Abstract
Objective
Drug exposure during critical periods of brain development may adversely affect nervous system function, posing a challenge for treating of infants. This is of particular concern for treating neonatal seizures, as early-life exposure to drugs such as phenobarbital is associated with adverse neurological outcomes in patients and induction of neuronal apoptosis in animal models. The functional significance of the preclinical neurotoxicity has been questioned due to the absence of evidence for functional impairment associated with drug-induced developmental apoptosis.
Method
We used patch-clamp recordings to examine functional synaptic maturation in striatal medium spiny neurons (MSNs) from neonatal rats exposed to antiepileptic drugs with proapoptotic action (phenobarbital, phenytoin, lamotrigine) and without proapoptotic action (levetiracetam). Phenobarbital-exposed rats were also assessed for reversal learning at weaning.
Result
Recordings from control animals revealed increased inhibitory and excitatory synaptic connectivity between postnatal day (P)10 and 18. This maturation was absent in rats exposed at P7 to a single dose of phenobarbital, phenytoin, or lamotrigine. Additionally, phenobarbital exposure impaired striatal-mediated behavior on P25. Neuroprotective pretreatment with melatonin, which prevents drug-induced neurodevelopmental apoptosis, prevented the drug-induced disruption in maturation. Levetiracetam was found not to disrupt synaptic development.
Interpretation
Our results provide the first evidence that exposure to antiepileptic drugs during a sensitive postnatal period impairs physiological maturation of synapses in neurons that survive the initial drug insult. These findings suggest a mechanism by which early-life exposure to AEDs can impact cognitive and behavioral outcomes, underscoring the need to identify therapies that control seizures without compromising synaptic maturation.
Introduction
The treatment of neurological disorders in infants is complicated by the fact that therapeutic interventions can derail the course of brain development. Because the developing brain is highly vulnerable to modifications of the molecular environment of neurons, even transient interventions during sensitive developmental periods can have long-lasting functional consequences.
This is especially true for interventions to control seizures, one of the most common neurological disorders of infancy.1 While treatment with antiepileptic drugs (AEDs) is usually necessary to avoid adverse outcomes associated with uncontrolled seizures, exposure to AEDs themselves has also been linked to adverse outcomes. In particular, phenobarbital exposure during gestation or early infancy has been associated with reduced IQ and decreased regional brain volumes in humans.2–5 Exposure to this drug may also contribute to the increased risk for neuropsychiatric disorders associated with early life seizures.6 However, conclusions from clinical studies are limited by the inability to fully dissociate the long term effects of drug treatment from the effects associated with the underlying clinical disorders that give rise to seizures.
Despite the potential risks, phenobarbital remains the treatment of choice for seizures in infancy.7,8 Over 80% of neonatal seizures are treated with phenobarbital, resulting in 15,000–20,000 pre-term and full-term newborns exposed to phenobarbital each year.1,7,8 In addition, phenobarbital is among the most commonly used medications for neonates with hypoxic-ischemic encephalopathy.9 This drug has also been used in infancy for the treatment of febrile seizures and opiate withdrawal.4,10,11 Nevertheless, questions about the clinical efficacy and safety of phenobarbital have prompted some to call for a reexamination of the use of this drug in infancy, and a consideration of potential therapeutic alternatives.4,12
Recent preclinical findings provide a further basis to question the wisdom of exposing the neonatal brain to phenobarbital. Excessive neuronal apoptosis, impaired neurogenesis, and dysregulation of the cortical proteome all result from phenobarbital exposure during a postnatal age in rodents corresponding to the perinatal/neonatal period in humans.13–15 However, because of a lack of evidence for a functional developmental impact of these molecular abnormalities, their clinical relevance has been disputed.16,17 Furthermore, because apoptotic neuronal death (i.e., programmed cell death) is a normal component of brain development during the first ten days of postnatal life in the rat13 the drug-induced amplification of this phenomenon cannot be taken as conclusive documentation of damage in the absence of evidence for functional impairment. Thus, the present study aimed to determine whether exposure to phenobarbital during the neonatal period would be detrimental to the normal pattern of maturation of synaptic function.
To assess the impact of phenobarbital on functional synaptic maturation in infancy, we employed the neonatal rat as a preclinical model. In pups between postnatal day (P)10 and P18, the maturation of inhibitory and excitatory postsynaptic currents were examined in striatum, a brain region that displays reduced volume in human imaging studies of adult patients exposed to AEDs early in life.5 The neurons in striatum are especially vulnerable to the pro-apoptotic action of AED exposure in rats during the first postnatal week.13,18,19 We used two different acute doses of phenobarbital (37.5 and 75mg/kg, given on P7), corresponding to the threshold and mid-range doses for inducing neuronal cell death;13 these doses fall within the moderate to high regions of the clinically-relevant range.20
We compared the effects of exposure to phenobarbital to the effects of exposure to three other AEDs: phenytoin, lamotrigine, and levetiracetam. Phenytoin is currently the second-line treatment for neonatal seizures,7 while lamotrigine and levetiracetam are newer-generation AEDs which are believed to have an especially favorable safety profile for clinical use in pregnancy and infancy.21–25 In addition, whereas doses of phenytoin within the therapeutic range increase neuronal apoptosis in neonatal rodents,26,27 lamotrigine has this effect only at doses higher than therapeutic,19 and levetiracetam is devoid of this effect even in doses well above the therapeutic range.18 To determine if a neuroprotective intervention can ameliorate drug-induced deficits in the maturation of synaptic transmission, we also evaluated the effect of phenobarbital in combination with melatonin, a neuroprotective treatment previously shown to prevent drug-induced developmental neuronal apoptosis.28
By using patch-clamp recordings and morphometric analysis of striatal medium spiny neurons (MSNs), we examined the maturation of GABAergic and glutamatergic synaptic connectivity during the second to third postnatal weeks in normal rat pups and in rat pups that had been exposed to one of the four AEDs at P7. P7 is a time point at which there is maximal vulnerability to the pro-apoptotic action of AEDs;13 this age corresponds closely to the perinatal/neonatal period of brain development in humans.29 During the second and third postnatal weeks, dendritic spine number markedly increases on MSNs, indicating that this is a key temporal window for striatal synaptic maturation.35
As the principle neurons of the striatum, MSNs constitute approximately 95% of striatal neurons and receive glutamatergic input from cortex34 and GABAergic input from interneurons and other MSNs.30 MSNs integrate cortical and subcortical input to modulate basal ganglia activity, guiding motor output and mediating procedural learning and memory.30 Disrupting the function of these neurons impairs procedural learning and memory,31,32 sensorimotor gating,33 and motor behavior.32
Materials and Methods
Animals
Timed-pregnant Sprague-Dawley rats were purchased from Harlan and housed on a standard 12h:12h Light:Dark cycle until parturition. Date of parturition was designated P0 for all pups. A minimum of 3 pups per treatment, derived from at least 2 litters was used for all groups.
