Abstract
Cyclic 3′,5′-adenosine monophosphate and cyclic 3′,5′-guanosine monophosphate are intracellular (second) messengers that are produced from the nucleotide triphosphates by a family of enzymes consisting of adenylyl and guanylyl cyclases. These enzymes are involved in a broad array of signal transduction pathways mediated by the cyclic nucleotide monophosphates and their kinases, which control multiple aspects of cell function through the phosphorylation of protein substrates. Here, we review the findings and working hypotheses on the role of the cyclic nucleotides and their kinases in the control of electrical activity of the endocrine pituitary cells and the plasma membrane channels involved in this process.
Keywords: cAMP, cGMP, protein kinase A, protein kinase G, voltage-gated channels
Introduction
Pituitary cells express several subtypes of adenylyl cyclases (ACs), including the calcium-inhibitable forms of these enzymes (1, 2), which generate cyclic 3′,5′-adenosine monophosphate (cAMP). These cells also express the particulate guanylyl cyclase (pGC) and soluble guanylyl cyclase (sGC), responsible for synthesis of cyclic 3′,5′-guanosine monophosphate (cGMP) (3). AC activity is controlled by numerous hypothalamic and intrapituitary agonists acting on G-protein-coupled receptors (GPCRs). Cyclic nucleotide production is stimulated by Gs-coupled growth hormone-releasing hormone (GHRH), corticotropin-releasing hormone (CRH), vasoactive intestinal polypeptide (VIP)/pituitary adenylate cyclase-activating peptide (PACAP) receptors and others. Conversely, it is inhibited by adenosine, dopamine, endothelin, gamma-aminobutyric acid, melatonin, neuropeptide Y, serotonin and somatostatin receptors (4). Pituitary cells also express several phosphodiesterases (5, 6), and multidrug resistance proteins operate as cyclic nucleotide transporters (7, 8). Cyclic nucleotides play numerous important roles in the control of pituitary cell functions, including control of the exocytotic pathway downstream of the voltage-gated calcium influx (VGCI) (9–11), and the transcriptional pathway (12–15), as well as the mitogenic pathway (16).
Endocrine pituitary cells express numerous voltage-gated Na+, Ca2+, K+, and Cl− channels and several ligand-gated channels. In vitro, they are silent or fire action potentials (APs) spontaneously. Depending on the cell type, this electrical activity can generate localized or global Ca2+ signals, the latter reaching the threshold for stimulus-secretion coupling (4, 17). The excitability of pituitary cells and accompanied voltage-gated calcium influx (VGCI) and hormone release are also influenced by cyclic nucleotides. Gs-coupled GPCRs stimulate electrical activity in pituitary cells, whereas Gi/o-coupled receptors inhibit it (4). Here, we summarise the current knowledge on the role of cyclic nucleotides in the control of pituitary cell excitability and VGCI.
Spontaneous Electrical Activity and Calcium Signalling
In vitro, isolated pituitary cells generate APs independently of external stimuli, a phenomenon termed spontaneous electrical activity. Initially, firing of APs was observed in lactotrophs and GH cells (4). It later became obvious that other secretory pituitary cell types also fire APs: melanotrophs (18), corticotrophs (19–21), somatotrophs (22), gonadotrophs (23), thyrotrophs (24). Firing of APs causes transient elevation in intracellular Ca2+ concentration ([Ca2+]i) and this was well documented in gonadotrophs, lactotrophs, somatotrophs (25) and immortalized pituitary cells (20, 26). However, not all pituitary cells fire APs; for example, fish pituitary cells are quiescent (27). In vivo, pituitary cells are organized as a large-scale network (28, 29). The existence of such network, however, does not argue against the relevance of electrical activity and VGCI in rapid hormone release, but indicates the complexity in coordination of calcium signaling and influence of other factors in this process (30, 31).
Forskolin-stimulated Electrical Activity and Calcium Signalling
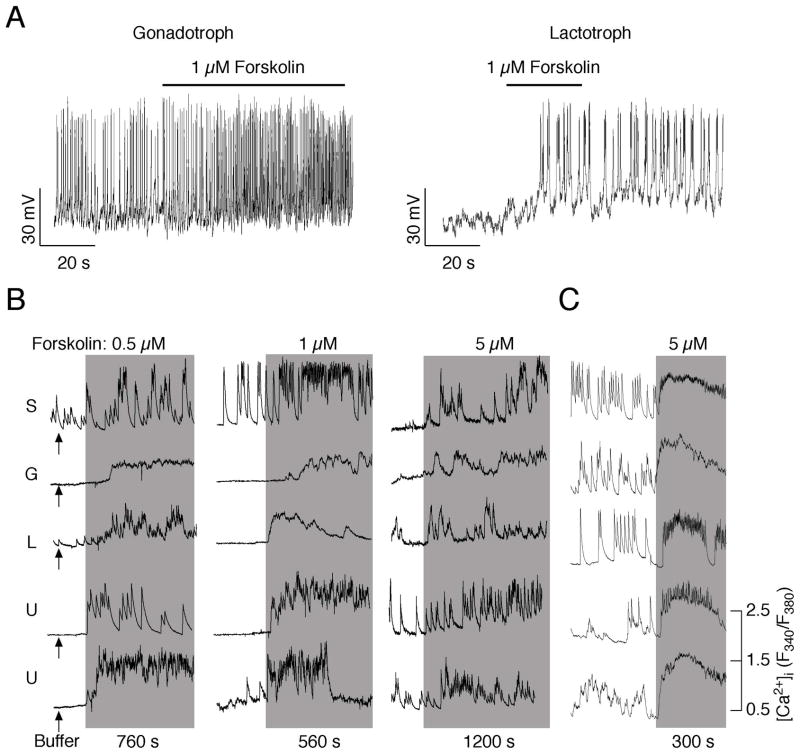
Forskolin is a labdane diterpene found in the roots of the plant Plectranthus barbatus (Coleus forskohlii). It is commonly used to raise levels of cAMP by directly activating ACs. In secretory anterior pituitary cells, forskolin stimulates electrical activity and VGCI in quiescent cells, and it increases the frequency of APs in spontaneously firing cells. Figure 1A shows the effects of forskolin on the electrical activity in gonadotrophs and lactotrophs. The inhibition of phosphodiesterases also increases the electrical activity of somatotrophs (22). The stimulatory effect of forskolin on Ca2+ influx through voltage-gated calcium (Cav) channels was observed in αT3-1 immortalised gonadotrophs (32). Forskolin also increases the Ca2+ influx in somatotrophs (33–35), corticotrophs (36), lactotrophs (2), and gonadotrophs (35). Figure 1B illustrates the stimulatory effect of forskolin on VGCI in lactotrophs, somatotrophs, gonadotrophs and GH3 cells. Thus, the facilitatory role of forskolin on electrical activity and VGCI is common feature of endocrine pituitary cells.
