Abstract
Background
Daptomycin is approved for the treatment of complicated skin and skin structure infections and Staphylococcus aureus bacteremia. We sought to characterize daptomycin single-dose pharmacokinetics and tolerability in young infants.
Methods
Subjects <120 days of age with suspected systemic infections were eligible for inclusion. Each subject was given a single 6 mg/kg intravenous dose of daptomycin. An average of 4 post-dose concentrations per infant was obtained.
Results
Data from 20 infants are presented. Median gestational age at birth and postnatal age were 32 weeks (range 23, 40) and 3 days (1, 85), respectively. The median area under the concentration curve at 24 hours, volume of distribution, total body clearance, and half-life of daptomycin were 262.4 mg*h/L (166.7, 340.2), 0.21 L/kg (0.11, 0.34), 0.021 L/hr/kg (0.016, 0.034), and 6.2 hours (3.7, 9.0), respectively. No adverse events related to daptomycin were observed, including changes in creatine phosphokinase concentrations.
Conclusions
Daptomycin clearance in young infants was similar to that in 2–6-year-olds and higher than that observed in adolescents and adults.
Keywords: daptomycin, complicated skin and skin structure infections, Staphylococcus aureus, pharmacokinetics
Daptomycin is a cyclic lipopeptide antibiotic agent approved in adults by the Food and Drug Administration and the European Medicines Agency for the treatment of complicated skin, skin structure, and bloodstream infections caused by Gram-positive organisms, including Staphylococcus aureus (including methicillin-resistant strains [MRSA]).1Staphylococcus aureus and coagulase-negative staphylococci are the most common causative organisms of late-onset sepsis in infants admitted to the neonatal intensive care unit and are associated with substantial morbidity and mortality.2
To prevent these dire consequences, infants diagnosed with staphylococcal infections are typically treated with vancomycin. However, vancomycin therapy requires therapeutic drug monitoring, which poses a challenge to clinicians caring for critically ill infants.3 In this setting, daptomycin is an attractive therapeutic alternative to vancomycin for the treatment of Gram-positive infections. However, daptomycin is not approved for use in infants, and scarce pharmacokinetic data suggest that daptomycin drug disposition in infants differs from that in adults.4 This finding has also been observed in other young pediatric populations.5 In addition, the safety profile of daptomycin has not been described in infants. In the present study, we evaluate the single-dose pharmacokinetics and tolerability of daptomycin in young infants.
METHODS
Study Design
This was a single-center, prospective, single-dose pharmacokinetic and tolerability study of daptomycin in young infants. Inclusion criteria included: <120 days of age; sufficient intravascular access to receive study drug; and suspected systemic infection. Infants were excluded for the following conditions: renal dysfunction evidenced by a serum creatinine >1.0 mg/dL; exposure to daptomycin in the month before the study; concomitant administration of tobramycin; or history of anaphylaxis attributed to daptomycin. Infants were administered daptomycin in addition to other antimicrobials given as standard of care. The Duke University Institutional Review Board approved this study (ClinicalTrials.gov NCT00942149). All study participants were enrolled after obtaining informed consent from the legal guardian.
Administration of Study Drug and Procedures
Subjects were stratified into 1 of 4 categories based on gestational age (GA) (<32 weeks and ≥32 weeks) and postnatal age (PNA) (<14 days and ≥14 days), and each subject received a single 6 mg/kg intravenous dose of daptomycin over 60 minutes. The recommended administration time in adults is 30 minutes. We chose 60 minutes to mitigate potential side effects associated with high maximum concentrations.
The protocol allowed for the administration of other antimicrobials per local standard of care. Medications received in the 24 hours before study drug administration and for 24 hours after study drug administration were documented. Appropriate blood, urine, or cerebrospinal fluid cultures were obtained as part of standard of care. Laboratory values (hematology, serum chemistries, liver function tests) obtained 72 hours before and 72 hours after the study drug dose were recorded if obtained per standard of care. Values closest to the study drug dose were recorded if there were multiple tests. A serum creatine phosphokinase (CPK) level was obtained within 72 hours before and after the dose of study drug.
Pharmacokinetic Sampling and Assay
Infants had blood (0.2 mL) collected 30 minutes–1 hour, 2–4 hours, 4–6 hours, 12–18 hours, and 20–24 hours from the end of daptomycin infusion. Plasma samples were also collected to determine daptomycin concentrations from blood left over from the clinical laboratories drawn per standard of care (scavenged samples). Daptomycin stability has been demonstrated in plasma at room temperature and refrigerated for 24 hours and 7 days, respectively (unpublished data, Cubist Pharmaceuticals Inc.). All samples were collected in heparinized collection tubes. An average of 4 post-dose concentrations was obtained per infant.