Drugs
Sodium phenobarbital (75 or 37.5mg/kg, 5-ethyl-5-phenyl-1,3-diazinane-2,4,6-trione; Sigma), lamotrigine (20mg/kg, 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine; GlaxoSmithKline, Research Triangle Park, NC), Levetiracetam (400mg/kg; Keppra oral solution, UCB Pharma, Smyrna, GA) were dissolved in saline, while phenytoin (50mg/kg, sodium 5,5-diphenylhydantoin, 5,5-diphenylimidazolidine-2,4-dione; Sigma) was dissolved in alkalinized saline, pH 10. All AEDs were injected at a volume of 0.01ml/g, ip. Melatonin (20mg/kg, s.c., N-Acetyl-5-methoxytryptamine, Sigma) was dissolved in 1% ethanol solution immediately before administration.
Relevance of doses used to human drug levels: The plasma levels of phenobarbital in human infants associated with therapeutic doses are 20–40ug/ml,36 but can reach 65ug/ml after acute loading doses.37 The doses of phenobarbital used (37.5 and 75mg/kg) were selected to approximate this range in the rat (20–40ug/ml for 37.5mg/kg, and 35–70ug/ml for 75mg/kg).13 The therapeutic range for phenytoin plasma levels in the human is 10–20ug/kg,38 the dose of phenytoin selected (50mg/kg) results in plasma levels results in peak plasma levels of 35ug/ml, with sustained plasma levels of 10ug/ml for at least 4h.13 In patients, plasma levels of lamotrigine have between reported between 1.8 and 11.1 ug/ml,41 the dose of lamotrigine (20mg/kg) was selected to result in plasma levels in the rat between 8–12ug/ml.39,40 Moreover, CSF levels of lamotrigine in patients reach 3.4–3.6ug/ml,41 while 20mg/kg of lamotrigine in rats results in CSF levels between 2.6 and 5ug/ml.40 The doses of phenobarbital and phenytoin selected produce cell death in neonatal rats, while the dose of lamotrigine selected is less than half the dose required to produce cell death.
Treatments
Pups were treated either once on P7 with phenobarbital, phenytoin, lamotrigine, levetiracetam, or vehicle, or once on P10 with phenobarbital via i.p. injection. A separate group of animals was treated with melatonin or vehicle 15 minutes prior to and 2 hours after treatment with 75mg/kg phenobarbital or vehicle.
Slice preparation
Rats were killed by decapitation at P10–11, P13–14, or P17–18 in compliance with the American Association for Accreditation of Laboratory Animal Care standards and the Georgetown University Animal Care and Use Committee. Corticostriatal brain slices were prepared as previously described.42 Additional details can be found in Supplemental Methods.
Whole-cell recordings
Electrophysiological recording procedures followed our previous protocols using KCl-based internal recording solution (see Supplement for details).42 Striatal MSNs were identified by size, shape, and firing pattern in response to hyperpolarizing and depolarizing current injections (10mV), as previously described.43 Voltage-clamp recordings were performed using the whole-cell configuration of the patch-clamp technique at a holding voltage of −60 mV using the Axopatch 200B and 1D amplifiers (Molecular Devices). Access resistance was monitored during the recordings and experiments with >15% change were discarded. Cells were held at −60 mV. Stock solutions of bicuculline methylbromide (BMR) and tetrodotoxin (TTX) (both from Sigma) were prepared in water. Stock solutions were diluted in aCSF and applied locally through a Y tube, modified for optimal solution exchange in brain slices.
Off-line data analysis, curve fitting, and figure preparation were performed with Clampfit 9 software (Molecular Devices). Spontaneous and miniature IPSCs (sIPSCs and mIPSCs) and mEPSCs were identified using a semiautomated threshold based minidetection software (Mini Analysis; Synaptosoft) and were visually confirmed. Event detection threshold was set at 5 times the RMS level of baseline noise. IPSC average values were based on >50 events in each cell studied, and IPSC decay kinetics were determined using double exponential curve fittings.44 All detected events were used for event frequency analysis; however, superimposing events were eliminated for the amplitude and decay analysis. NBQX was not included in sIPSC measurements to not perturb the network activity. AMPA-mediated spontaneous EPSCs could easily be identified by the rapid decay kinetics (<8 ms) and were excluded from the IPSC analysis, as we have previously described.45–47 mIPSCs were isolated by application of TTX (0.5 µM). mEPSCs were identified during application of TTX and BMR (25 µM). sEPSCs were not pharmacologically isolated in these experiments. It has been previously documented that the frequency of mEPSCs does not differ from the frequency of sEPSCs in acute corticostriatal slices,48 because the majority of corticostriatal projections to striatum have been disconnected from their cell bodies in the slice. When we analyzed the frequency of mEPSCs and sEPSCs in 32 control neurons the frequencies were equivalent (Supplemental Fig 2) confirming previous observations.
Details of anatomical reconstruction can be found in Supplemental Methods.
Statistical Analysis
Data were analyzed using SPSS Statistics Software (Ver 19, IBM) and GraphPad Prism (Ver 5, Graphpad). Analysis of variance (ANOVA) with Fisher’s Least Significant Difference post-hoc test, t-tests, and least squares regression were used as appropriate to analyze the data. Criterion for significance in all tests was set at P<0.05.
For Methods related to Caspase-3 western blots and T-Maze reversal learning, see supplemental methods.