Fig. 1.
Effects of forskolin on the electrical activity and VGCI in pituitary cells. A, Forskolin initiates electrical activity in quiescent cells and increases the frequency of APs in spontaneously firing pituitary cells. B, Forskolin-stimulated calcium transients in cultured pituitary cells. The addition of a medium was used as a control (left panel - arrows). S, somatotrophs; G, gonadotrophs; L, lactotrophs; U, unidentified cells. C, Forskolin-stimulated calcium influx in GH3 cells. Horizontal bars (A) and gray areas (B) indicate the duration of forskolin stimulation. Derived from (2, 35).
Agonist-stimulated Electrical Activity and Calcium Signalling
CRH is the main regulator of ACTH release in normal and immortalised corticotrophs. It acts on CRF-R1 receptors coupled to the Gs signalling pathway, leading to the stimulation of cAMP production (37, 38). One of the main functions of CRH in corticotrophs is to depolarise the plasma membrane and facilitate VGCI influx. Both patterns of electrical activity, including steady depolarisation and depolarisation accompanied with firing of APs, were observed (19–21, 36, 39). The GHRH receptor is expressed in somatotrophs and is coupled to the Gs signalling pathway. In rats, there are two splice forms of this receptor showing similar sensitivity to GHRH, but only the short receptor isoform stimulates cAMP production (40). Like CRH in corticotrophs, GHRH facilitates electrical activity and Ca2+ influx through the L-type Cav channels of the silent or already active somatotrophs (22, 41–47). An increase in electrical activity after GHRH application is also observed in pituitary slices (48). Pituitary cells also express PAC1 and VPAC2 receptors. Gonadotrophs express the PAC1 receptor, linked to the phospholipase C signalling pathway, and somatotrophs, lactotrophs and melanotrophs express VPAC2 receptors, coupled to the Gs signalling pathway (49, 50). In αT3-1 cells, PACAP also stimulates cAMP production and facilitates extracellular Ca2+ influx through dihydropyridine-sensitive Cav channels (51, 52). PACAP also stimulates cAMP production and α-MSH release from melanotrophs and ACTH release from AtT-20 cells (53). In the melanotrophs, PACAP stimulates electrical activity and Ca2+ influx through L-type Cav channels (54). In somatotrophs, PACAP also stimulates the Ca2+ influx through Cav channels (55, 56). Furthermore, PACAP causes extracellular Ca2+ influx in lactotrophs (57). In GH3 cells, VIP evokes a modest VGCI via an increase in cAMP (58), and both VIP and PACAP increase cAMP levels in GH4C1 cells (59).
Role of cAMP and cGMP in Electrical Activity and Calcium Signalling
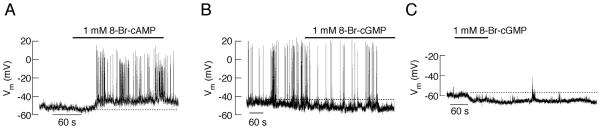
There is consensus that the stimulatory effect of forskolin and Gs-coupled receptors on the electrical activity of secretory anterior pituitary cells is mimicked by the application of cAMP. Figure 2A shows the depolarising effects of 8-bromoadenosine- 3′, 5′-cyclic monophosphate (8-Br-cAMP), a cell-permeable cAMP analogue, in the thyrotrophs. The application of 8-Br-cAMP also facilitates the firing of APs in lactotrophs (2) and gonadotrophs (24). In somatotrophs, the stimulation of GH release by 8-Br-cAMP depends on Ca2+ influx (60, 61). In melanotrophs, 8-Br-cAMP stimulates Ca2+ oscillations that are dependent on Ca2+ influx (62). In corticotrophs, 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphate (8-CTP-cAMP), another cell permeable cAMP analogue, was shown to depolarise the cell membrane and facilitate Ca2+ influx (36).
Fig. 2.
Opposite effects of cAMP and cGMP on the excitability of pituitary thyrotrophs. A, Depolarising effect of cAMP, leading to the initiation of electrical activity in quiescent cells. B and C, Hyperpolarising effects of cGMP in spontaneously active (B) and quiescent (C) thyrotrophs. Derived from (24).
