Abstract
BH3 mimetic drugs induce cell death by antagonizing the activity of anti-apoptotic Bcl-2 family proteins. Cyclin-dependent kinase (CDK) inhibitors that function as transcriptional repressors down-regulate the Bcl-2 family member Mcl-1 and increase the activity of selective BH3-mimetics that fail to target this protein. In this study, we determined whether CDK inhibitors potentiate the activity of pan-BH3 mimetics by directly neutralizing Mcl-1. Specifically, we evaluated interactions between the prototypical pan-CDK inhibitor flavopiridol and the pan-BH3-mimetic obatoclax in multiple myeloma (MM) cells in which Mcl-1 is critical for survival. Co-administration of flavopiridol and obatoclax synergistically triggered apoptosis in both drug-naive and drug-resistant MM cells. Mechanistic investigations revealed that flavopiridol inhibited Mcl-1 transcription but increased transcription of Bim and its binding to Bcl-2/Bcl-xL. Obatoclax prevented Mcl-1 recovery and potentiated release of Bim from Bcl-2/Bcl-xL and Mcl-1, accompanied by activation of Bax/Bak. Whether administered singly or in combination with obatoclax, flavopiridol also induced up-regulation of multiple BH3-only proteins, including BimEL, BimL, Noxa, and Bik/NBK. Notably, shRNA knock-down of Bim or Noxa abrogated lethality triggered by the flavopiridol/obatoclax combination in vitro and in vivo. Together, our findings demonstrate that CDK inhibition potentiates pan-BH3-mimetic activity through a cooperative mechanism involving up-regulation of BH3-only proteins with coordinate down-regulation of their anti-apoptotic counterparts. These findings have immediate implications for the clinical trial design of BH3 mimetic-based therapies that are presently being studied intensively for the treatment of diverse hematopoietic malignancies, including lethal multiple myeloma.
Keywords: BH3-only protein, Bim, Cdk inhibitor, BH3-mimetic, myeloma
Introduction
Multiple myeloma (MM) is an incurable accumulative disease of plasma cells characterized by dysregulation of Bcl family members1. These apoptosis regulatory proteins are divided into pro- and anti-apoptotic groups. The former consists of multi-domain proteins (e.g., Bak and Bax) and BH3-only proteins (e.g., Bim, Bid, Puma, Noxa, Bad, Bik, Bmf, and Hrk etc.). The latter include multi-domain proteins e.g., Bcl-2, Bcl-xL, Mcl-12. Whereas Bax/Bak are absolutely required for apoptosis, BH3-only proteins, which convert noxious stimuli into death signals2, consist of “activators” (e.g., Bim) and “sensitizers/derepressors” (e.g., Noxa, Bik)2. Evidence implicating BH3-only proteins in anti-cancer agent-induced apoptosis3;4 prompted the development of BH3-mimetics that bind to and inactivate anti-apoptotic Bcl proteins5.
One such agent, ABT-737, binds avidly to Bcl-2/Bcl-xL, but not Mcl-16: consequently, relative levels of Bcl-2/Bcl-xL versus Mcl-1 determine susceptibility to this agent7. Mcl-1 is highly expressed in MM (e.g., in 51% patients at diagnosis and 81% at relapse), while high Mcl-1 expression correlates with poor clinical outcome8. Mcl-1 also plays an important role in resistance to agents such as bortezomib9. Recently, the novel pan-BH3-mimetic obatoclax has been developed which in addition to other anti-apoptotic proteins, antagonizes the activity of Mcl-1 in various tumors types10;11, including hematological malignancies such as MM. Preclinical in vitro studies in MM demonstrated single-agent activity and additivity with other agents, but limited in vivo bioactivity when administered alone12.
Cyclin-dependent kinases (Cdks) regulate cell cycle progression and transcription13. Pan-Cdk inhibitors such as flavopiridol (FP; alvocidib) act in part by inhibiting Cdk9, a kinase involved in RNA polymerase II (Pol II)-mediated transcription elongation13. Consequently, Cdk inhibitors block gene transcription and down-regulate short-lived proteins including Mcl-1, promoting apoptosis14;15. Recently, several new-generation pan-Cdk inhibitors (e.g., CYC202, SCH727965), which also target Cdk9, have entered clinical trials13.
Although pan-Cdk inhibitors have been shown to potentiate ABT-737 lethality in transformed cells by down-regulating Mcl-17, it is unknown whether synergistic interactions would occur with pan-BH3-mimetics like obatoclax, which bind to/inactivate Mcl-110. To address this question, we examined interactions between the protoyptical pan-Cdk inhibitor FP and obatoclax in human MM cells. Here we report that FP synergistically increases obatoclax lethality in diverse MM cells, including those resistant to novel agents, in the presence of stromal cell factors, and in primary CD138+ MM samples, but not in their normal counterparts. Significantly, obatoclax/FP co-administration, in sharp contrast to obatoclax alone, displays marked in vivo activity and increases survival in multiple murine systems. From a mechanistic standpoint, the unexpected up-regulation of multiple BH3-only proteins, including BimEL, BimL, Noxa, and Bik/NBK, cooperates with down-regulation of anti-apoptotic proteins (e.g., Mcl-1, Bcl-xL) to play a significant functional role in lethality. Collectively, these findings provide proof of principle for a novel anti-MM strategy in which pan-Cdk inhibitors are combined with pan-BH3 mimetics, and highlight the critical importance of interplay between pro- and anti-apoptotic proteins in synergistic interactions between such agents.
Materials and Methods
Cells and reagents
Human MM U266 and RPMI8226 cells were obtained from ATCC and maintained as before19. Both were authenticated (Basic STR Profiling Service, ATCC® 135-X) by ATCC immediately after this study was completed. Bortezomib-resistant cells (PS-R) were generated by continuously culturing U266 cells in increasing concentrations of bortezomib (beginning at 0.5nM and increasing in stepwise increments of 0.2nM) until 20nM, and maintained in medium containing 15nM bortezomib. A revlimid-resistant RPMI8226 (R10R) cell line was similarly established and maintained in 10 μM revlimid20. Dexamethasone-sensitive (MM.1S) and -resistant (MM.1R) cell lines were provided by Dr Steven T. Rosen (Northwestern University, Chicago, Ill). U266/Mcl-1 and RPMI8226/Bcl-xL cells were established by stably transfecting full-length human Mcl-1 and Bcl-xL cDNA, respectively19. All experiments utilized logarithmically growing cells (3–5×105 cells/ml). MycoAlert (Lonza, Allendale, NJ) assays were performed, demonstrating that all cell lines were free of mycoplasma contamination.
Bone marrow (BM) samples were obtained with informed consent according to the Declaration of Helsinki and Virginia Commonwealth University IRB approval from four patients with MM undergoing routine diagnostic aspirations. CD138+ cells were separated using a MACS magnetic separation technique (Miltenyi Biotech, Auburn, CA). Normal CD34+ hematopoietic progenitor cells were isolated from two cord blood (CB) samples; purity and viability were > 90%, by flow cytometry and trypan blue exclusion, respectively,
The pan-BH3-mimetic obatoclax (GX015-070) were provided by GeminX Pharmaceuticals (Malvern, PA). The pan-Cdk inhibitors flavopiridol (alvocidib) and SCH727965 (Merck, Whitehouse Station, N.J.) were provided by the NCI. Cycloheximide (CHX) and MG-132 were purchased from Sigma and Calbiochem (San Diego, CA) respectively, dissolved in DMSO, aliquoted, and stored at −20°C. In all experiments, final DMSO concentrations did not exceed 0.1%. Recombinant human Il-6, IGF-1, BAFF, and APRIL were obtained from PeproTech (Rocky Hill, NJ).