Results
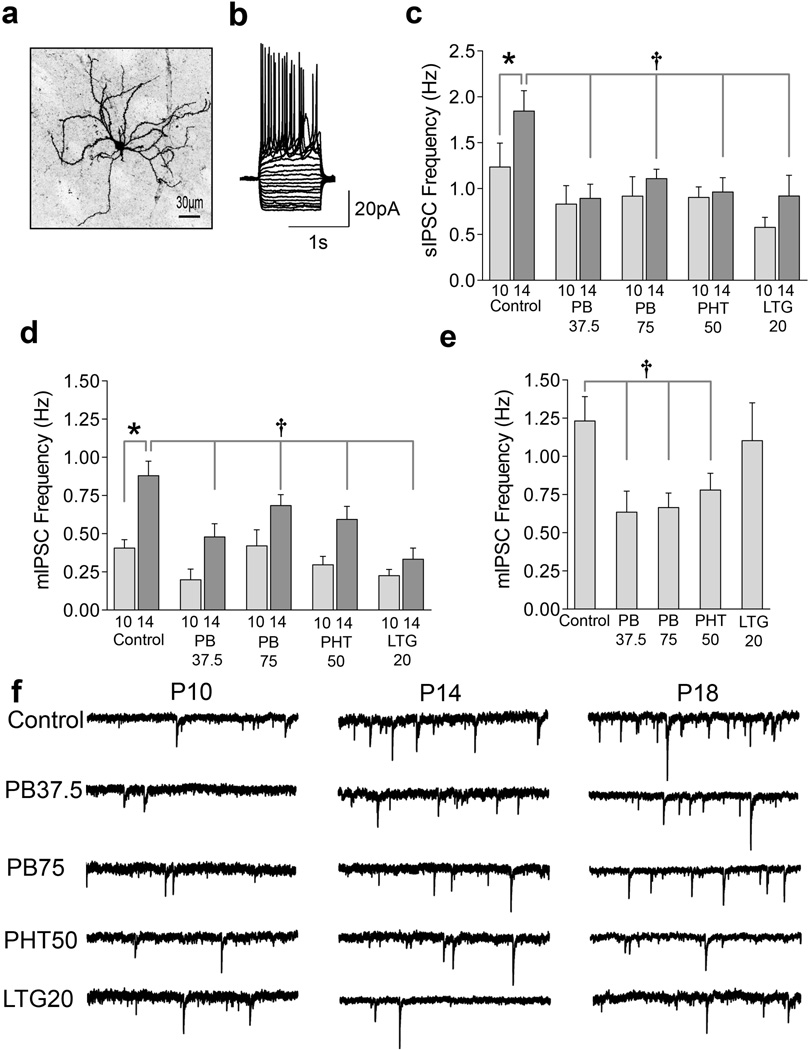
We first characterized the time course of MSN synaptic development in drug-naïve animals, because this had not yet been established. We then examined the effects of P7 exposure to AEDs. Whole-cell patch clamp recordings were made from striatal MSNs (representative, biocytin filled MSN, Fig 1a) in acute slices prepared from rats at P10–11, P13–14, or P17–18. MSNs were identified based on size and their characteristic delayed, non-adapting firing pattern (Fig 1b). We first investigated spontaneous inhibitory post-synaptic currents (sIPSCs, Fig 1c), as a measure of GABAergic synaptic connectivity in MSNs. Control animals displayed a significant increase (P<0.05) in sIPSC frequency between P10 and P14. This pattern of age-dependent increase in frequency also appeared when miniature (m)IPSCs were analyzed (Fig 1c, d) in the presence of tetrodotoxin (TTX, 0.5 µM). In control animals, the frequency of mIPSCs doubled between P10 and P14, and tripled by P18. IPSC amplitude decreased from P10 to P14 (P<0.005), with further decreases by P18; while IPSC decay kinetics became faster between P10 and P14 (P<0.05, Supplemental Table 1 and 2). This is the first report describing the pattern of IPSC development in the neonatal striatum, demonstrating that IPSCs became more frequent with faster decay kinetics and decreased amplitude. This developmental pattern is remarkably similar to that reported for other brain regions, albeit with different timing 49–53.
Figure 1.
AED exposure disrupts IPSC development during the second postnatal week. (A) Representative confocal image of a biocytin-filled striatal medium spiny neurons (MSNs) with local dendritic arborization and spiny dendrites. Calibration bar, 30µm (B) Representative current clamp recording from a MSN, showing the characteristic repetitive, non-adapting firing pattern in response to a series of 1s hyperpolarizing and depolarizing current injections (10 pA steps) from a holding potential of −60 mV. (C) Quantification (mean±S.E.M.) of spontaneous inhibitory postsynaptic current (sIPSC) frequency at P10 and P14 showing the effect of P7 exposure to saline (control, n=28, n=32), phenobarbital (PB, 37.5 mg/kg, n=11, n=9), phenobarbital (PB, 75 mg/kg, n=9, n=23), phenytoin (PHT, 50 mg/kg, n=12, n=23), and lamotrigine (LTG, 20 mg/kg, n=11, n=11). (D) Quantification (mean±S.E.M.) of miniature inhibitory postsynaptic current (mIPSC) frequency at P10 and P14 showing effects of P7 exposure to saline (n=21, n=30), phenobarbital (37.5 mg/kg, n=6, n=6), phenobarbital (75 mg/kg, n=6, n=23), phenytoin (50 mg/kg, n=12, n=21), and lamotrigine (20 mg/kg, n=9, n=8) (E) Quantification (mean±S.E.M.) of mIPSC frequency at P18 showing effects of P7 exposure to saline (n=22), phenobarbital (37.5 mg/kg, n=6), phenobarbital (75 mg/kg, n=9), phenytoin (50 mg/kg, n=8), and lamotrigine (20 mg/kg, n=10). (F) Representative mIPSC traces from each group at P10, P14 and P18, following P7 treatment with saline, phenobarbital (75 mg/kg), phenobarbital (37.5 mg/kg), phenytoin (50 mg/kg), and lamotrigine (20 mg/kg). Data were analyzed by ANOVA with Fisher’s Least Significant Difference test for multiple comparisons. * indicates significantly different than P10 value within treatment. † Indicates significantly different than control at same age. See Supplemental Statistics Discussion for a complete discussion of ANOVA results.
The pattern of IPSC maturation was then examined in MSNs from animals exposed to phenobarbital (75 or 37.5mg/kg), phenytoin (50 mg/kg) or lamotrigine (20mg/kg), once on P7. Strikingly, all three AEDs caused later abnormalities in GABAergic synaptic connectivity.
At P10, all treatment groups displayed equivalent IPSC frequency, amplitude, and decay. By P14, IPSC frequency differed across groups (P<0.005, Fig 1b, and c). In sharp contrast to drug-naïve controls, the AED-exposed groups lacked the age-dependent increases in IPSC frequency (Fig 1c, d, and f): AED-exposed groups showed significantly lower IPSC frequency compared to controls (see Fig 1 legend for details). AED-exposed groups were not different from controls with respect to IPSC amplitude or decay between P10 and P14 (Supplemental Table 1 and 2).