Earlier work with somatotrophs suggested that 8-bromoguanosine- 3′, 5′-cyclic monophosphate (8-Br-cGMP) facilitates GH release (63–65), which could indicate that [Ca2+]i is elevated. Consistent with this hypothesis, the application of dibutyryl cGMP was suggested to stimulate VGCI in GH3 cells (66). However, others observed that dibutyryl-cGMP causes a progressive reduction in the frequency and amplitude of the spontaneous AP-driven [Ca2+]i oscillations in these cells (67). Furthermore, 8-Br-cGMP-stimulated GH release also caused a small reduction in [Ca2+]i (65), suggesting that cGMP stimulates GH release in a Ca2+-independent manner. The application of nitric oxide (NO) donors to GH3 cells also causes an inhibition of VGCI (68).
Recently, we systematically investigated the effects of 8-Br-cGMP on electrical activity in a primary culture of pituitary cells (24). These experiments revealed a consistent inhibitory effect of 8-Br-cGMP on electrical activity in all endocrine cell types. Figs. 2B and C illustrate the hyperpolarising action of 8-Br-cGMP, which slows the AP frequency in the spontaneously firing thyrotrophs and hyperpolarises the cell membrane in both firing and quiescent cells. These observations are consistent with the inhibitory effect of the NO signalling pathway on hormone release by cultured pituitary cells (69–71). Thus, it is reasonable to conclude that cAMP and cGMP have opposite effects on the excitability of pituitary cells and the accompanied VGCI; specifically, cGMP hyperpolarises the cell membrane, whereas cAMP depolarises cell membranes.
The cyclic nucleotides can facilitate electrical activity and VGCI directly by activating cyclic nucleotide-gated (CNG) and/or hyperpolarization-activated cyclic nucleotide-regulated (HCN) channels (72), or indirectly through their kinases that phosphorylate several channels, including the inwardly rectifying K+ (Kir), Cav, voltage-gated Na+ (Nav) and non-selective cation channels (73). The cyclic nucleotides could also inhibit the electrical activity and Ca2+ influx by activating Ca2+-controlled K+ channels (74). In the following sections, we will discuss in detail the direct and indirect effects of cyclic nucleotides on the plasma membrane channel functions in the endocrine pituitary cells.
Cyclic Nucleotide-gated Channels
In vertebrates, there are six CNG subunits, termed CNGA1, CNGA2, CNGA3, CNGA4, CNGB1, and CNGB3, and several splicing forms of primary transcripts have been identified. The CNGA1-3 subunits can form homomeric channels in the heterologous expression systems, and other subunits can co-assemble to form functional heteromeric channels. These channels are expressed in olfactory neurons and the outer segments of rod and cone photoreceptors, where they play a critical role in sensory transduction. Photoreceptors have a strong preference for cGMP, whereas the olfactory channel is almost equally sensitive to both ligands. The channels are permeable to Na+, K+, and Ca2+, but not to Cl− or other anions. The low levels of mRNA transcripts for these channels are also found in the brain, testes, kidneys, and heart (72). The mRNA transcripts for the rod CNG were also detected in rat pituitary cells by RT-PCR analysis (41) and RNA blot hybridisation (75). The zebrafish-specific CNGA5 mRNA and protein transcripts are also expressed in the pituitary cells (76). Further studies are required to clarify their expression at the protein level and their potential role in electrical activity and Ca2+ signalling in pituitary cells.
Hyperpolarisation-activated Cyclic Nucleotide-regulated Channels
Structure and function
In mammals, the HCN channel family is comprised of four subunit isoforms, encoded by the four genes, HCN1-4. Each subunit forms functional homomeric channels, which are tetramers; however, the native channels are probably organised as heterotetramers. The HCN channels are activated by hyperpolarisation beyond −60 mV, thereby generating a current termed Ih (h stands for hyperpolarisation), which does not inactivate, and these channels conduct both sodium and potassium. In the cells expressing these channels, their activation leads to a slow depolarisation, which is an action consistent with their equilibrium potential of about −30 mV. Their voltage-sensitivity is modulated by cAMP. HCN channels serve the following three principal functions in excitable cells: i) they contribute to the resting potential, ii) they generate or contribute to the pacemaker depolarisation that controls the rhythmic activity in spontaneously firing cells, and iii) they compensate for inhibitory postsynaptic potentials. A small fraction of HCN channels are tonically activated at rest, and this subset produces the former two functions of these channels (77).