Procedures for in vitro studies
For procedures related to flow cytometry, TUNEL staining, quantitative RT-PCR (qPCR), immunoblot, co-immunoprecipitation, subcellular fractionation, Bak and Bax conformational change, RNA interference see Supplemental Materials and Methods7.
Animal studies
Animal studies were approved by the Virginia Commonwealth University IACUC, and performed in accordance with the U.S. Department of Agriculture and Department of Health and Human Services, and the NIH. Three mouse models were employed in this study.
Model #1 - subcutaneous (s.c.) flank murine model: Athymic NCr-nu/nu mice (Jackson Laboratories, Bar Harbor, ME) were subcutaneously inoculated in the right rear flank with 5×106 RPMI8226 cells stably transfected with a construct encoding luciferase. Treatment was administrated after luciferase activity was detected.
Model #2 – subcutaneous (s.c.) dual-side flank murine model: NOD/SCID-gamma mice (Jackson Laboratories) were subcutaneously inoculated in two opposite flanks with 1×107 U266 cells stably transfected with constructs encoding shRNA targeting either Bim (shBim, left flank) or scrambled sequence negative control (shNC, right flank).
Model #3 – intravenous (i.v.) orthotopic murine model: NOD/SCID gamma mice (Jackson Laboratories) were intravenously injected with 5×106 U266 cells stably transfected with constructs encoding luciferase.
Obatoclax mesylate (GX15-070MS) was freshly reconstituted with 5% Dextrose for Injection (USP) and administrated via intramuscular (i.m.) or intraperitoneal (i.p.) injection. FP in DMSO was diluted in 0.9% saline and administrated via i.p. injection. Control animals were injected with equal volumes of vehicle. Mice were monitored for tumor growth every other day visually or with the use of an IVIS 200 imaging system (Xenogen Corporation, Alameda, CA). Measurement of animal body weight was performed every other day throughout the study to monitor toxicity. Tumor volumes were calculated using the formula (L × W2)/2, with L and W representing length and width respectively, and when tumor size reached 2,000 mm3, mice were euthanized in accordance with institutional guidelines.
Statistical analysis
Values represent the means ± SD for at least three independent experiments performed in triplicate. The significance of differences between experimental variables was determined using the Student's t test or One-way ANOVA with Tukey-Kramer Multiple Comparisons Test. The significance of P values was < 0.05 (*), < 0.01 (**), or < 0.001 (***) wherever indicated. Analysis of synergism was performed according to Median Dose Effect analysis using the software Calcusyn (Biosoft, Ferguson, MO). Kaplan-Meier analysis of mouse survival or hind-leg paralysis was performed using IBM SPSS Statistics software.
Results
Cdk inhibitors synergistically potentiate BH3-mimetic lethality by engaging the mitochondria-related apoptotic cascade
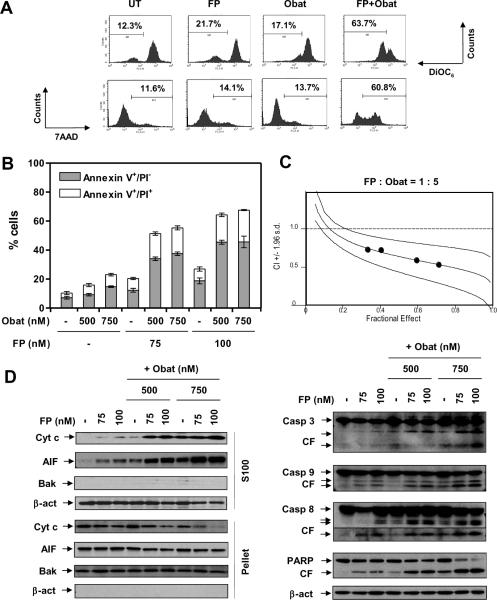
Co-administration (24hr) of minimally toxic concentrations of obatoclax and FP sharply reduced mitochondrial membrane potential and increased 7-AAD uptake in U266 cells (Fig 1A), accompanied by a pronounced increase in both early (annexin V+/PI−) and late (annexin V+/PI+) apoptosis (Fig 1B). Interactions occurred at obatoclax concentrations as low as 300nM (data not shown). Median Dose Effect analysis revealed CI values less than 1.0, indicating synergism (Fig 1C). Whereas individual treatment had only modest effects, combined exposure markedly increased AIF and cytochrome c cytosolic release (Fig 1D, left panels), and caspase−3, −8, −9, and PARP cleavage (right panels). Apoptosis was confirmed by TUNEL staining (data not shown). Concordant results were observed in other human MM lines (Fig 5C). Similar interactions were observed when other pan-Cdk inhibitors (e.g., SCH727965) and pan-Bcl-2 antagonists (e.g., HA14-1) were employed (Suppl Fig S1A).
Figure 1. The pan-Cdk inhibitor flavopiridol (FP) interacts synergistically with the pan-Bcl antagonist obatoclax to induce mitochondria-dependent apoptosis in MM cells.
Human MM U266 cells were exposed (24 hr) to (A) 100 nM FP +/− 500 nM obatoclax (Obat); (B, D) 75 – 100 nM FP +/− 500 – 750 nM obatoclax; or (C) 80 – 110 nM FP +/− 400 – 550 nM obatoclax at a fixed ratio (1 : 5). After drug treatment, cell death and related signaling pathways were analyzed by flow cytometry after stained with (A) DiOC6 (Δψm) and 7AAD (cell death), or (B, C) Annexin V-FITC/PI (early and late apoptosis); and (D) immunoblot for cytosolic S-100, mitochondria-enriched fractions, or for whole cell lysates. β-actin = loading controls for S-100 and whole cell lysate; Bak = loading control for mitochondrial fraction; CF = cleaved fragments.
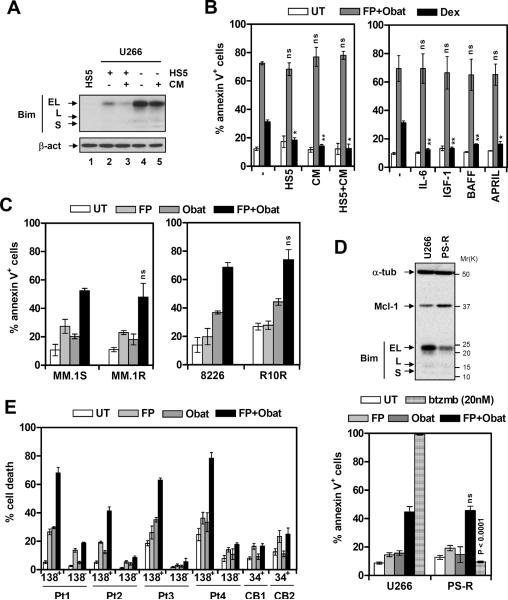
Figure 5. FP/obatoclax circumvents various forms of MM-related drug resistance.