We next extended our analysis of IPSC frequency to a later age, P18. At this time point, phenobarbital- and phenytoin-exposed groups continued to show a significantly lower frequency of IPSCs compared to controls, indicating a lack of recovery in GABAergic synaptic connectivity. In contrast, the lamotrigine-exposed group showed recovery by P18, with IPSC frequency reaching controls levels (Fig 1e).
In the same cells in which we analyzed IPSC frequency, we also evaluated excitatory (glutamatergic) synaptic transmission, in the presence of TTX and the GABAA receptor antagonist, bicuculline methylbromide (BMR). Control animals showed a significant (270%) increase in frequency of miniature excitatory postsynaptic currents (mEPSCs) between P14 and P18 (P<0.000005, Fig 2a), indicating that the maturation of glutamatergic synaptic transmission occurs later than that of GABAergic transmission.
Figure 2.
AED exposure disrupts excitatory postsynaptic current development and alters spine morphology. (A) Quantification of glutamatergic (miniature excitatory postsynaptic currents, mEPSCs) development in striatal MSNs from P10 to P18, as a function of drug treatment, showing effects of P7 exposure to saline (n=19, n=29, n=21), phenobarbital (PB, 37.5 mg/kg, n=6, n=6, n=7), phenobarbital (PB, 75 mg/kg, n=7, n=19, n=8), phenytoin (PHT, 50 mg/kg, n=9, n=19, n=8), and lamotrigine (LTG, 20 mg/kg, n=6, n=9, n=19). Data were analyzed by ANOVA with Fisher's Least Significant Difference post-hoc test. * indicates significantly different than P10 value within treatment (P<0.05), † indicates significantly different than control at same age (P<0.05). (B1) Quantification of spine and (B2) filopodia number on P18 MSNs from animals exposed to saline (control, n=8 neurons) or phenobarbital (75mg/kg, n=5 neurons) on P7; while the number of spines did not differ between treatments (t-test, P=0.9246), the number of filopodia where significantly greater in phenobarbital-exposed animals (n=6 neurons per treatment, t-test, * = P<0.0001). (B3) Quantification (performed using ImageJ) of spine length (left y-axis) and (B4) spine width (right y-axis) in P18 MSNs from animals exposed to control (n=6 neurons) or PB (75mg/kg, n=6 neurons) on P7. (C) Representative confocal images of dendritic branch used for quantification of spines and filopodia. Only mature (mushroom shaped) spines were counted (black arrow). Filopodia were classified as long thin processes (grey arrow) and were not included in spine width or length measurements.
The maturation of excitatory synaptic transmission after AED exposure exhibited deficits similar to those observed with inhibitory transmission. Each of the AED-exposed groups showed a lower frequency of mEPSCs at P18 as compared to controls (P<0.05). Moreover, when the AED-exposed groups were analyzed for age-dependent increases in mEPSC frequency, this maturation was absent.
The disruption of developmental increases in both IPSC and EPSC frequency, which manifest at least a week after a single drug exposure, reflects a profound impact of AEDs on synaptic maturation. Because both IPSC and EPSC amplitudes are unchanged across treatments (Supplemental Tables 1, 2 and 3), it is likely that the number of postsynaptic receptors per synapse is not altered by drug exposure. Because the frequency of events is a reflection of the number of presynaptic sites and/or release probability, our data suggest a presynaptic site of action accounts for the disruption in maturation.
We next hypothesized that MSNs deprived of normal synaptic activity would exhibit alterations in dendritic morphology. We measured the number and size of dendritic spines and filopodia on P18 MSNs after P7 exposure to phenobarbital (Fig 2b, c). Phenobarbital exposure did not alter either spine density or length, but decreased spine width (P<0.01) and increased filopodia density (P<0.0001) as compared to controls. The persistence of filopodia, the most immature form of spine, may indicate continued “searching” for synaptic partners by neurons receiving a lower frequency of excitatory synaptic events. This resembles the increase in filopodia seen in cortical neurons deprived of synaptic input by treatment with tetrodotoxin.54
When we exposed P7 rat pups to phenobarbital (75mg/kg) and tested them on a reversal learning task that has been previously validated as striatal-dependent in weanling rats,31 we found that they were impaired as compared to vehicle controls (Supplemental Fig 1, p<0.05). Both control and phenobarbital-exposed animals rapidly acquired the initial task but phenobarbital-exposed animals were significantly impaired in the reversal phase (Supplemental Fig 1, p<0.05).