Expression in pituitary cells
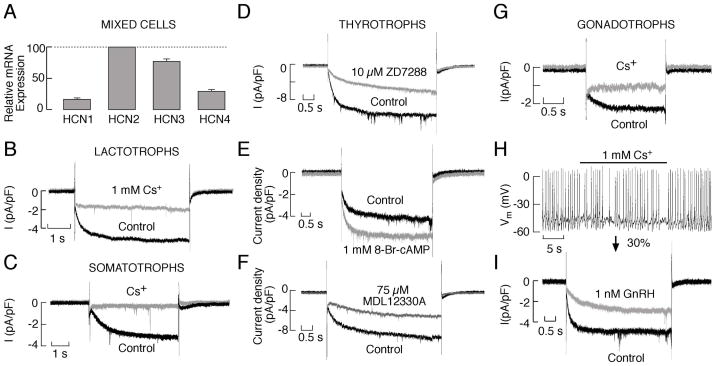
HCN channels were first identified in cardiac sinoatrial node cells, and subsequently they have been found in a variety of peripheral and central neurons. Qualitative RT-PCR analysis suggests that AtT-20 cells express mRNA transcripts for HCN1 (78). GH3 cells express the mRNA transcripts for HCN2, HCN3, and HCN4, but not for HCN1 (79). Quantitative RT-PCR analysis showed higher levels of expression of mRNA transcripts for HCN2 and HCN3 subunits and lower expression of HCN1 and HCN4 subunits in rat anterior pituitary cells (Fig. 3A). Electrophysiological experiments confirmed the presence of a hyperpolarisation-activated current in GH3 cells (79, 80), AtT-20 cells (78), melanotrophs (81), somatotrophs (80), lactotrophs (82), thyrotrophs, and gonadotrophs (24). The biophysical properties of this current (including activation of this current by hyperpolarising voltages, the time course of activation, a lack of inactivation during sustained recording, a rapid deactivation, and the activation curve obtained by tail current analysis) are consistent with the expression of the Ih current in pituitary cells. The pharmacological profile of this current (e.g., its reversible inhibition by 1 mM Cs+, the low and irreversible inhibition by ZD7288, and the sensitivity to tramadol, a synthetic analgesic used to relieve pain) also supports the functional expression of HCN channels in endocrine pituitary cells (24, 78–80, 83). Figure 3 shows the inhibition of the Ih current by 1 mM Cs+ in lactotrophs (B), somatotrophs (C), and gonadotrophs (G), and with 10 μM ZD7288 in thyrotrophs (D). Although the current density was relatively low in both normal and immortalised pituitary cells, such a current may still profoundly influence the resting membrane potential, because the input resistance of these cells is usually > 5 GΩ. In gonadotrophs, the voltage response to a hyperpolarising current pulse showed a slowly developing inward rectification, which was blocked by extracellular Cs+ and was absent in cells lacking Ih. This could indicate that Ih currents in pituitary cells contribute to the control of the resting membrane potential in the range where other channels can act as pacemakers (24). Ih may also limit the excessive hyperpolarisation in response to hyperpolarising stimuli (78–80). Furthermore, we observed a decrease in the AP frequency in the spontaneously firing gonadotrophs with inhibited Ih, indicating that these channels contribute to the pacemaking (Fig. 3H) in a manner comparable to that observed in the gonadotropin-releasing hormone (GnRH) neurons (84). The abolition of spontaneous electrical activity was not observed in any of these experiments, further indicating the role of other channels in slow depolarisation.
Fig. 3.
Expression of HCN channels in pituitary cells. A, Quantitative RT-PCR analysis of the HCN mRNA transcript expression in cultured anterior pituitary cells. B and C, Electrophysiological and pharmacological characterisation of HCN channels in lactotrophs (B) and somatotrophs (C). D – F, Characterisation of the Ih current in rat pituitary thyrotrophs. D) Inhibition of the Ih current by ZD7288. E, Stimulation of Ih by 8-Br-cAMP. F, Inhibition of Ih by MDL12230A, an AC inhibitor. G–I, Properties of the HCN channels in pituitary gonadotrophs. G) Blockade of the Ih current by the addition of 1 mM Cs+ to the extracellular solution. H, Effects of Ih blockade on the frequency of spontaneous firing of APs in gonadotrophs. I, Inhibition of Ih by GnRH. In B, C, D, E, F, G and I, representative traces of the whole-cell current response to a hyperpolarizing voltage step to −120 mV from a holding potential of −40 mV are shown. Derived from (24).
cAMP-dependent facilitation
The HCN channels in AtT-20 pituitary cells are weakly modulated by forskolin (78). The cultured anterior pituitary cells also exhibit a weak facilitation by forskolin and 8-Br-cAMP (24). Figure 3DE shows a current facilitation by 8-Br-cAMP, in thyrotrophs. In GH3 cells, the forskolin-induced changes in the properties of the Ih current were not observed (79, 80). However, in both normal and immortalised GH3 pituitary cells, the inhibition of the basal cAMP production significantly attenuated the Ih current, which fully recovered with the application of 8-Br-cAMP (24, 79). Figure 3F illustrates the effects of MDL12330A, an inhibitor of ACs, on the amplitude of the Ih current in thyrotrophs. In AtT-20 cells, the current is also robustly inhibited by a cAMP antagonist (78). These results suggest that, in pituitary cells, in vitro HCN channels are tonically activated by the basal AC activity. Because basal cAMP production is down-regulated in vivo by several hypothalamic and intrapituitary factors, including dopamine and somatostatin (4), this suggests that the facilitation of AC activity, under physiological conditions, could lead to the activation of HCN channels and the firing of APs This hypothesis should be addressed in the future. The activity of these channels in pituitary cells is not only determined by the status of AC activity, but it is also affected by GPCR signalling through the phospholipase C pathway. As documented for the GnRH receptor, this family of receptors inhibits HCN channels (Fig. 3I), thereby reflecting the depletion of phosphatidylinositol 4,5 bisphosphate (24), which is required for normal HCN function (85, 86).