Plasma samples were analyzed using a liquid chromatography/mass spectrometry (LC/MS) assay. The analytical reference standard for daptomycin and internal standard CB-186,253 were provided by Cubist Pharmaceuticals Inc. (Lexington, MA, USA). The LC/MS instrumentation included an Applied Biosystem API-4000 coupled with an Agilent 1100 Quaternary Pump and a Leap CTC Autosampler. Daptomycin analogue CB-183,253 was used as an internal standard.
Multiple ions Reactions Monitor (MRM) transitions on mass-spectrometer of 811–159 and 837–365 were used to monitor daptomycin and the internal standard, respectively. Human sodium heparin plasma purchased from Bioreclamation Inc. (NY, USA) was used for plasma assay as matrix blanks. The calibration curve was 1.0–100 μg/mL for the plasma assay. The plasma extraction method was solid-phase extraction. A Waters HLB 96 (30 mg) well plate was used, and final extracts were analyzed by LC/MS. A Waters XBridge C18 column 4.6 × 50 mm, 5 μM was used. The mobile phase A was water, mobile phase B was acetonitrile, and mobile phase C was 10% formic acid in water. A mobile phase gradient was used as follows: 55:35:10 (mobile phase A:B:C, respectively) at time zero, held for half minute, changed to 40:50:10 for 2.5 minutes, and then changed to initial condition for a total run of 4.6 minutes. The formic acid in final gradient was 1.0% in a 35%–50% mobile phase B run over 4.6 minutes. For the 3 levels of quality control samples at 3, 15, and 80 μg/mL concentrations, % coefficient of variation ranged from 11.3% to 12.6%; % bias ranged from −3.4% to 2.6%.
Pharmacokinetic and Statistical Analysis
Non-compartmental methods (WinNonlin Phoenix; Pharsight Corp., Cary, NC) were used to calculate single-dose pharmacokinetic indices from plasma concentration data. The terminal elimination rate constant (kel) was obtained from a log linear regression of the plasma concentration versus time data in the terminal post distribution phase. Twenty-four–hour area under the drug concentration curve (AUC24) was calculated by the linear trapezoidal rule. Area under the plasma drug concentration versus time curve from zero to infinity (AUCinf) was obtained from AUClast + Ct/kel where Ct was the last measurable concentration and kel was the terminal elimination rate constant. Total body clearance (CL) was obtained from dose/AUCinf. Volume of distribution (Vd) was calculated as Vd = CL/kel. A univariable screen (scatter visual plots) was performed for potential associations between demographic covariates and pharmacokinetic indices. The following potential covariates were included in this analysis: serum creatinine (SCR), PNA, GA, and postmenstrual age (PMA). We used the Wilcoxon signed-rank test to compare laboratory values and CPK levels pre- and post-study drug dosing. We used correlation analysis to compare daptomycin concentrations in timed versus scavenged samples collected within 30 minutes of each other.
RESULTS
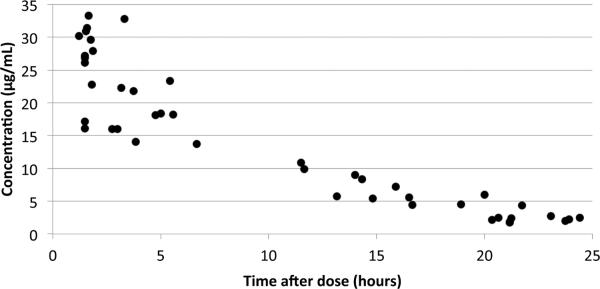
Twenty subjects were enrolled, and 85 evaluable plasma samples (Figure 1) were obtained following daptomycin administration (Table 1). Twenty-three (27%) of the 85 samples were scavenged samples. The correlation (r2) of daptomycin concentrations in these samples compared with the timed samples was >0.97 (N=10 pairs).
FIGURE 1.
Concentration–time curve.
TABLE 1.
Subject Demographics
| Gestational age (weeks) | 32 (23, 40) |
| Birth weight (g) | 1750 (560, 3950) |
| Postnatal age (days) | 3 (1, 85) |
| Dosing weight (g) | 1785 (600, 3984) |
| Female sex, n (%) | 10 (50) |
| Race, n (%) | |
| White | 14 (70) |
| African-American | 5 (25) |
| Other | 1 (5) |
| Hispanic ethnicity, n (%) | 2 (11) |
Data are median (range), unless otherwise stated.
The median (range) AUC24, Vd, CL, and half-life of daptomycin were 262.4 mg*h/L (166.7, 340.2), 0.21 L/kg (0.11, 0.34), 0.021 L/hr/kg (0.016, 0.034), and 6.2 hours (3.7, 9.0), respectively (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B234 which shows individual subject pharmacokinetic indices). The median maximum concentration (Cmax) was 25.5 μg/mL (16.0, 33.3). No relationship between GA, PNA, PMA, or SCR with daptomycin CL or Vd was observed.