The disruption in striatal MSN synaptic maturation we have described is not specific to a particular mechanism of AED action, given the fact that the three AEDs tested have distinct actions. Phenobarbital potentiates GABAergic transmission,55 phenytoin and lamotrigine block voltage-gated sodium channels,56 and lamotrigine also activates HCN channels.57 However, one feature that these three drugs share is their ability to increase neuronal apoptosis in striatum and cortex of P7 neonatal rats.18,58 This raises the prospect that the effects we have observed may either be an intrinsic feature of AEDs or a reflection of the proapoptotic actions of a subset of AEDs. To distinguish between these possibilities, we next tested levetiracetam, an AED devoid of proapoptotic actions even at doses several-fold above therapeutic levels.18,59
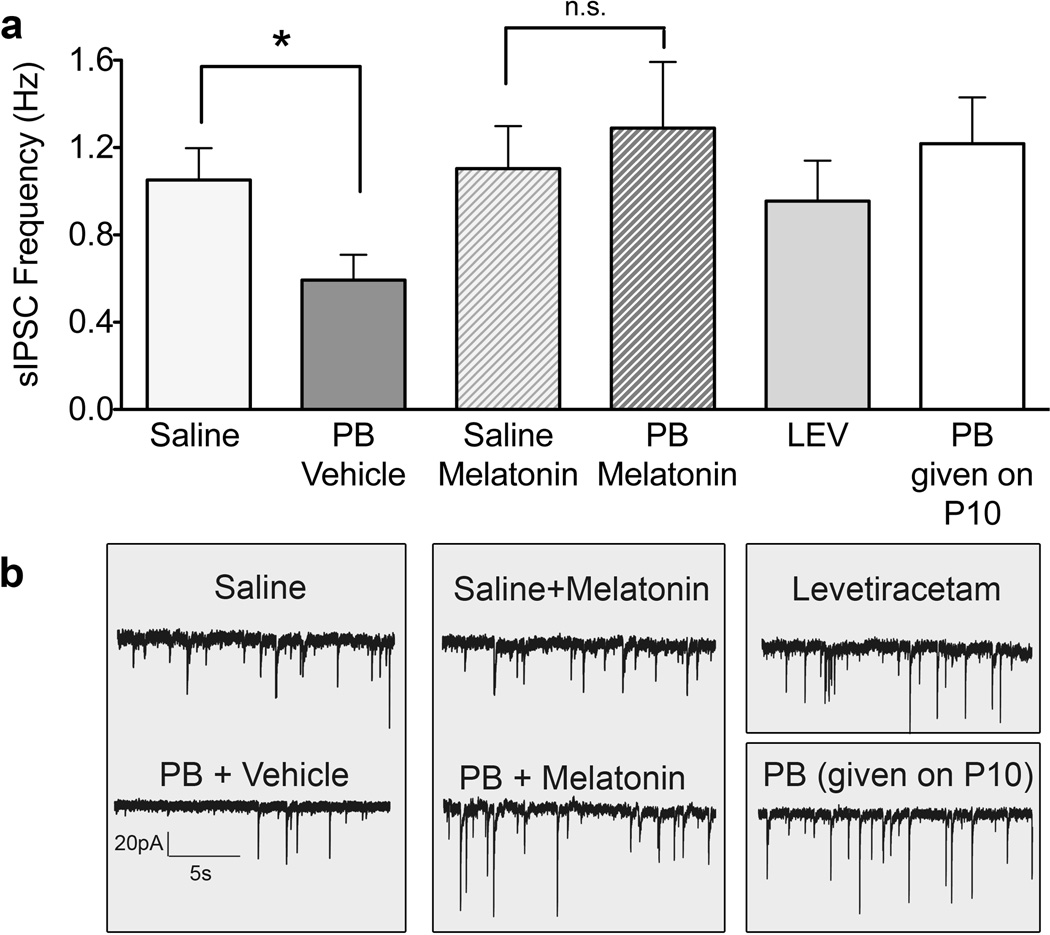
In contrast to the other AEDs, exposure to levetiracetam on P7 had no effect on MSN IPSC frequency measured at P14 (Fig 3). Thus, maturational disruption is not intrinsic to antiepileptic action; instead, proapoptotic actions may be required for this disruption. Because proapoptotic drug effects are evident during a restricted postnatal period, which closes around P10, we next tested the hypothesis that phenobarbital exposure at P10 would not disrupt later synaptic maturation. In animals treated with phenobarbital at P10, we observed IPSC frequency at P14 that was equivalent to that seen in drug naïve controls (Fig 3). This was in clear contrast to the robust disturbance in maturation, which had occurred following P7 exposure to phenobarbital.
Figure 3.
Treatments that avoid cellular toxicity do not disrupt development. Spontaneous inhibitory postsynaptic current (sIPSC) frequency, measured at P14, in medium spiny neurons (MSNs) from animals exposed on P7 to saline (n=24), phenobaribtal/vehicle (n=10), saline/melatonin (n=13), phenobarbital/melatonin (n=14), levetiracetam (n=9) or exposed on P10 to phenobarbital (n=16). (* = p<0.05, t-test with Bonferroni’s correction for multiple comparisons).
To further address the relationship between proapoptotic mechanisms and the disruption in synaptic maturation, we next employed melatonin, a neuroprotective treatment, that has been used clinically in supraphysiological doses to attenuate oxidative stress in neonates.28,60–62 Melatonin is neuroprotective in a variety of neonatal injury models, including hypoxia-ischemia,63 nerve transection,64 and drug-induced neuronal apoptosis.28 We verified the neuroprotective action of melatonin in our conditions by evaluating the effect of melatonin on the induction of cleaved caspase-3 (a marker of apoptosis) following phenobarbital (75mg/kg). Melatonin significantly (P<0.05) attenuated the induction of cleaved caspase-3 (normalized to beta-actin), as detected by western blot (mean+SEM in arbitrary units; phenobarbital: 1.54 ± 0.41 N=9 phenobarbital+melatonin: 0.58 ± 0.07 N=8). Pups pretreated with melatonin prior to phenobarbital exposure at P7 did not exhibit a deficit in IPSC frequency at P14 (Fig 3). Furthermore, IPSC frequency at P14 was significantly greater in the phenobarbital group pretreated with melatonin, compared to the group that received phenobarbital alone (P<0.05). These data provide compelling evidence that proapoptotic neurotoxicity is necessary for the adverse effect of phenobarbital exposure on synaptic maturation.
Discussion
The concern that AED therapy during infancy might give rise to long-term abnormalities by altering neural development was an important motivation for our study. Our examination of the maturation of striatal neuronal physiology provides the first evidence that neonatal exposure to AEDs delays or stunts the development of both excitatory and inhibitory synapse function. Moreover, our study has revealed a functional neurotoxicity that is measured in surviving neurons, therefore representing a sublethal injury that eludes histopathological analysis. As the principle output neurons of the striatum, MSNs are responsible for relaying cortical control to the brainstem,65 regulating sensorimotor integration,33 and supporting implicit learning and memory.66 These behaviors may be disrupted by the changes in maturation we have detected. Supporting a behavioral consequence of neonatal AED exposure, we observed an impairment of striatal-dependent reversal learning31 in weanling animals after exposure to phenobarbital at P7 (Supplemental Fig 1). Furthermore, we have recently reported abnormalities in motor behavior, sensory motor gating, and memory in adult rats treated with phenobarbital, phenytoin, or lamotrigine as neonates.67–70 This extends a previous study with phenobarbital showing impaired water maze performance.15 These abnormalities could result from the deficits we have described in striatal synaptic transmission, along with similar deficits that may occur in other brain regions vulnerable to AED developmental toxicity.71
The neurons in which we observed delayed or stunted synaptic maturation survived the insult of drug exposure in a presumably compromised state. This may be analogous to hippocampal neurons that were rescued from the terminal stages of cell death, yet exhibited impaired synaptic function.72 As such, the functional impairments we have described here may be more pervasive than cell death, and may therefore serve as an especially sensitive indicator of drug toxicity in the developing brain. Moreover, the fact that we observed a delay in synaptic maturation following exposure to a dose of lamotrigine that is subthreshold for the induction of neuronal apoptosis,19 suggests that the threshold for functional toxicity is lower than that required for cell death. It is therefore likely that the activation of apoptotic events upstream from irreversible execution stages is sufficient to disrupt synaptic maturation 73. Accordingly, this disruptive action could be avoided by using drugs that lack proapoptotic effects. Levetiracetam is one such drug; even doses well above therapeutic, it does not cause neuronal apoptosis.18 The fact that levetiracetam did not impede normal synaptic maturation supports the relationship between a proapoptotic mechanism and the functional developmental neurotoxicity we have described.