PKA/PKG-dependent effects of cyclic nucleotides
Pharmacological approaches
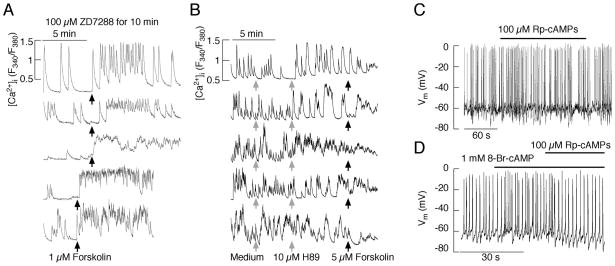
To identify the indirect effects of cAMP and cGMP on electrical activity, cAMP-dependent protein kinase (PKA) and cGMP-dependent protein kinase (PKG) inhibitors are frequently used. Among the PKA inhibitors, the most commonly used is N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesuslvonamide dihydrochloride (H-89), which inhibits both types of PKA (I and II). In vitro, the residual PKA activity is about 2% of baseline levels in the presence of 10 μM H-89. It is important to emphasise that this compound also inhibits S6K1 (100%), ROCK-II (100%), MSK1 (97%), PKBa (83%), AMPK (81%), CHK1 (79%), SGK (75%), and PHK (49%) (87). The 8-Br-cAMP-induced Ca2+ oscillations in melanotrophs that are dependent on Ca2+ influx are abolished by H-89 (62). The forskolin-stimulated Ca2+ influx in gonadotrophs, lactotrophs, somatotrophs and αT3-1 gonadotrophs is also inhibited by 10 μM H-89 (32, 35); these observations are consistent with earlier studies with somatotrophs (42, 88). Figure 4B shows the lack of response to a forskolin application in pituitary cells bathed in a medium containing 10 μM H-89. The CRH and 8-CPT-cAMP-induced Ca2+ influx in corticotrophs is blocked by adenosine cyclic 3′,5′-(Rp)-phosphothioate (Rp-cAMPS), another PKA inhibitor (36). However, some effects of CRH were resistant to the PKA inhibitor H-89, raising the possibility that CRH might act through an additional G-protein pathway (39, 89). Our experiments with Rp-cAMPs also revealed that spontaneous electrical activity in the pituitary cells was not affected by the inhibition of PKA, but that the 8-Br-cAMP-stimulated increase in the frequency of APs was abolished in the presence of this compound (Fig. 4C). In melanotrophs, PACAP stimulates a Ca2+ influx through L-type Cav channels, which is inhibited by a blockade of PKA (54). In somatotrophs, the PACAP-stimulated Ca2+ influx through Cav channels is also blocked by the PKA antagonists (90).
Fig. 4.
Effects of HCN channel and PKA inhibitors on the forskolin-stimulated calcium influx in anterior pituitary cells. A, The stimulation of Ca2+ influx by forskolin in cells with the HCN channels, inhibited by pretreatment with ZD7288, which was applied for 10 minutes prior to recording. B, The lack of an effect of forskolin on Ca2+ influx after treatment of cells with 10 μM H89, a PKA inhibitor. Notice the change in the pattern of calcium signalling after addition of H89 (two top traces). C and D, PKA inhibitor Rp-cAMPs does not alter the pattern of spontaneous firing of APs (C), but it does abolish the 8-Br-cAMP-induced increase in the firing frequency (D). Derived from (35).
PKA animal models
To more directly investigate the role of PKA in the regulation of these channels, we recently used the following two mouse models with altered PKA signalling: Prkar1a+/− and Prkar1a+/− Prkaca+/− (91, 92). We expected that Prkar1a haploinsufficiency would be accompanied by an elevated PKA activity in the pituitary cells, because the down-regulation of the regulatory subunit type 1A leads to endocrine and other tumours (93). This, in turn, should induce a loss of forskolin’s stimulatory action on electrical activity and Ca2+ signalling. In contrast, we expected that the basal and stimulated PKA activity be normalised in cells from Prkar1a+/−Prkaca+/− mice, and that forskolin’s stimulatory action on electrical activity and Ca2+ influx would not be affected. Consistent with these predictions, we found that basal and forskolin-stimulated cAMP production is significantly reduced in pituitary cells from Prkar1a+/− mice. We further showed that these cells expressed AC5/6, whose phosphorylation, by PKA, causes an inhibition of the enzyme activity. This observation indirectly suggests that Prkar1a haploinsufficiency is accompanied by an elevated PKA activity; this hypothesis was confirmed by the direct measurement of PKA activity. The Prkar1a haploinsufficiency also caused the loss of stimulatory action of the forskolin on Ca2+ influx. In contrast to Prkar1a+/− mice, basal and stimulated PKA activity was normalised in cells from Prkar1a+/− Prkaca+/− mice, and the stimulatory action of forskolin on Ca2+ influx was restored (35).
Calcium-controlled Potassium Channels as Effectors for cGMP
Structure and function
Calcium-controlled K+ channels (KCa) are composed of two families. One family of these channels includes three small-conductance (SK) channels (KCa2.1, 2.2 and 2.3) and one intermediate-conductance (IK) channel (KCa3.1) (94, 95). The high-conductance K+ (BK) channels represent the other family of KCa channels, only distantly related to SK and IK channels. The BK channels are composed of four pore-forming KCa1.1 (Slo1) α subunits that share the same six transmembrane topology of the voltage-gated K+ channels; however, they also contain an additional transmembrane segment at the N-terminus. In contrast to the small-conductance channels, the BK channels form macromolecular complexes with Cav channels and establish a prototypic Ca2+ nanodomain. This provides an effective mechanism for the control of the activity of these channels by Ca2+ influx through Cav channels. The co-localisation of BK and Cav channels thereby facilitates the spike repolarisation and influences the frequency of AP-driven [Ca2+]i transients by slowing the pacemaker depolarisation. Both actions result in the reduction of VGCI. The activation of these channels may also remove the steady inactivation of Nav and Cav channels, which may stimulate or enhance AP generation in some cells. BK channels may also play a role in the generation of the bursting type of electrical activity in pituitary cells (96, 97).
Expression in pituitary cells
Whole-cell current recordings showed the presence of the BK current in normal somatotrophs and lactotrophs (98), as well as in the GH3 and AtT-20 pituitary cell lines (99–102). The activation of PKC inhibits these channels, which could account for the sustained excitability of the pituitary cells during the prolonged activation of Gq/11-coupled GPCRs (103). Single channel recordings also showed that the BK channels are expressed in melanotrophs (104) and lactotrophs (105). The mslo transcripts that encode the pore-forming α-subunit of the BK channels are robustly expressed in the AtT-20 cells, and the native channels are not functionally coupled to the β-subunits (106).