(A) U266 cells were cultured (24 hr) under either regular conditions (RPMI1640 with 10% FBS, lane 4) or with human BM stromal HS-5 cells (lane 2), HS-5-conditional medium (lane 5), or both (lane 3). Immunoblot analysis was performed to monitor Bim expression. HS-5 cell lysate (lane 1) was loaded for comparison. (B) U266 cells were exposed (24 hr) to 100 nM FP + 500 nM obatoclax under the indicated conditions (left), or in the presence of human recombinant 100 ng/mL IL-6, 400 ng/ml IGF-1, 200 ng/ml BAFF, or 200 ng/ml APRIL (right, ns = not significant, P > 0.05). In parallel, cells were treated with 10 μM dexamethasone for comparison (* P < 0.05, ** P < 0.01). The percentage of apoptotic (annexin V+) cells was then determined by flow cytometry. (C) Dexamethasone- (MM.1R) and revlimid-resistant (R10R) cells, as well as their drug-naïve counterparts (MM.1S and RPMI8226), were treated (24 hr) with FP (1S and 1R, 75 nM; 8226 and R10R, 100 nM) +/− obatoclax (1S and 1R, 500 nM; 8226 and R10R, 750 nM). The percentage of apoptotic cells was then determined by flow cytometry. (D) The bortezomib-resistant U266 cell line (PS-R) was generated by continuously culturing U266 cells in gradually increasing concentrations of bortezomib. Immunoblot analysis was performed to assess expression of Mcl-1 and Bim on the same membrane (upper). PS-R and its parental cell line were treated (24 hr) with 75 nM FP +/− 500 nM obatoclax, or 20 nM bortezomib for comparison (P < 0.0001), after which the extent of apoptosis was determined by flow cytometry. (E) CD138+ and CD138− cells were isolated from BM samples of four patients with MM (Pt 1 – 4), and CD34+ cells isolated from two cord blood (CB) samples. Cells were exposed (24 hr) to 75 – 100 nM FP +/− 300 – 500 nM obatoclax, after which percentage of cell death was assessed by 7AAD staining followed by flow cytometry.
Cdk inhibitor/BH3-mimetic interactions are associated with Mcl-1 and Bcl-xL down-regulation
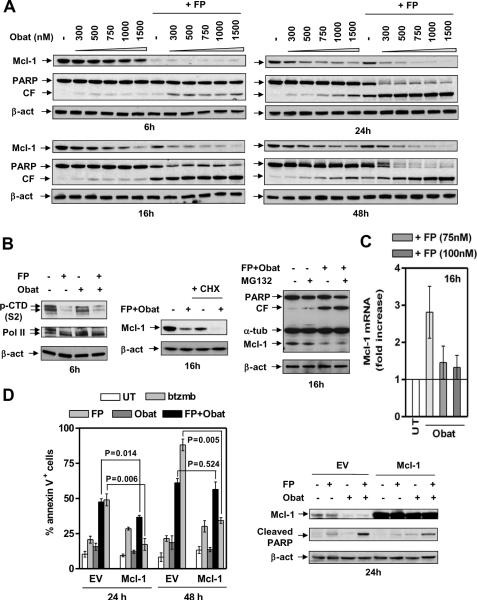
Immunoblot analysis revealed Mcl-1 down-regulation 6h after FP exposure, with recovery at 16h (Fig 2A) despite the fact that inhibition of Pol II CTD phosphorylation (serine 2) persisted (Suppl Fig S1B), a phenomenon observed in an earlier study14. Obatoclax alone clearly decreased Mcl-1 levels in a dose- and time-dependent manner21. Notably, FP/obatoclax co-administration prevented recovery from Mcl-1 down-regulation at later intervals (e.g., 16h – 48h; Fig 2A). Treatment with FP +/− obatoclax markedly diminished serine 2 phosphorylation of Pol II CTD at 6h (Fig 2B, left panels) and 16h (Suppl Fig S1B), indicating Cdk9/cyclin T (P-TEFb) inhibition. Moreover, in FP/obatoclax-treated cells, co-administration of the translation inhibitor CHX further reduced Mcl-1 levels (Fig 2B, middle panels), whereas the proteasome inhibitor MG-132 failed to restore Mcl-1 expression (Fig 2B, right panels), arguing against translational or post-translational mechanisms of Mcl-1 down-regulation. Quantitative RT-PCR (qPCR) revealed a clear increase in Mcl-1 mRNA levels in obatoclax-treated U266 and RPMI8226 cells, as described earlier21, a phenomenon largely attenuated by FP (Fig 2C and Suppl Fig S1C).
Figure 2. FP/obatoclax down-regulates Mcl-1.
U266 cells were treated as follows, (A) 300 nM – 1.5 μ-M obatoclax +/− 100 nM FP for 6, 16, 24, and 48 hr; (B, left) 100 nM FP +/− 500 nM obatoclax for 6 hr; (B, middle) 100 nM FP + 500 nM obatoclax, in the presence or absence of 1 μM CHX, for 16 hr; (B, right) 100 nM FP + 500 nM obatoclax, with or without the addition of 300 nM MG-132, for 16 hr. After drug treatment, Immunoblot analysis was performed to monitor Mcl-1 protein levels and PARP cleavage, or total and phosphorylated (serine 2, CTD) RNA Pol II. (C) U266 cells were incubated with 500 nM obatoclax +/− 75 – 100 nM FP for 16 hr, after which Mcl-1 mRNA levels were assessed by quantitative RT-PCR, using GAPDH as control (see Supplemental Materials and Methods). (D) U266 cells were stably transfected with human full length Mcl-1 or empty vector (EV). Cells were exposed (24 hr) to 100 nM FP +/− 500 nM obatoclax, or 4 nM bortezomib for comparison, and then subjected to flow cytometry (left) or immunoblot analysis (right).
Effects on Bcl-xL, which cooperates with Mcl-1 to tether and inactivate Bak22, were then examined. Exposure of U266 cells to FP +/− obatoclax reduced Bcl-xL levels in a time-dependent manner (Suppl Fig S2A), while CHX failed to further down-regulate Bcl-xL (Suppl Fig S2B). In contrast, Bcl-2 protein levels remained unchanged with all treatments (Suppl Fig S2C, left panels). Analogous results were obtained in RPMI8226 cells (Suppl Fig S2C, right panels).
Ectopic expression of Mcl-1 partially but significantly attenuated FP/obatoclax lethality at 24h (Fig 2D and Suppl Fig S2D). However, protection was not statistically significant at 48h (Fig 2D). In contrast, cells overexpressing Mcl-1 were substantially resistant to bortezomib at both 24h and 48h (Fig 2D), consistent with previous reports9. Bcl-xL over-expression partially but significantly protected cells from FP/obatoclax lethality at both 24h and 48h (Suppl Fig S2E). Together, these findings suggest that Mcl-1 and Bcl-xL down-regulation plays a significant but limited functional role in FP/obatoclax lethality.
Up-regulation of BH3-only proteins in MM cells exposed to Cdk inhibitor/BH3-mimetic
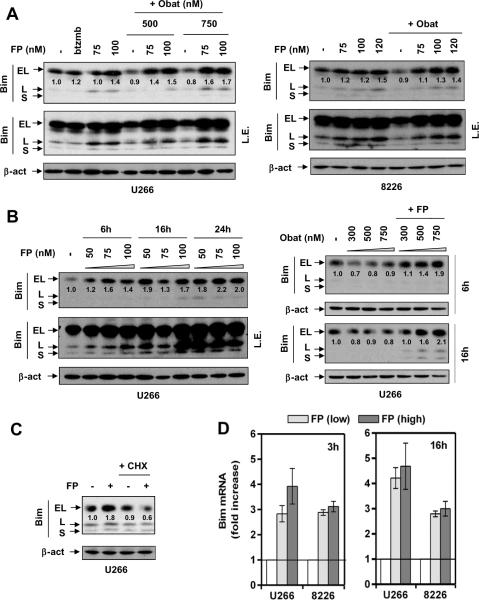
Because Mcl-1 or Bcl-xL over-expression only partially protected cells from FP/obatoclax, effects of the regimen were then examined in relation to expression of proapoptotic BH3-only proteins using a BH3-only detection kit. Unexpectedly, 24h exposure of MM cells to FP +/− obatoclax resulted in marked up-regulation of several BH3-only proteins, including Bim (EL and L isoforms, Fig 3A), Bik/NBK (Suppl Fig S3A), and Noxa (Suppl Fig S3B).