All 20 infants had negative peripheral blood cultures obtained before daptomycin administration. One infant had a positive urine culture for Enterococcus sp., and 1 infant had a positive urine culture for Escherichia coli. The most commonly administered concomitant medications were ampicillin (95%), gentamicin (85%), and caffeine (55%).
There were 34 adverse events reported in 11 (55%) subjects (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B235 which shows adverse events by body systems). None were determined to be related to study drug. Five of the adverse events were classified as serious adverse events (1: abnormal abdominal radiograph; 2: pneumatocele; 1: questionable thoracic lesion; and 1: possible Morgagni congenital diaphragmatic hernia).
No clinically significant differences in laboratory values (hematology, serum chemistry, serum bilirubin) obtained before and after the daptomycin dose were observed. There was a statistical difference in calcium levels before and after the daptomycin dose (median 9.3 [range 6.8, 10.7] and 9.7 [8.5, 10.6], respectively; P=0.01). No significant changes in CPK values before and after the dose of daptomycin were observed (median 132 IU/L [range; 33, 628] and 86 [35, 506], respectively; P=0.14). No significant changes in respiratory rate, temperature, heart rate, or mean blood pressure were observed during the daptomycin infusion.
DISCUSSION
Pharmacokinetic studies with single and multiple dosing regimens of daptomycin have been performed in healthy adults and in subjects with acute bacterial infections.6–8 In these populations, daptomycin exhibits linear kinetics with daily doses of 4–6 mg/kg per day.7 Excretion of daptomycin occurs primarily through the renal system, with 50% of the administered dose excreted within 24 hours as intact drug.7 Consequently, impairment of renal function influences CL of daptomycin, and adjustment in dose regimen is required among patients with renal failure.8 Age,6 moderate liver impairment,9 and comorbidities (e.g., ascites, hypertension, diabetes, and congestive heart failure) do not significantly alter the pharmacokinetics of daptomycin.8 Daptomycin does not have an effect on the hepatic cytochrome P450 enzyme system, suggesting a reduced risk for drug interactions.10
In adults with complicated skin and skin structure infections, a single 4 mg/kg dose yielded a mean exposure (AUCinf) of 417 mg*h/L.1 Differences in extracellular fluid volume, plasma protein levels, protein affinity to antimicrobials, rate, and mechanism of elimination are observed between young infants, older children, and adults.11 Daptomycin's high degree of protein-binding, renal elimination, and extracellular fluid distribution suggests dosing adjustment is necessary for these patients. This was observed in a study that evaluated the single-dose pharmacokinetics of daptomycin (4 mg/kg) in 25 children (2–17 years of age). In this study, daptomycin CL changes were associated with age; CL was lower in the 12–17-year-old cohort (0.011 L/h/kg) compared with the 2–6-year-old cohort (.020 L/h/kg; P<0.05).5 The lower exposures seen in the younger age groups prompted a subsequent study evaluating single-dose (8–10 mg/kg) daptomycin pharmacokinetics in children 2–6 years of age.12 Mean exposures (AUCinf) following the single dose were 429 mg*h/L +/− 113 and 550 mg*h/L +/− 139 for the 8 mg/kg and 10 mg/kg doses, respectively. Reports of 3 infants receiving daptomycin at 6 mg/kg q 12 hours, from whom daptomycin concentrations were obtained during the course of treatment for MRSA infections, concluded that this dosing was equivalent to adults treated with 4 mg/kg q 24 hours.4,13 Exposures in all 20 of the infants in our cohort were below the mean exposure observed in adults given a similar 4 mg/kg dose, and CL was nearly identical to that observed in the previous study of 2–6-year-olds.1,5
Daptomycin is well tolerated in healthy adults6,7 and adults diagnosed with acute bacterial infections.14,15 Elevation in serum values of CPK were observed when daptomycin was administered to healthy individuals.7 However, the alterations were transient and not associated with clinical symptoms.7 In a large clinical trial, elevation of CPK concentrations was noted in 2.1% (11/534) of patients with complicated skin and skin structure infections treated with daptomycin and in 1.4% (8/558) of cases in the comparator group.14 However, true muscle toxicity attributable to daptomycin that required cessation of therapy was documented in only 1 of the 11 patients with CPK abnormalities. In a recent series of studies of the use of daptomycin (6 mg/kg) for Staphylococcus aureus bacteremia or infective endocarditis, 6.7% (8/120) of patients exhibited CPK elevations versus 0.9% (1/116) in the comparator group.15 The only documented serious adverse event to have occurred in >1% of patients treated with daptomycin for complicated skin and skin structure infections was cellulitis (1.3%, 7/534).14 Although excretion of daptomycin occurs through the kidneys, the drug does not cause renal toxicity.6,7,14 Daptomycin was well tolerated in our small cohort of young infants with no increases in CPK observed after a single dose.