PKG-dependent facilitation
In numerous cell types, it is well established that cGMP activates the BK channels (107–111). The α subunit of BK channels is directly phosphorylated by PKG, causing the activation of these channels (74). Multiple PKG-dependent serine phosphorylation sites have been identified (112, 113). In the posterior pituitary, the NO enhances KCa activity by stimulating sGC and PKG (114), whereas the role of the cGMP-PKG signalling pathway in BK channel activation in the endocrine anterior pituitary cells has not been studied.
Inwardly Rectifying Potassium Channels
Structure and function
The term “inward rectifier” describes the activation of inward current under hyperpolarisation, leading to K+ influx, with almost no K+ efflux under depolarisation. Because of these unusual activation properties, these channels are also known as anomalous rectifiers. Although the amplitude of the outward currents following through these channels is limited, they profoundly influence the resting membrane potential. Among voltage-gated channels, the structures of Kir channels and bacterial K+ channels, known as KcsA, are the most simple. The α subunit of these channels is a tetramer, each monomer of which contains two transmembrane segments, connected with a re-entry P loop. The Kir channels are expressed in numerous tissues, including the brain, heart, kidney, endocrine cells, ears, and retina. They participate in the control of the resting potentials and are closed by strong depolarisations. There are 15 members of this family of channels that are divided into three groups, based on their regulation patterns. Some of these channels are constitutively active at rest, leading to K+ leaking from cells, whereas the activity of others is influenced by certain modulators, such as G proteins and nucleotides. The long cytoplasmic pore of these channels plays a critical role for inward rectification, and it provides the structural basis for the modulation of gating by G proteins and phosphatidylinositol 4,5 bisphosphate (115).
Expression and role in pituitary cells
RT-PCR analysis showed the presence of Kir 3.1, 3.2, and 3.4 mRNA transcripts in the rat pituitary cells (116), and Kir3.1–3.4 mRNA transcripts in GH3/B6 mammosomatotrophs (117). The presence of Kir3.1 and 3.2 proteins in AtT-20 cells was also confirmed by western blot analysis (118). RT-PCR analysis also revealed the presence of the Kir 1.1, 2.2, 4.1, 6.1, and 6.2 mRNA transcripts in the GH3/B6 cells (117). Electrophysiological studies confirmed the functional expression of G protein-regulated Kir channels in rat pituitary lactotrophs, where they are activated by dopamine (119) and endothelin (120). In somatotrophs, they are activated by somatostatin (121) and ETs (122), whereas the AtT-20 corticotrophs are activated by somatostatin and muscarinic receptors (118, 123–125). The G protein-dependent activation of the Kir currents in AtT-20 cells and human GH-secreting adenoma cells was demonstrated by the down regulation of their expression by antisense oligonucleotides (126, 127). In the GH3 cells, the constitutively active Kir channels were also identified and found to play an important role in the maintenance of the resting membrane potential (128, 129). The presence of the ATP-sensitive K+ channels in the pituitary cells has also been reported (130).
PKA-dependent inhibition
A key element in the control of spontaneous firing of APs in the pituitary cells, including corticotrophs, is the control of the resting membrane potential and slow membrane depolarisation, called the pacemaking depolarisation. The CRH changes the resting membrane potential and the rate of the slow depolarisation, leading to an increase in the firing rate of spontaneously active cells and causing silent cells to become active (19, 20, 39). The slow membrane depolarisation is caused, in large part, by a reduction in a background K+ conductance, mediated by a member of the Kir channel family (36, 131). The inhibition of Kir, including its associated depolarisation and increases in spike frequency, lasts up to 15 minutes after the removal of CRH from the physiological solution. This relationship suggests that phosphorylation of Kir channels could account for this memory (131). These effects of CRH and their time courses are mimicked by the application of forskolin and by membrane-permeable analogues of cAMP (36, 131). No CRH-induced depolarisation is observed in the presence of intracellular Rp-cAMPS, a blocker of PKA (36), thereby confirming that the effects of CRH on the pacemaking depolarisation are mediated through the cAMP activation of PKA. However, some effects of CRH were resistant to the PKA inhibitor H-89, raising the possibility that the CRH might act through an additional G-protein pathway (39, 89). It is possible that the GHRH decreases the intrinsic activity of a Kir channel in somatotrophs, just as CRF does in corticotrophs. This was shown in experiments that blocked these channels in spontaneously firing cells and model simulations (22). In ovine somatotrophs, GH-releasing peptide-2 reduces the Kir current via the PKA-dependent signalling pathway (132). Kir channels are also inhibited by the activation of the thyrotropin-releasing hormone (TRH) receptor, presumably through their cross-coupling to the Gs signalling pathway (133). The TRH also inhibits Kir channels in lactotrophs from lactating rats (134).
The K2P Channel TREK-1
Depolarisation alone is not sufficient to induce spiking in quiescent cell (39), and the firing frequency of cells depolarised by CRH is higher than that for corticotrophs depolarised to the same voltage level by blocking the Kir current. This indicates that a component of the depolarisation might be mediated by the reduction of another type of background K+ conductance, or by the facilitation of an inward current (131). Among the K+ conducting channels, two members of the two pore-forming domain channels, TASK-1 (KCNK3) and TASK-3 (KCNK9), are constitutively open at rest, whereas TREK-1 (KCNK2) requires physical and chemical stimulation to open (135). At the present time, there are no pharmacological agents available that are selective for these channels (136). Based on the sensitivity of a background K+ current on fluoxetine, chlorpromazine, extracellular acidification, and arachidonic acid application, it has been recently suggested that TREK-1 channels are important in setting the resting potential in mouse corticotrophs. Furthermore, both CRH and 8-CTP-cAMP inhibited this current (137). In the presence of fluoxetine, the CRH-induced depolarisation was significantly reduced, but not abolished. This finding could be explained by an incomplete inhibition of TREK-1 channels, or the contribution of Kir channels in CRH action. Further work should be focused on the molecular identification of TREK-1 channels in corticotrophs and other pituitary cells, and on a clarification of the role of PKA in the action of cAMP on activity of these channels.