Figure 3. FP induces Bim expression at the transcriptional level.
(A) U266 cells were exposed (24 hr) to 75 – 100 nM FP +/− 500 – 750 nM obatoclax, or 4 nM bortezomib for comparison (left); RPMI8226 cells were exposed (24 hr) to 75 – 120 nM FP +/− 750 nM obatoclax (right). (B) U266 were incubated with 50 – 100 nM FP for 6, 16, and 24 hr (left); or 300 – 750 nM obatoclax +/−100 nM FP for 6 and 16 hr (right). (C) U266 cells were treated with 100 nM FP +/− 1 μM CHX for 24 hr. After drug treatment, immunoblot analysis was performed to monitor protein expression of three isoforms (EL, L, and S) of Bim. For changes in BimEL protein levels, the density of blots was quantified; values reflect the ratio of integrated densitometric determinations between untreated and drug-treated cells, with normalization against β-actin. L.E. = long exposure. (D) Cells were exposed to low (U266, 75 nM; 8226, 100 nM) or high (U266, 100 nM; 8226, 120 nM) concentrations of FP for 3 hr and 16 hr, respectively, after which quantitative RT-PCR was performed to determine mRNA levels of Bim, using β-actin as control.
Consistent with results in other tumor cell types21, obatoclax played a major role in Noxa up-regulation. Time course analysis of U266 cells revealed that obatoclax alone sharply increased Noxa levels as early as 6h after exposure, but this effect was no longer apparent after 16h (data not shown). Notably, obatoclax-induced Noxa up-regulation was sustained for longer intervals (e.g., at least 24h) in the presence of FP (Suppl Fig S3B, upper panels). Similar events occurred in RPMI8226 cells (Suppl Fig S3B, lower panels). Importantly, Noxa shRNA dramatically blocked apoptosis induced by either bortezomib23 or FP/obatoclax (Suppl Fig S3C), arguing that up-regulation of the BH3-only protein Noxa plays a significant functional role in FP/obatoclax lethality..
Up-regulation of Bim at the transcriptional level plays a significant functional role in Cdk inhibitor/BH3-mimetic interactions
The functional significance of up-regulation of the direct activator Bim24 was then examined. FP induced Bim expression (EL and L isoforms), with or without obatoclax, in U266 (Fig 3A, left panels) and RPMI8226 cells (right panels). Immunoblot analysis confirmed increased expression of both Bim isoforms after FP treatment alone (Fig 3B, left panels) or in combination with obatoclax (right panels), events occurring at 6h and sustained for at least 24h after treatment. In contrast, obatoclax alone did not up-regulate Bim (data not shown). FP-induced Bim induction was largely blocked by CHX (Fig 3C), suggesting a requirement for de novo protein synthesis. Moreover, qPCR demonstrated significant increases in Bim mRNA levels at 3h, 6h, and 16h after FP treatment with or without obatoclax in both U266 and RPMI8226 cells (Fig 3D and Suppl Fig S3D), arguing that Bim up-regulation by FP occurs at the transcriptional level.
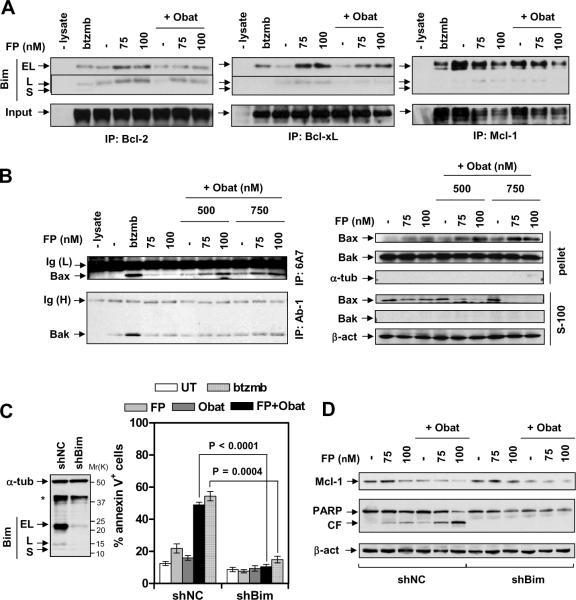
Co-immunoprecipitation was performed to examine interactions between Bim and anti-apoptotic Bcl proteins. Whereas bortezomib increased BimEL rather than BimL bound to Bcl-2 and particularly to Bcl-xL, FP alone clearly increased Bim (EL and L) binding to Bcl-2 and BclxL (Fig 4A). The latter events were markedly attenuated by obatoclax. Interestingly, FP or obatoclax alone modestly diminished BimEL bound to Mcl-1, an effect only slightly enhanced with the combination (Fig 4A). In FP-treated cells, unleashing of Bim from both Bcl-2 and BclxL by obatoclax was associated with conformational activation of Bax and to a lesser extent Bak (Fig 4B, left panels), as well as Bax mitochondrial translocation (right panels), triggering mitochondrial outer membrane permeabilization (MOMP), caspase activation, and pronounced apoptosis (Fig 1D). Moreover, transient transfection of a construct encoding Bax shRNA significantly diminished FP/obatoclax lethality (Suppl Fig S3E).
Figure 4. Obatoclax unleashes Bim from Bcl-2, Bcl-xL, and Mcl-1 in FP-treated cells, activating Bax/Bak.
(A) After 24 hr-treatment with 75 – 100 nM FP +/− obatoclax (A, 500 nM; B, 500 – 750 nM), or 4 nM bortezomib for comparison, U266 cells were lysed in 1% CHAPS buffer and subjected to co-immunoprecipitation (IP). Blots were stripped and subsequently reprobed with Bcl-2, Bcl-xL, or Mcl-1 (input) respectively. (B) U266 cells were treated as described in 4A, IP was performed with 6A7 or AB-1 monoclonal antibodies which specifically recognize conformationally-changed Bax and Bak respectively, and immunobloted for Bax or Bak (left). IP without cell lysate was performed as a control. Alternatively, cytosolic (S-100) and mitochondria-enriched membrane (pelleted) fractions were separated and subjected to immunoblot for Bax and Bak (right). (C, D) U266 cells were stably transfected with shRNA targeting human Bim (shBim, clone ID #1) or scramble sequence as negative control (shNC) (C, left). Cells were then treated (24 hr) with FP (C, 100 nM; D, 75 – 100 nM) +/− 500 nM obatoclax, or 4 nM bortezomib for comparison, after which apoptosis and Mcl-1 expression were monitored by flow cytometry (C, right panel) or immunoblot analysis (D) respectively.
To define the functional role of Bim up-regulation, U266 cells were stably transfected with a constructs encoding shRNA targeting Bim (shBim). In these cells, both BimEL and BimL were substantially knocked down, compared to (shNC) scrambled sequence negative controls (Fig 4C, left panel). Notably, shBim essentially abrogated FP/obatoclax-mediated lethality (Fig 4C, right panel), analogous to its ability to protect cells from bortezomib25. Consistent with these findings, shBim prevented Bax conformational change and translocation (Suppl Fig S3F), caspase activation, and PARP cleavage (Fig 4D) induced by FP/obatoclax. However, co-exposure to FP/obatoclax induced nearly equivalent Mcl-1 down-regulation in both shNC and shBim cells (Fig 4D). Analogous results were obtained when another Bim shRNA was employed (Suppl Fig S3C). Together, these findings raise the possibility that unleashing of up-regulated Bim from anti-apoptotic proteins (e.g., Bcl-2, Bcl-xL, Mcl-1) by obatoclax contributes to synergistic interactions. They also argue that in the setting of down-regulation of anti-apoptotic proteins, up-regulation of BH3-only proteins such as Bim plays a critical functional role in lethality.