Additional support for the concept that a proapoptotic mechanism accounts for the drug-induced disruption in synaptic maturation comes from our results with melatonin. The protective effect that we observed with melatonin is consistent with the well-established neuroprotective action of this drug in a variety of neonatal injury models, including hypoxia-ischemia,63 nerve transection, 64 and drug-induced neuronal apoptosis.28 The early upstream events in the apoptotic cascade are the likely targets of melatonin's neuroprotective action.28,60,61
In the clinical context, with the exception of in utero exposure, AED exposure typically occurs against the background of seizure episodes, whereas our studies examined the impact of AED exposure in otherwise normal animals. Previous studies found that exposure to repeated seizures did not change the severity of AED-induced neuronal apoptosis in P7 rats,59,74 although neuronal cell death induced by the N-methyl-D-aspartate (NMDA)-receptor antagonist MK-801 was significantly attenuated by the same seizure regimen.59,74 This suggests that while MK-801 neurotoxicity is sensitive to a protective effect of seizure-evoked stimulation, AED neurotoxicity is not. On the other hand, in neonatal rats, AEDs can exacerbate the adverse long term impact of focal brain damage,67 raising the possibility that infants with underlying neurological abnormalities may be more vulnerable to AED-induced neurotoxicity. Thus, further studies are needed to characterize the functional impact of AED-induced neurotoxicity in animal models of developmental disorders.
Our data also raise an obvious question: Given that the proapoptotic actions of AEDs impact multiple brain areas (including hippocampus, thalamus, cerebellum and cortex),58 will AED exposure also cause alterations in synaptic maturation in other brain regions? As several regions exhibit a pattern of synaptic maturation resembling the pattern we have described for striatum,49–53 it is likely that our results may generalize to other brain regions.
Our data with levetiracetam indicate that developmental neurotoxicity is not inherent to AED action, offering a prospect for selecting AEDs that do not disrupt functional maturation for use in infancy. Moreover, our findings with melatonin suggest that adjunct neuroprotective therapies that attenuate oxidative stress and proapoptotic processes may minimize developmental neurotoxicity, thereby allowing a broader choice of AEDs for use in neonates and infants. In this regard, a recent clinical trial demonstrated efficacy of levetiracetam for the treatment of neonatal seizures25 suggests that this drug may be a promising alternative to phenobarbital and phenytoin.
Our data provide the first evidence for a deleterious impact of neonatal AED exposure on synaptic physiology in the developing brain. This functional disruption in brain maturation is linked to the proapoptotic action of AEDs, and may provide a mechanistic explanation at the level of synaptic physiology for behavioral deficits in children exposed to AEDs during development.21,22,75 These findings underscore the need to identify AEDs that do not disrupt synaptic maturation in the developing brain.
Supplementary Material
Acknowledgements
The authors are grateful to Drs. John Huegenard, Molly Huntsman, Alexei Kondratyev, Daniel Pak, and Barry Wolfe for their critical reading of previous drafts of this manuscript, and Mr. Cameron Sweeney for his technical assistance. Funding: This work was supported by a Predoctoral Fellowship from the Epilepsy Foundation and F31NS066822 (PAF), a research grant from GlaxoSmithKline, NIH research grants R21MH079991, and R01MH64797 (SV), and NIH training grants T32DA007291 and T32NS041231. All procedures were approved by the Georgetown University Animal Care and Use Committee.
Footnotes
Author Contributions. PAF and MJJ conducted the experiments. PAF, MJJ, SV, and KG designed the experiments, analyzed the data and wrote the paper.
References
- 1.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(Suppl 2):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 2.Parisi P, Francia A, Vanacore N, et al. Psychomotor development and general movements in offspring of women with epilepsy and anticonvulsant therapy. Early Hum. Dev. 2003;74(2):97–108. doi: 10.1016/s0378-3782(03)00083-5. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 3.Reinisch JM, Sanders SA, Mortensen EL, Rubin DB. In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA. 1995;274(19):1518–1525. [cited 2011 Jun 10] [PubMed] [Google Scholar]
- 4.Farwell JR, Lee YJ, Hirtz DG, et al. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N. Engl. J. Med. 1990;322(6):364–369. doi: 10.1056/NEJM199002083220604. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 5.Ikonomidou C, Scheer I, Wilhelm T, et al. Brain morphology alterations in the basal ganglia and the hypothalamus following prenatal exposure to antiepileptic drugs. Eur. J. Paediatr. Neurol. 2007;11(5):297–301. doi: 10.1016/j.ejpn.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Vestergaard M, Pedersen C, Christensen J, et al. Febrile seizures and risk of schizophrenia. Schizophrenia Research. 2005;73(2–3):343–349. doi: 10.1016/j.schres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37(2):85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Blume HK, Garrison MM, Christakis DA. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. J Child Neurol. 2009;24(2):148–154. doi: 10.1177/0883073808321056. [DOI] [PubMed] [Google Scholar]