Daptomycin CL in this population of young infants was similar to the CL observed in 2–6-year-old children and greater than the CL observed in older children and adults.5 As a result, young infants may require a higher dosage of daptomycin to achieve the efficacious exposures observed in adults. This study was limited by the fact that PK and tolerability were evaluated around a single dose of daptomycin. However, additional pharmacokinetic (multiple and higher doses) and safety studies are needed to determine the appropriate, safe dosing regimen of daptomycin in this population.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Lihong Gao and Lynn Chevette for their role in the assay of plasma samples.
Sources of funding This study was supported by an investigator-initiated research grant from Cubist Pharmaceuticals, Inc., Lexington, MA, USA. Dr. Smith received support from NICHD 1K23HD060040-01, DHHS-1R18AE000028-01, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Cohen-Wolkowiez receives support from NICHD 1K23HD064814-01, from the non-profit organization Thrasher Research Foundation for his work in pediatric clinical pharmacology, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Watt received support from a T-32 Multidisciplinary Pediatric Training Grant (5T32HD043029-09, PI St. Geme) and the Thrasher Research Foundation for his work in pediatric research. Dr. Benjamin receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the non-profit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org), and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
Footnotes
conflicts of interest: Mr. Hornik has no potential conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Daptomycin (Cubicin®) package literature. Cubist Pharmaceuticals, Inc.; Lexington, MA: 2003. [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Oudin C, Vialet R, Boulamery A, Martin C, Simon N. Vancomycin prescription in neonates and young infants: toward a simplified dosage. Arch Dis Child Fetal Neonatal Ed. 2011;96:F365–370. doi: 10.1136/adc.2010.196402. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Wolkowiez M, Smith PB, Benjamin DK, Jr, Fowler VG, Jr, Wade KC. Daptomycin use in infants: report of two cases with peak and trough drug concentrations. J Perinatol. 2008;28:233–234. doi: 10.1038/sj.jp.7211898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Rahman SM, Benziger DP, Jacobs RF, Jafri HS, Hong EF, Kearns GL. Single-dose pharmacokinetics of daptomycin in children with suspected or proved Gram-positive infections. Pediatr Infect Dis J. 2008;27:330–334. doi: 10.1097/INF.0b013e318160edfc. [DOI] [PubMed] [Google Scholar]
- 6.Dvorchik B, Damphousse D. Single-dose pharmacokinetics of daptomycin in young and geriatric volunteers. J Clin Pharmacol. 2004;44:612–620. doi: 10.1177/0091270004265646. [DOI] [PubMed] [Google Scholar]
- 7.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother. 2003;47:1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorchik B, Arbeit RD, Chung J, Liu S, Knebel W, Kastrissios H. Population pharmacokinetics of daptomycin. Antimicrob Agents Chemother. 2004;48:2799–2807. doi: 10.1128/AAC.48.8.2799-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorchik B. Moderate liver impairment has no influence on daptomycin pharmacokinetics. J Clin Pharmacol. 2004;44:715–722. doi: 10.1177/0091270004266619. [DOI] [PubMed] [Google Scholar]
- 10.Oleson FB, Berman CL, Li AP. An evaluation of the P450 inhibition and induction potential of daptomycin in primary human hepatocytes. Chem Biol Interact. 2004;150:137–147. doi: 10.1016/j.cbi.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Burr B, Stringham D, Arrieta A. Commonly used antibacterial and antifungal agents for hospitalised paediatric patients: implications for therapy with an emphasis on clinical pharmacokinetics. Paediatr Drugs. 2001;3:733–761. doi: 10.2165/00128072-200103100-00003. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Rahman SM, Chandorkar G, Akins RL, et al. Single-dose pharmacokinetics and tolerability of daptomycin 8 to 10 mg/kg in children aged 2 to 6 years with suspected or proved Gram-positive infections. Pediatr Infect Dis J. 2011;30:712–714. doi: 10.1097/INF.0b013e31820fc8e1. [DOI] [PubMed] [Google Scholar]
- 13.Sarafidis K, Iosifidis E, Gikas E, Tsivitanidou M, Drossou-Agakidou V, Roilides E. Daptomycin use in a neonate: serum level monitoring and outcome. Am J Perinatol. 2010;27:421–424. doi: 10.1055/s-0029-1243370. [DOI] [PubMed] [Google Scholar]
- 14.Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI, Daptomycin 98–01 and 99–01 Investigators The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis. 2004;38:1673–1681. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration (FDA) Cubicin® (daptomycin for injection) for the treatment of Staphylococcus aureus bacteremia, including those with known or suspected infective endocarditis: FDA Briefing Document for Anti-Infective Drugs Advisory Committee Meeting, March 6, 2006. FDA; Rockville, MD: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.