Voltage-gated Calcium Channels
Structure and function
Electrophysiologically, Cav channels are separated into two groups. The first group of channels is known as the high-voltage activated Cav channels, because these channels require moderate to strong membrane depolarisations to open. Among this group, biophysical and pharmacological studies have identified the L-, N-, P/Q-, and R-type Ca2+ channels, which are distinguished by their single-channel conductance, pharmacology, and metabolic regulation. The second group is known as the low-voltage activated Cav channels, because they require less depolarisation for their activation and their subsequent inactivation, as compared to the high-voltage activated channels. Furthermore, a strong membrane hyperpolarisation is required to bring them out of their steady inactivation. Because of their gating properties, these channels are often referred to as the transient or T-type Cav channels. The purification of Ca2+ channels has identified the following five subunits: a pore forming large α1 subunit, and four smaller ancillary subunits, including α2, β, γ, and δ. In addition to the voltage sensor, the gating machinery, and the channel pore, the α1 subunit also contains most of the known sites of channel regulation provided by the intracellular messengers, drugs, and toxins. These sites include Gβγ domains and multiple PKA phosphorylation sites (138). The Cav channels serve two major functions in cells: electrogenic and regulatory. The major function of the T-type Cav channels is electrogenic; at the resting potential, these channels depolarise cells to the threshold level for a Na+ or Ca2+ spike. In contrast, the high-voltage activated channels will inactivate incompletely and help to keep the cells depolarised for a prolonged period. In some neurons and many neuroendocrine cells, these channels give rise to APs in the same way as Nav channels, although typically with slower kinetics and lower amplitudes. In other neurons, Cav channels shape the Na+-dependent APs. Such APs increase in [Ca2+]i of a sufficient amplitude to trigger the Ca2+-dependent processes (138, 139). In cells that are not firing AP, depolarisation also facilitates Ca2+ influx through Cav channels. The regulatory function of these channels is based on the Ca2+ influx during the transient depolarisation, which acts as an intracellular messenger, controlling a variety of cellular functions.
Expression in pituitary cells
Progress has been made in the identification of Cav-α subunit transcripts that are present in pituitary cells. The Cav3.1 and Cav3.3 mRNAs were detected in GH3 cells exhibiting a prominent T-type Ca2+ current (140). Several pore forming subunits of Cav channels are also present in GH3/B6 pituitary cells, and these subunits account for the formation of T-type (Cav3.1), L-type (Cav1.1, 1.2, and 1.3), and P/Q (Cav2.1) type currents. The mRNA transcripts for the β1, β2, and β3 Cav subunits were also detected in these cells (141). Immunocytochemical analyses confirmed the expression of the Cav1.2, 1.3, 2.2, and 3.1-α subunits in mouse anterior pituitary cells (142). The functional expression of both the inactivating and non-inactivating Cav currents is well documented in rat (98, 143–145), ovine (146) , and fish (147) gonadotrophs, as well as in genetically-labelled mouse gonadotrophs (148) and αT-3 immortalised mouse gonadotrophs (149). These currents are also present in the rat somatotrophs and lactotrophs (98, 150), fish somatotrophs (151), frog melanotrophs (152), and in the immortalized GH cells (141, 153, 154). In the GH3 cells, there are multiple conductance levels of the L-type Cav channels (155), and estrogens stimulate the expression of these T-type channels (156). The properties of the inactivating Cav channels are consistent with the expression of the T-type Ca2+ channels. In contrast, the non-inactivating Ca2+ current in the pituitary cells is mediated by the dihydropyridine-sensitive and -insensitive Cav channels (144, 145, 153, 157).
Role in pituitary cells
Cav channels in pituitary cells not only give rise to APs in the same way as Nav channels, but they also provide an effective pathway for Ca2+ influx during transient depolarisations, called VGCI. The patterns of the spontaneous electrical activity in different cell types (e.g., single spiking vs. plateau bursting) largely impact intracellular Ca2+ dynamics and overall Ca2+ levels. In single spiking gonadotrophs, the bulk Ca2+ levels are 20 nM to 70 nM, whereas in spontaneously bursting lactotrophs and somatotrophs, the levels are much higher, are variable between 300 nM to 1.2 μM, and are clearly oscillatory (22, 25). Corticotrophs (20) and immortalised pituitary cells (26, 128, 153) also exhibit plateau bursting type electrical activity and high amplitude Ca2+ transients. Both types of APs depolarise the membrane sufficiently to activate the various types of Cav channels that are expressed in pituitary cells. However, the majority of the Ca2+ influx occurs through dihydropyridine-sensitive L-type Cav channels (98, 157). In cells not exhibiting firing of APs, a plateau depolarisation also results in Ca2+ entry via Cav channels, but in a non-oscillatory manner (36). The T-type channels may also contribute to the slow depolarisation between spikes (158).