The Cdk inhibitor/BH3-mimetic regimen is active against MM cells displaying conventional or novel forms of drug resistance, as well as primary MM cells
In addition to drug resistance due to Bcl family dysregulation, microenvironmental factors also confer resistance in MM26. To address these issues, U266 cells were cultured in the presence of HS-5 cells (a human BM stromal cell line)27, HS-5-conditional medium (CM), or both. Whereas CM slightly reduced Bim levels, co-culture with HS-5 markedly down-regulated Bim (Fig 5A). Notably, HS-5 plus CM essentially abolished Bim expression, raising the possibility that Bim down-regulation represents a mechanism underlying stromal cell-mediated drug resistance28. Importantly, neither HS-5 ± CM prevented FP/obatoclax lethality (P > 0.05, Fig 5B, left panel). Furthermore, addition of IL-6, BAFF, APRIL, or IGF-1 also failed to attenuate FP/obatoclax lethality (P > 0.05, Fig 5B, right panel), suggesting that the FP/obatoclax regimen overcomes drug-resistance related to microenvironmental factors (e.g., stromal cells, cytokines, and growth factors).
Dexamethasone-resistant (MM.1R) and revlimid-resistant (R10R) cells exhibited roughly equivalent sensitivity to FP/obatoclax, compared to their drug-naïve counterparts (MM.1S and RPMI8226) respectively (Fig 5C). Interestingly, bortezomib-resistant U266 cells (PS-R), which were resistant to 20 nM bortezomib (Fig 5D, lower panel), displayed a clear increase in Mcl-1 levels, accompanied by a dramatic reduction in Bim expression24, particularly the EL isoform (upper panel). Significantly, PS-R cells exhibited no cross-resistance to FP/obatoclax, compared to parental cells (Fig 5D, lower panel), suggesting that MM cells exhibiting either conventional or novel forms of drug-resistance remain fully susceptible to this regimen.
Whereas sensitivity to individual agents varied between primary CD138+ MM specimens isolated from different patients, co-treatment with FP/obatoclax sharply increased cell death (Fig 5E). However, toxicity toward CD138− BM cells was minimal in all samples. Interestingly, despite differences between samples, CD138+ cells displayed relatively higher levels of Bim compared with their CD138− counterparts (data not shown)29. Furthermore, FP or obatoclax alone displayed only modest toxicity toward normal CB CD34+ cells, while combined treatment did not increase lethality (Fig 5E), suggesting that this regimen is active against and relatively selective toward primary MM cells.
The Cdk inhibitor/BH3-mimetic regimen displays marked in vivo anti-tumor activity through a Bim-dependent mechanism
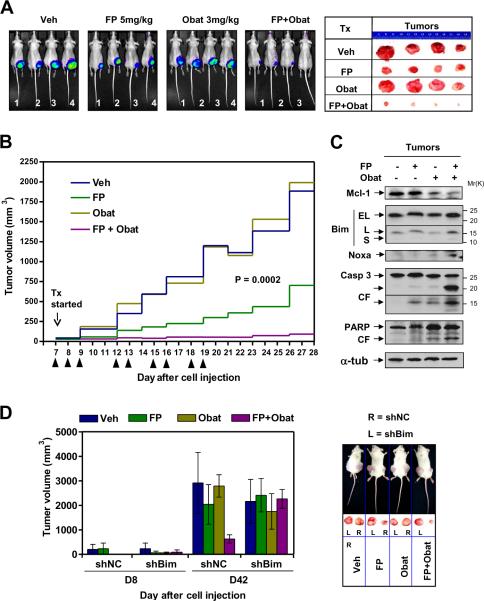
Obatoclax reportedly lacked in vivo single agent bioactivity in mice bearing subcutaneous KMS12PE human MM tumors12. To determine whether the FP/obatoclax regimen exhibited in vivo activity, athymic nude mice were inoculated in the flank with RPMI8226 cells carrying a luciferase gene. When tumors became visible, mice were treated with FP (5mg/kg, i.p.) ± obatoclax (3mg/kg, i.m.). Consistent with previous reports12, obatoclax (3mg/kg) alone had no effect on tumor growth, manifested by luciferase activity (Fig 6A, left panels). However, whereas FP (5mg/kg) alone exerted modest but discernible effects, combined treatment substantially suppressed tumor growth. The size of tumors excised from mice confirmed pronounced tumor growth suppression with combined treatment (Fig 6A, right panels). Moreover, tumor size measurements yielded concordant results (P = 0.0002, Fig 6B). Interestingly, immunoblot analysis of tumor tissues revealed that FP/obatoclax co-administration down-regulated Mcl-1 and up-regulated Bim (particularly the L isoform) and Noxa, accompanied by caspase-3 activation and PARP cleavage (Fig 6C), consistent with in vitro observations.
Figure 6. FP/obatoclax suppresses MM tumor growth in subcutaneous flank murine models, an event diminished by Bim shRNA.
(A–C) Mouse model #1 - subcutaneous (s.c.) flank RPMI8226 model: athymic NCr-nu/nu mice were subcutaneously inoculated in the right rear flank with 5×106 RPMI8226 cells carrying luciferase. Treatment was initiated after luciferase activity was detected (7 days after injection of tumor cells). Mice were treated (indicated by arrow heads) with FP (5mg/kg, i.p.) ± obatoclax (3mg/kg, i.m.), daily for the first three days. After a two-day interval (due to poor absorption of obatoclax after i.m. injection), the schedule was adjusted to twice every three days for an additional three cycles. n = 4 per group. Tumor growth was monitored every other day using the IVIS 200 imaging system (A, left, images shown were captured at day 28), and tumor size measured by calipers (B). When tumor size reached 2,000 mm3, mice were euthanized, and tumors removed from mice (A, right), after which tumor tissues were homogenized and subjected to immunoblot analysis (C). A discernible loss of body weight (< 10% of initial weight) occurred in both obatoclax and FP/obatoclax groups during the first week of treatment, which recovered soon after the treatment schedule was adjusted. There was no discernible loss of body weight in another two groups throughout the experiment. (D) Mouse model #2 – s.c. dual-side flank U266 model: NOD scid gamma mice were subcutaneously inoculated in each flank with 1×107 U266 cells carrying shRNA targeting Bim (shBim, left flank) or negative control (shNC, right flank). Treatment was initiated after tumors were visible (8 days after injection of tumor cells). FP (3mg/kg, i.p.) ± obatoclax (3mg/kg, i.p.) were administered daily for the first four days, followed by once every two days for an additional seven cycles. n = 4 per group. Tumor size was measured every other day (left); when tumor size reached 2,000 mm3, mice were euthanized, and tumors removed from mice (right). There was no significant loss of body weight in any groups throughout the experiment. Tumor volumes were calculated using the formula (L × W2)/2, with L and W representing length and width respectively.
