOI] [PubMed] [Google Scholar]
- 20.Adkins D, Johnston M, Walsh J, Spitzer G, Goodnough T. Hydroxyethylstarch sedimentation by gravity ex vivo for red cell reduction of granulocyte apheresis components. J Clin Apheresis. 1998;13:56–61. doi: 10.1002/(sici)1098-1101(1998)13:2<56::aid-jca2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Price TH, editor. Standards for blood banks and transfusion services. 25. Bethesda (MD): AABB; 2008. p. 45. [Google Scholar]
- 22.McCullough J, Clay M, Loken M, Hurd D. Effect of ABO incompatibility on the fate in vivo of 111-indium granulocytes. Transfusion. 1988;28:358–61. doi: 10.1046/j.1537-2995.1988.28488265267.x. [DOI] [PubMed] [Google Scholar]