- 9.Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9(6):414–423. doi: 10.1007/s11940-007-0043-0. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 10.Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010;(10):CD002053. doi: 10.1002/14651858.CD002053.pub3. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17. doi: 10.1038/sj.jp.7211427. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 12.Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr. Neurol. 2003;28(4):277–280. doi: 10.1016/s0887-8994(02)00621-5. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 13.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaindl AM, Koppelstaetter A, Nebrich G, et al. Brief alteration of NMDA or GABAA receptor-mediated neurotransmission has long term effects on the developing cerebral cortex. Mol Cell Proteomics. 2008;7(12):2293–2310. doi: 10.1074/mcp.M800030-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Stefovska VG, Uckermann O, Czuczwar M, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64(4):434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 16.McCann ME, Soriano SG. Is anesthesia bad for the newborn brain? Anesthesiol Clin. 2009;27(2):269–284. doi: 10.1016/j.anclin.2009.05.007. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 17.Loepke AW, McGowan FX, Jr, Soriano SG. CON: The toxic effects of anesthetics in the developing brain: the clinical perspective. Anesth. Analg. 2008;106(6):1664–1669. doi: 10.1213/ane.0b013e3181733ef8. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J. Pharmacol. Exp. Ther. 2007;323(1):165–173. doi: 10.1124/jpet.107.126250. [cited 2011 Jun 13] [DOI] [PubMed] [Google Scholar]
- 19.Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J. Pharmacol. Exp. Ther. 2007;322(2):494–500. doi: 10.1124/jpet.107.123133. [cited 2011 Jun 10] [DOI] [PubMed] [Google Scholar]
- 20.Kubova H, Mares P. Anticonvulsant effects of phenobarbital and primidone during ontogenesis in rats. Epilepsy Res. 1991;10(2–3):148–155. doi: 10.1016/0920-1211(91)90007-3. [cited 2011 Jul 18] [DOI] [PubMed] [Google Scholar]
- 21.Meador KJ, Baker GA, Browning N, et al. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134(Pt 2):396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McVearry KM, Gaillard WD, VanMeter J, Meador KJ. A prospective study of cognitive fluency and originality in children exposed in utero to carbamazepine, lamotrigine, or valproate monotherapy. Epilepsy Behav. 2009;16(4):609–616. doi: 10.1016/j.yebeh.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305(19):1996–2002. doi: 10.1001/jama.2011.624. [cited 2011 Jun 13] [DOI] [PubMed] [Google Scholar]
- 25.Ramantani G, Ikonomidou C, Walter B, et al. Levetiracetam: safety and efficacy in neonatal seizures. Eur. J. Paediatr. Neurol. 2011;15(1):1–7. doi: 10.1016/j.ejpn.2010.10.003. [cited 2011 Jun 13] [DOI] [PubMed] [Google Scholar]
- 26.Bernásková K, Mares P. Similar effects of lamotrigine and phenytoin against cortical epileptic foci in immature rats. Physiol Res. 2010;59(1):113–119. doi: 10.33549/physiolres.931563. [cited 2011 Jul 18] [DOI] [PubMed] [Google Scholar]
- 27.Stankova L, Kubova H, Mares P. Anticonvulsant action of lamotrigine during ontogenesis in rats. Epilepsy Res. 1992;13(1):17–22. doi: 10.1016/0920-1211(92)90003-c. [DOI] [PubMed] [Google Scholar]
- 28.Yon J, Carter L, Reiter R, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21(3):522–530. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 30.Tepper JM, Abercrombie ED, Bolam JP. Basal ganglia macrocircuits. Prog Brain Res. 2007;160:3–7. doi: 10.1016/S0079-6123(06)60001-0. [DOI] [PubMed] [Google Scholar]
- 31.Watson DJ, Stanton ME. Spatial discrimination reversal learning in weanling rats is impaired by striatal administration of an NMDA-receptor antagonist. Learn. Mem. 2009;16(9):564–572. doi: 10.1101/lm.1448009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beutler LR, Eldred KC, Quintana A, et al. Severely impaired learning and altered neuronal morphology in mice lacking NMDA receptors in medium spiny neurons. PLoS ONE. 2011;6(11):e28168. doi: 10.1371/journal.pone.0028168. [cited 2012 Mar 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodsi MH, Swerdlow NR. Prepulse inhibition in the rat is regulated by ventral and caudodorsal striato-pallidal circuitry. Behav Neurosci. 1995;109(5):912–928. doi: 10.1037//0735-7044.109.5.912. [DOI] [PubMed] [Google Scholar]
- 34.Sharpe NA, Tepper JM. Postnatal development of excitatory synaptic input to the rat neostriatum: an electron microscopic study. Neuroscience. 1998;84(4):1163–1175. doi: 10.1016/s0306-4522(97)00583-6. [DOI] [PubMed] [Google Scholar]
- 35.Sharpe NA, Tepper JM. Postnatal development of excitatory synaptic input to the rat neostriatum: an electron microscopic study. Neuroscience. 1998;84(4):1163–1175. doi: 10.1016/s0306-4522(97)00583-6. [DOI] [PubMed] [Google Scholar]
- 36.Painter MJ, Minnigh MB, Gaus L, et al. Neonatal phenobarbital and phenytoin binding profiles. J Clin Pharmacol. 1994;34(4):312–317. doi: 10.1002/j.1552-4604.1994.tb01999.x. [cited 2011 Jul 18] [DOI] [PubMed] [Google Scholar]
- 37.Filippi L, la Marca G, Cavallaro G, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia. 2011;52(4):794–801. doi: 10.1111/j.1528-1167.2011.02978.x. [cited 2012 Mar 4] [DOI] [PubMed] [Google Scholar]
- 38.Hawcutt DB, Sampath S, Timmis A, et al. Serum phenytoin concentrations in paediatric patients following intravenous loading. Arch. Dis. Child. 2011;96(9):883–884. doi: 10.1136/adc.2011.215269. [cited 2012 Mar 4] [DOI] [PubMed] [Google Scholar]
- 39.Castel-Branco MM, Falcão AC, Figueiredo IV, et al. Lamotrigine kidney distribution in male rats following a single intraperitoneal dose. Fundam Clin Pharmacol. 2004;18(1):51–55. doi: 10.1046/j.0767-3981.2003.00210.x. [cited 2012 Mar 2] [DOI] [PubMed] [Google Scholar]
- 40.Walker MC, Tong X, Perry H, et al. Comparison of serum, cerebrospinal fluid and brain extracellular fluid pharmacokinetics of lamotrigine. Br. J. Pharmacol. 2000;130(2):242–248. doi: 10.1038/sj.bjp.0703337. [cited 2012 Mar 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambeck B, Jürgens UH, May TW, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47(4):681–694. doi: 10.1111/j.1528-1167.2006.00504.x. [cited 2012 Mar 2] [DOI] [PubMed] [Google Scholar]
- 42.Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci. 2009;29(16):5116–5126. doi: 10.1523/JNEUROSCI.4737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J. Neurophysiol. 1989;62(5):1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- 44.Vicini S, Ferguson C, Prybylowski K, et al. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21(9):3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortinski PI, Lu C, Takagaki K, et al. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J. Neurophysiol. 2004;92(3):1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- 46.Janssen MJ, Yasuda RP, Vicini S. GABA(A) Receptor β3 Subunit Expression Regulates Tonic Current in Developing Striatopallidal Medium Spiny Neurons. Front Cell Neurosci. 2011;5:15. doi: 10.3389/fncel.2011.00015. [cited 2012 Mar 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Partridge JG, Janssen MJ, Chou DYT, et al. Excitatory and inhibitory synapses in neuropeptide Y-expressing striatal interneurons. J. Neurophysiol. 2009;102(5):3038–3045. doi: 10.1152/jn.00272.2009. [cited 2012 Mar 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang KC, Lovinger DM. Role of pertussis toxin-sensitive G-proteins in synaptic transmission and plasticity at corticostriatal synapses. J. Neurophysiol. 2000;83(1):60–69. doi: 10.1152/jn.2000.83.1.60. [cited 2012 Mar 2] [DOI] [PubMed] [Google Scholar]