To determine whether BH3-only protein up-regulation (e.g., Bim) plays a significant functional role in FP/obatoclax lethality in vivo, NOD/SCID-gamma mice were inoculated s.c. with U266 cells stably transfected with shBim or shNC respectively (Fig 4C) in each flank, after which FP (3mg/kg, i.p.) ± obatoclax (3mg/kg, i.p.) was administered. FP/obatoclax co-administration markedly suppressed growth of shNC tumors (Fig 6D, right flank), analogous to results observed in the previously described flank model (Fig 6A–C). Notably, whereas a slight reduction in tumor size was observed in the obatoclax group, no obvious growth suppression was observed by FP alone or with obatoclax in shBim tumors (Fig 6D, left flank), demonstrating an important functional role for Bim in FP/obatoclax lethality in vivo.
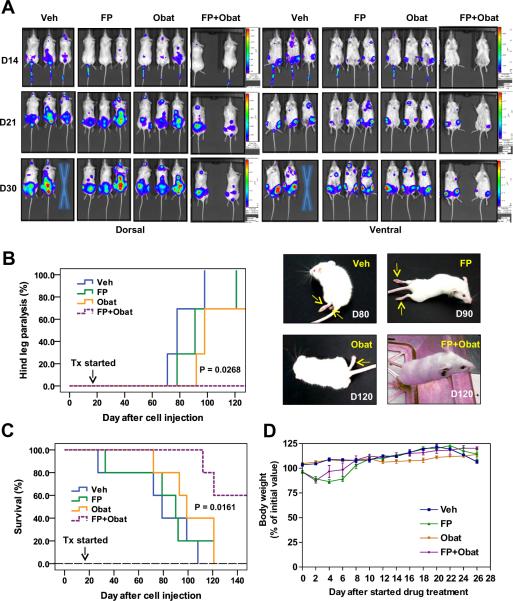
The BM microenvironment plays a critical role in survival, growth, and drug-resistance of MM cells26. The activity of the FP/obatoclax regimen was therefore assessed in an animal model in which human MM cells form BM lesions, leading to bone disease at late intervals. In this orthotopic murine model, NOD/SCID-gamma mice were injected with U266 cells stably transfected with a luciferase gene, after which homing and growth of tumor cells were dynamically monitored by imaging luciferase activity. Notably, U266 cells homed to BM and then formed lesions at skeletal sites (Fig 7A), without detectable lesions in other organs, findings confirmed by IHC staining for human CD138 (data not shown). At later intervals (9 – 13 weeks after cell injection), inoculated mice displayed hind-leg paralysis (Fig 7B, right panels), a classic indicator of bone disease. After luciferase signals were visible, FP (5mg/kg, i.p.) ± obatoclax (3mg/kg, i.p.) were administered daily for five days, followed by FP (3mg/kg) ± obatoclax (3mg/kg) twice every three days. Tumor growth was monitored every two days. As shown in Fig 7A, combined treatment substantially reduced tumor burden compared to agents administered individually. Kaplan-Meier hazard analysis demonstrated that whereas obatoclax alone clearly delayed the appearance of hind-leg paralysis, FP alone had only minimal effects (Fig 7B). Notably, hind-leg paralysis did not appear in any mice in the FP/obatoclax group throughout the entire four month observation period (P = 0.0268, Fig 7B, Suppl Video). Moreover, Kaplan-Meier survival analysis revealed more prolonged survival of mice receiving combined treatment compared to mice treated with FP or obatoclax alone (P = 0.0161, Fig 7C). Finally, significant neurologic toxicity (e.g., onset of agitation and hyperactivity), which has been noted immediately after a rapid i.v. injection of obatoclax (4mg/kg)12, was not observed in mice after either i.m. or i.p. obatoclax (3mg/kg) alone or in combination with FP, similar to results with i.v. injection of 2mg/kg obatoclax in mice bearing solid tumors11. Moreover, there was no significant loss of body weight (Fig 7D) or other signs of toxicity observed after FP and obatoclax administrated alone or in combination in these murine systems. Together, these findings argue that an anti-MM regimen combining a pan-Cdk inhibitor with a pan-BH3-mimetic is active in vivo, and suggest that mechanisms identified in vitro (e.g., up-regulation of BH3-only proteins such as Bim, Mcl-1 down-regulation) may be operative in vivo.
Figure 7. The FP/obatoclax regimen is active in an intravenous BM-homing murine model.
(A–D) Mouse model #3 – intravenous (i.v.) orthotopic U266 model: NOD scid gamma mice were intravenously injected via tail vein with 5×106 U266 carrying luciferase. After luciferase signals were visible (14 days after injection of tumor cells), FP (5mg/kg, i.p.) ± obatoclax (3mg/kg, i.p.) were administered daily for first five days, followed by FP (3mg/kg) ± obatoclax (3mg/kg) twice every three days for additional seven cycles. n = 5 per group. Tumor growth was monitored every other day by imaging luciferase activity (A). Cross = dead. Kaplan-Meier analysis was performed to analyze occurrence of hind-leg paralysis (B, arrows), as well as survival of animals (C). Loss of body weight (< 20% of initial weight) occurred within the first week of treatment in the FP and FP/obatoclax groups, indicating toxicity of 5mg/kg FP in these mice, which recovered after reduction of the FP dose to 3mg/kg and adjustment of the treatment schedule (D).
Discussion
Tumor cells are commonly characterized by over-expression of anti-apoptotic Bcl proteins (e.g., Bcl-2, Bcl-xL, or Mcl-1), which contributes to drug-resistance by disabling the death machinery30;31. Transformed cells also frequently express pro-apoptotic Bcl proteins including multi-domain (e.g., Bax and Bak) and some, if not all, BH3-only proteins such as Bim3;4. While tumor cells may be fully equipped with anti- and pro-apoptotic machinery, the latter are often inactivated through binding to their highly-expressed anti-apoptotic counterparts2. Due to the balance between anti- and pro-death Bcl proteins, tumor cells may be “primed” for cell death32 by disrupting this balance, e.g., by up-regulating or mimicking pro-apoptotic protein actions, or by down-regulating/antagonizing the function of anti-apoptotic proteins33;34. The present findings demonstrate that pan-Cdk inhibitors markedly potentiates the lethality of pan-BH3 mimetics (e.g., obatoclax) in vitro and in vivo in MM cells, including those displaying conventional or novel forms of drug-resistance. They also reveal that these agents interact synergistically by interfering with both arms of the apoptotic regulatory balance, i.e., down-regulating/disabling Bcl-2, Bcl-xL, and Mcl-1, and up-regulating BH3-only proteins including Bim, Noxa, and Bik/NBK.
Pan-Cdk inhibitors like FP act as transcriptional repressors by inhibiting Cdk9, a catalytic subunit of the cyclin T/Cdk9 complex (P-TEFb), preventing serine 2 phosphorylation of RNA Pol II CTD, thereby blocking transcription elongation13 and inducing down-regulation of short-lived proteins such as Mcl-114, an essential survival factor for MM cells35. The present results demonstrate that FP down-regulates Mcl-1 at early intervals (e.g., 6h) in association with diminished CTD serine 2 phosphorylation. However, Mcl-1 expression recovered after 16h, indicating that FP rapidly but only transiently inhibits Mcl-1 transcription in MM cells14. Recently, obatoclax was shown to down-regulate Mcl-1 through induction of Noxa, which binds to Mcl-1 and triggers its degradation, while qPCR revealed more than a 5-fold increase in Mcl-1 mRNA levels after obatoclax exposure (6h)21. While similar events were observed in the present study, mRNA up-regulation was largely blocked by FP. Although the mechanism underlying these phenomena is uncertain, increases in Mcl-1 mRNA may represent a compensatory response to down-regulation of the protein. Importantly, obatoclax strikingly blocked recovery from Mcl-1 protein down-regulation after prolonged FP exposure (e.g., 16 – 48h), suggesting that persistent Mcl-1 down-regulation following FP/obatoclax co-exposure may reflect two separate but cooperative mechanisms: a) Mcl-1 mRNA transcription inhibition by FP; and b) promotion of Mcl-1 protein degradation through Noxa induction by obatoclax. However, Mcl-1 over-expression only partially attenuated FP/obatoclax-mediated apoptosis at 24h, but not at intervals ≥ 48h, raising the possibility that the FP/obatoclax regimen may circumvent Mcl-1-related drug-resistance.
MM cells exposed to FP +/− obatoclax exhibited sharp increases in expression of BH3-only proteins including Bim (EL and L isoforms), Noxa, and Bik/NBK. Unlike Bax/Bak, protein levels of which are relatively stable2, and which must be activated to trigger apoptosis, expression of BH3-only proteins is tightly regulated, and thus represents a candidate target3;36. Many novel agents induce expression (e.g., HDAC inhibitors19) or prevent degradation (e.g., proteasome inhibitors37) of BH3-only proteins such as Noxa, Puma, and Bim, thereby directly or indirectly activating Bax/Bak2. Obatoclax antagonizes Mcl-1 anti-apoptotic functions by unleashing Bim or Bak from Mcl-1, and also down-regulates Mcl-1 via induction of Noxa21. The present findings demonstrate that obatoclax induced Noxa up-regulation at early intervals (e.g., 6h), and that this event was enhanced and rendered more sustained by FP. Functionally, both Noxa and Bim shRNA profoundly diminished FP/obatoclax lethality. Thus, while down-regulation of anti-apoptotic proteins (e.g., Mcl-1 and Bcl-xL) may contribute to the lethality of this regimen to a limited extent, up-regulation of BH3-only proteins (e.g., Bim and Noxa) clearly plays a critical role in Cdk inhibitor/pan-BH3-mimetic interactions.
The marked increase in Bim (both EL and L isoforms) by FP ± obatoclax has, to the best of our knowledge, not been described previously. Transcriptional regulation of Bim represents a major mechanism of apoptosis regulation38. Several findings argue that FP up-regulates Bim at the transcriptional level. First, translation inhibition by CHX largely blocked Bim up-regulation induced by FP. Second, qPCR indicated that FP induced a marked increase (e.g., 3 – 5 fold) in Bim mRNA levels. In this context, transcriptional regulation of Bim is complex and multifactorial. For example, growth factor withdrawal-induced Bim up-regulation requires JNK activation in neurons, while depends on the forkhead transcription factor FKHR-L1 in hematopoietic cells3. Moreover, the bim promoter is regulated by stress-related transcriptional factors such as FOXO and AP-1 family members38. The mechanism by which a pan-Cdk inhibitor transcriptionally up-regulates BH3-only proteins such as Bim is not intuitively obvious. One possibility is that FP may activate JNK in the presence of pan-CDK inhibitors up-regulate BH3-only proteins to sensitize human myeloma cells to BH3 mimetic therapies Bcl-2 antagonists (e.g., HA14-1)39. Another possibility is that Cdk2 inhibition by pan-Cdk inhibitors may activate FOXO1, which induces Bim transcription41. In addition to transcriptional induction, Bim also exhibits multifactorial regulation at the levels of mRNA stability, post-translational modifications, proteasomal degradation, and cellular localization38;42. Consequently, the possibility that additional mechanisms contribute to Cdk inhibitor-mediated Bim up-regulation cannot be excluded.
The observation that Bim shRNA knock down essentially abrogated apoptosis indicates a critical functional role for Bim up-regulation in Cdk inhibitor/pan-BH3 mimetic lethality. Mechanisms of Bim-mediated cell death may reflect direct actions following displacement/de-repression of anti-apoptotic proteins by BH3-only “sensitizers”, leading to Bax/Bak activation. Alternatively, in the neutralization model, Bim binds to and neutralizes/inactivates all anti-apoptotic Bcl proteins that repress constitutively active Bax/Bak. Obatoclax releases Bim from Bcl-2, Bcl-xL, and Mcl-1 binding, thereby promoting apoptosis10;12. Interestingly, FP up-regulated expression of Bim but increased its binding to Bcl-2 and Bcl-xL, suggesting that up-regulated Bim may “prime” cells for death43;44. Indeed, obatoclax unleashed Bim from Bcl-2, Bcl-xL, and Mcl-1, leading to Bax/Bak activation and cell death in Cdk inhibitor-treated cells. Significantly, the latter event (i.e., Bax/Bak activation) did not occur in cells in which Bim was knocked down by shRNA. Notably, in a dual-sided flank murine model that circumvents problems in interpretation related to mouse-to-mouse variability45, FP/obatoclax failed to suppress the growth of tumors carrying Bim shRNA, demonstrating a functional in vivo role for Bim in lethality. Finally, the observation that obatoclax released Bim from multiple anti-apoptotic proteins (e.g., Bcl-2, Bcl-xL, and Mcl-1), suggests that as observed in the case of BH3-mimetics (e.g., ABT-737) administered alone43, interactions between Bcl family members, rather than simply their expression profiles, may be critical predictors of sensitivity to the Cdk inhibitor/pan-BH3 mimetic regimen in MM.
MM cells interact with the BM microenvironment to promote cell proliferation, survival, migration, and drug resistance. Genetic profiling studies revealed that Bcl family members represent microenvironment-specific chemotherapeutic response determinants in malignant hematopoietic cells, and that BH3-only “activators” are necessary for cell death45. IL-6 and IGF-1, which are critical for MM cell/microenvironment interactions, promote MM cell survival and confer drug-resistance through a) Mcl-1 induction46;47 and b) Bim down-regulation34;48. The latter involves at least two mechanisms e.g., post-translational phosphorylations and/or transcription48. Notably, FP/obatoclax was fully active in the presence of these cytokines or other autocrine MM survival/growth factors (e.g., BAFF and APRIL)49. Direct MM cell-stroma contact also contributes to MM drug-resistance28. Interestingly, HS-5 co-culture and conditioned medium largely abrogated Bim expression in MM cells, implicating this phenomenon as a mechanism of stromal cell-mediated drug resistance. Significantly, FP/obatoclax lethality persisted under these conditions. FP/obatoclax also suppressed growth of MM cells that homed to bone marrow, arguing further that the Cdk inhibitor/pan-BH3 mimetic strategy may circumvent BM microenvironment-mediated drug-resistance. Finally, FP/obatoclax was active against multiple drug-resistant cell lines. Interestingly, bortezomib-resistant cells (PS-R), exhibited marked Bim down-regulation accompanied by modestly increased Mcl-1, supporting a recent concept that Bcl-2 family member dysregulation determines MM cell susceptibility to therapeutic interventions34. Importantly, these cells were also fully susceptible to FP/obatoclax. Collectively, these findings argue that those resistance mechanisms fail to protect MM cells from Cdk inhibitor/pan-BH3 mimetic regimens which coordinately target both pro- and anti-apoptotic Bcl family members.