- 49.Hollrigel GS, Soltesz I. Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. J Neurosci. 1997;17(13):5119–5128. doi: 10.1523/JNEUROSCI.17-13-05119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks MI, Hardie JB, Pearce RA. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88(6):3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- 51.Chudomel O, Herman H, Nair K, et al. Age- and gender-related differences in GABAA receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neuroscience. 2009;163(1):155–167. doi: 10.1016/j.neuroscience.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABA(A) receptor alpha 6 subunit. J. Neurosci. 1996;16(11):3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huntsman MM, Huguenard JR. Nucleus-specific differences in GABA(A)-receptor-mediated inhibition are enhanced during thalamic development. J. Neurophysiol. 2000;83(1):350–358. doi: 10.1152/jn.2000.83.1.350. [DOI] [PubMed] [Google Scholar]
- 54.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23(18):7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald RL, Barker JL. Enhancement of GABA-mediated postsynaptic inhibition in cultured mammalian spinal cord neurons: a common mode of anticonvulsant action. Brain Res. 1979;167(2):323–336. doi: 10.1016/0006-8993(79)90826-6. [DOI] [PubMed] [Google Scholar]
- 56.Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986;27(5):490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 57.Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat. Neurosci. 2002;5(8):767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- 58.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann. N. Y. Acad. Sci. 2003;993:103–114. doi: 10.1111/j.1749-6632.2003.tb07517.x. discussion 123–124.[cited 2011 Jul 18] [DOI] [PubMed] [Google Scholar]
- 59.Kim JS, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007;48(Suppl 5):19–26. doi: 10.1111/j.1528-1167.2007.01285.x. [DOI] [PubMed] [Google Scholar]
- 60.Radogna F, Cristofanon S, Paternoster L, et al. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J Pineal Res. 2008;44(3):316–325. doi: 10.1111/j.1600-079X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 61.Reiter RJ, Tan DX, Manchester LC, Tamura H. Melatonin defeats neurally-derived free radicals and reduces the associated neuromorphological and neurobehavioral damage. J Physiol Pharmacol. 2007;58(Suppl 6):5–22. [PubMed] [Google Scholar]
- 62.Gitto E, Romeo C, Reiter RJ, et al. Melatonin reduces oxidative stress in surgical neonates. J. Pediatr. Surg. 2004;39(2):184–189. doi: 10.1016/j.jpedsurg.2003.10.003. discussion 184–189.[cited 2011 Jun 13] [DOI] [PubMed] [Google Scholar]
- 63.Carloni S, Perrone S, Buonocore G, et al. Melatonin protects from the long-term consequences of a neonatal hypoxic-ischemic brain injury in rats. J. Pineal Res. 2008;44(2):157–164. doi: 10.1111/j.1600-079X.2007.00503.x. [DOI] [PubMed] [Google Scholar]
- 64.Rogério F, de Souza Queiroz L, Teixeira SA, et al. Neuroprotective action of melatonin on neonatal rat motoneurons after sciatic nerve transection. Brain Res. 2002;926(1–2):33–41. doi: 10.1016/s0006-8993(01)03286-3. [DOI] [PubMed] [Google Scholar]
- 65.Gale K, Proctor M, Veliskova J, Nehlig A. Ganglia and Brainstem Anatomy and Physiology. In: Engel JJ, Pedley T, editors. Epilepsy: A Comprehensive Textbook. New York, NY: Lippincott Williams & Wilkins; 2007. pp. 367–384. [Google Scholar]
- 66.Belin D, Jonkman S, Dickinson A, et al. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav. Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [cited 2011 Jun 13] [DOI] [PubMed] [Google Scholar]
- 67.Bhardwaj S, Forcelli P, Palchik G, et al. Neonatal exposure to phenobarbital potentiates schizophrenia-like behavioral outcomes in the rat. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forcelli PA, Janssen MJ, Stamps LA, et al. Therapeutic strategies to avoid long-term adverse outcomes of neonatal antiepileptic drug exposure. Epilepsia. 2010;51(Suppl 3):18–23. doi: 10.1111/j.1528-1167.2010.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forcelli PA, Gale K, Kondratyev A. Early postnatal exposure of rats to lamotrigine, but not phenytoin, reduces seizure threshold in adulthood. Epilepsia. 2011;52(4):e20–e22. doi: 10.1111/j.1528-1167.2010.02971.x. [cited 2011 Jun 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Forcelli PA, Kozlowski R, Snyder C, et al. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats [Internet] The Journal of Pharmacology and Experimental Therapeutics. 2011 doi: 10.1124/jpet.111.188862. [cited 2011 Dec 3] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22129597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forcelli P, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011;52:e207–e211. doi: 10.1111/j.1528-1167.2011.03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumas TC, McLaughlin JR, Ho DY, et al. Gene therapies that enhance hippocampal neuron survival after an excitotoxic insult are not equivalent in their ability to maintain synaptic transmission. Exp. Neurol. 2000;166(1):180–189. doi: 10.1006/exnr.2000.7500. [DOI] [PubMed] [Google Scholar]
- 73.D’Amelio M, Cavallucci V, Middei S, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2011;14(1):69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 74.Kim J. Effects of repeated brief seizures and antiepileptic drugs in the developing rat brain. 2007 [Google Scholar]
- 75.Bromley RL, Baker GA, Meador KJ. Cognitive abilities and behaviour of children exposed to antiepileptic drugs in utero. Curr Opin Neurol. 2009;22(2):162–166. doi: 10.1097/WCO.0b013e3283292401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.