The marked lethality of FP/obatoclax in primary CD138+ MM specimens, with minimal toxicity toward their normal CD138− counterparts or CD34+ hematopoietic cells, raises the possibility that MM cells may be particularly susceptible to this strategy. Significantly, in marked contrast to obatoclax administrated alone, combined treatment produced marked suppression of tumor growth in vivo in both subcutaneous flank and intravenous orthotopic models. Particularly in the latter, in which MM cells selectively homed to the bone marrow and produced MM-specific bone disease, co-administration of FP/obatoclax markedly improved outcomes for these animals, e.g., significantly preventing hind-leg paralysis and prolonging survival. Such findings argue that whereas pan-BH3-mimetics like obatoclax may not be sufficient by themselves to induce cell death in vitro or in vivo, additional perturbations (e.g., prolongation of anti-apoptotic protein down-regulation in conjunction with up-regulation of proapoptotic BH3-only proteins) induced by pan-Cdk inhibitors may trigger a constellation of events that exceed the apoptotic threshold. In summary, the present findings provide a preclinical justification for combining pan-Cdk inhibitors, possibly including new-generation Cdk inhibitors that have recently entered the clinic13, with pan-BH3-mimetics (e.g., obatoclax)50 in MM and other hematological malignancies. They also highlight the critical importance of coordinate disruption of pro- and anti-apoptotic arms of the apoptotic regulatory machinery in promoting transformed cell death.
Supplementary Material
Acknowledgements
Grant Support This work was supported by awards CA100866, CA 93738, 1 P50 CA142509-01, and 1 P50 CA130805-01 from the National Institutes of Health, award 6181-10 from the Leukemia and Lymphoma Society of America, and awards from the V Foundation and the Multiple Myeloma Research Foundation. Plasmid preparation was performed at the VCU Macromolecule Core Facility, supported, in part, with the funding from NIH-NCI Cancer Center Core Grant 5P30CA016059-29.
Footnotes
Disclosure of Potential Conflicts of Interest: There is no potential conflict of interest to disclose.
Reference List
- 1.Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 2.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkholi R, Floros KV, Chipuk JE. The Role of BH3-Only Proteins in Tumor Cell Development, Signaling, and Treatment. Genes Cancer. 2011;2:523–37. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol.Hematol. 2009;71:89–101. doi: 10.1016/j.critrevonc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Emerging Bcl-2 inhibitors for the treatment of cancer. Expert Opin Emerg Drugs. 2011;16:59–70. doi: 10.1517/14728214.2010.515210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 8.Wuilleme-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19:1248–52. doi: 10.1038/sj.leu.2403784. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, Trichet V, Robillard N, Philippe M, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67:5418–24. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- 10.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104:19512–17. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–38. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 13.Stellrecht CM, Chen LS. Transcription Inhibition as a Therapeutic Target for Cancer. Cancers. 2011;3:4170–90. doi: 10.3390/cancers3044170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–38. [PubMed] [Google Scholar]
- 15.MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65:5399–407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- 16.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–18. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woyach JA, Lozanski G, Ruppert AS, Lozanski A, Blum KA, Jones JA, et al. Outcome of patients with relapsed or refractory chronic lymphocytic leukemia treated with flavopiridol: impact of genetic features. Leukemia. 2012 Jan 13; doi: 10.1038/leu.2011.375. doi:10.1038/leu.2011.375. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holkova B, Perkins EB, Ramakrishnan V, Tombes MB, Shrader E, Talreja N, et al. Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory B-cell neoplasms. Clin.Cancer Res. 2011;17:3388–97. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–69. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM, et al. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. J Biol Chem. 2011;286:11009–20. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, et al. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–95. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–93. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama T, Dass CR, Choong PF. Bim-targeted cancer therapy: a link between drug action and underlying molecular changes. Mol Cancer Ther. 2009;8:3173–80. doi: 10.1158/1535-7163.MCT-09-0685. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Li R, Grandis JR, Johnson DE. Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2008;7:1647–55. doi: 10.1158/1535-7163.MCT-07-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson KC. Oncogenomics to target myeloma in the bone marrow microenvironment. Clin Cancer Res. 2011;17:1225–33. doi: 10.1158/1078-0432.CCR-10-3366. [DOI] [PubMed] [Google Scholar]
- 27.Perez LE, Parquet N, Shain K, Nimmanapalli R, Alsina M, Anasetti C, et al. Bone marrow stroma confers resistance to Apo2 ligand/TRAIL in multiple myeloma in part by regulating c-FLIP. J Immunol. 2008;180:1545–55. doi: 10.4049/jimmunol.180.3.1545. [DOI] [PubMed] [Google Scholar]
- 28.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–82. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 29.Faber A, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, et al. BIM expression in treatment naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1:352–65. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–24. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 32.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–32. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 33.Romagnoli M, Seveno C, Wuilleme-Toumi S, Amiot M, Bataille R, Minvielle S, et al. The imbalance between Survivin and Bim mediates tumour growth and correlates with poor survival in patients with multiple myeloma. Br J Haematol. 2009;145:180–9. doi: 10.1111/j.1365-2141.2009.07608.x. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Bougie P, Bataille R, Amiot M. The imbalance between Bim and Mcl-1 expression controls the survival of human myeloma cells. Eur J Immunol. 2004;34:3156–64. doi: 10.1002/eji.200424981. [DOI] [PubMed] [Google Scholar]
- 35.Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–9. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 36.Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008;111:5152–62. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27:1189–97. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 38.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–25. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pei XY, Dai Y, Grant S. The small-molecule Bcl-2 inhibitor HA14-1 interacts synergistically with flavopiridol to induce mitochondrial injury and apoptosis in human myeloma cells through a free radical-dependent and Jun NH2-terminal kinase-dependent mechanism. Mol Cancer Ther. 2004;3:1513–24. [PubMed] [Google Scholar]
- 40.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–7. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Zhao Y, Liao W, Yang J, Wu L, Zheng Z, et al. Acetylation of FoxO1 activates Bim expression to induce apoptosis in response to histone deacetylase inhibitor depsipeptide treatment. Neoplasia. 2009;11:313–24. doi: 10.1593/neo.81358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon PM, Fisher DE. Role for the proapoptotic factor BIM in mediating imatinib-induced apoptosis in a c-KIT-dependent gastrointestinal stromal tumor cell line. J Biol Chem. 2010;285:14109–14. doi: 10.1074/jbc.M109.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–39. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillings AS, Balmanno K, Wiggins CM, Johnson M, Cook SJ. Apoptosis and autophagy: BIM as a mediator of tumour cell death in response to oncogene-targeted therapeutics. FEBS J. 2009;276:6050–62. doi: 10.1111/j.1742-4658.2009.07329.x. [DOI] [PubMed] [Google Scholar]
- 45.Pritchard JR, Gilbert LA, Meacham CE, Ricks JL, Jiang H, Lauffenburger DA, et al. Bcl-2 family genetic profiling reveals microenvironment-specific determinants of chemotherapeutic response. Cancer Res. 2011;71:5850–8. doi: 10.1158/0008-5472.CAN-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jourdan M, De VJ, Mechti N, Klein B. Regulation of Bcl-2-family proteins in myeloma cells by three myeloma survival factors: interleukin-6, interferon-alpha and insulin-like growth factor 1. Cell Death Differ. 2000;7:1244–52. doi: 10.1038/sj.cdd.4400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang B, Potyagaylo V, Fenton RG. IL-6-independent expression of Mcl-1 in human multiple myeloma. Oncogene. 2003;22:1848–59. doi: 10.1038/sj.onc.1206358. [DOI] [PubMed] [Google Scholar]
- 48.De BE, Bos TJ, Schuit F, Van Valckenborgh E, Menu E, Thorrez L, et al. IGF-1 suppresses Bim expression in multiple myeloma via epigenetic and posttranslational mechanisms. Blood. 2010;115:2430–40. doi: 10.1182/blood-2009-07-232801. [DOI] [PubMed] [Google Scholar]
- 49.Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.