PKA-dependent facilitation
In somatotrophs, GHRH depolarises the plasma membrane and facilitates Ca2+ influx through Cav channels (42, 159). GHRH was also suggested to increase the L- and T-type Ca2+ conductance in rat and ovine somatotrophs (42, 160). The L- and T-type Cav channel currents are also expressed in the human GH-secreting pituitary adenomas, and both GHRH and 8-Br-cAMP increase the amplitude of both currents. The stimulatory action of GHRH on the L- and T-type currents is abolished in the cells with inhibited PKA, indicating a role of PKA in this action (161). The synthesised GH releasing peptide, GH releasing peptide-2, also depolarises the plasma membrane of the ovine somatotrophs, and this agonist enhances the L- and T-type Cav conductance (162). Other synthesised GH releasing peptides also depolarise the somatotrophic membrane (163). In corticotrophs, the Cav channels can be activated with a sufficiently strong pacemaking depolarisation, and their opening produces the upstroke of the AP spike and a robust Ca2+ influx. It appears that the main channel involved is the L-type Cav channel, but P-type Cav channels also play a role in the regulation of spike frequency. Indeed, another high-voltage activated and toxin-resistant Cav channel may do so as well (19). In these cells, the rapid PKA-mediated phosphorylation of L-type Cav channels has not been studied and should not be excluded. Corticotrophs also express T-type Cav channels, but their role in CRH action has also not been studied. These results are consistent with the finding that the L-type Ca2+ channels are rapidly stimulated by the PKA-dependent phosphorylation of their α subunits (138). Because PKA does not phosphorylate the T-type channel (139), the indirect action of PKA probably accounts for effects on those channels. Furthermore, cAMP stimulates the expression of the L-type Cav channels in AtT-20 cells at the mRNA and protein levels, presumably through PKA (164). Ghrelin and GH releasing peptide-6 also upregulate L-type Cav channels after a long-term exposure in GH cell lines (165), indicating a role of cAMP/PKA in the transcriptional regulation of Cav channels.
Voltage-gated Sodium Channels
Structure and function
Mammals express nine genes for the Na+ channel α subunit, termed Nav1.1-Nav1.9, and the closely related Na+ channel-like proteins (Nax) share approximately 50% of their structural similarity with Nav1 channels. The α subunit contains four homologous domains, each consisting of a six transmembrane domain and a re-entry P loop between 5S and 6S. The P loop contains the tetrodotoxin (TTX) binding site, a voltage gate and sensor, and the sites for phosphorylation by the protein kinases on the intracellular surface. Nav 1.5, 1.8 and 1.9 are insensitive to TTX in a nanomolar concentration range. Four auxiliary subunits have been identified so far, termed NaVβ1, NaVβ2, NaVβ3, and NaVβ4. They belong to a single family of proteins, which interact with different α subunits and alter their physiological properties. The main function of Nav channels is to depolarise cells and to generate the upstroke of the AP, thereby controlling the firing amplitude in excitable cells, including nerve, muscle, and neuroendocrine cell types. In some cells, these channels are solely responsible for the rapid and regenerative upstroke of an AP. In others, they act in conjunction with Cav channels to depolarise cells. The Nav channels are also expressed in non-excitable cells at a lower level, where their physiological role is unclear (166).
Expression and role in pituitary cells
The expression of TTX-sensitive and -insensitive Nav channels has been extensively studied in endocrine pituitary cells. Rat melanotrophs express the mRNA transcripts of seven α subunits, including the TTX-insensitive Nav1.8 and 1.9 subunit mRNAs, and the β1 and β2 auxiliary subunit mRNAs (167). The mRNA transcripts for the α-subunit of Nav1.1, Nav1.2, Nav1.3, and Nav1.6, as well as the β1 and β3-subunits of Na+ channels, are present in GH3 cells (168). The expression of the Nav1.7-α subunit in the rat anterior pituitary was confirmed by in situ hybridisation and immunohistochemistry (169). Somatotrophs from GH-green fluorescent protein transgenic mice express mRNA transcripts for Nav1.5, 1.8, and 1.9, as well as the TTX-sensitive and TTX-resistant Na+ currents (170). Electrophysiological experiments revealed that both freshly dispersed and cultured rat melanotrophs express functional channels that are composed of TTX-sensitive and TTX-resistant components (167, 171). These channels are also identified in goldfish somatotrophs (151) and Xenopus laevis melanotrophs (172, 173). The single cell Ca2+ measurements further indicated the presence of functional Nav channels in frog melanotrophs (172, 173). The TTX-sensitive current has also been identified in rat (98, 143, 145), mouse (174), ovine (175, 176) and fish (147, 177) gonadotrophs, as well as in αT3-1 mouse gonadotrophs (149, 178). Rat lactotrophs (179, 180), somatotrophs (98), corticotrophs (143), and GH3 cells (181), as well as fish lactotrophs (182), also express Nav channels. In a fraction of ovine gonadotrophs (175) and bovine lactotrophs (183), the Nav channels are also responsible for AP generation. TTX-sensitive Nav channels may contribute to the firing of APs and the accompanied VGCI in frog and rat melanotrophs (172). However, in the majority of rat anterior pituitary cells in vitro, these channels do not contribute to the spike depolarisation because they are inactivated at the resting membrane potential. In hyperpolarised cells, which is tonically maintained in vivo by a GPCR coupled to the Gi/o signalling pathway, Nav channels could play an important role in the transition from the quiescent to the firing modes (4).
PKA-dependent facilitation
Several investigations have suggested that the Na+-conducting channels are responsible for the facilitation of electrical activity and Ca2+ influx in the pituitary somatotrophs, by GHRH receptors. Kato and colleagues showed that GHRH-stimulated hormone secretion by somatotrophs was suppressed in cells bathed in a Na+-deficient medium (88). The same group also showed that GHRH depolarised somatotrophs, which was greatly suppressed by the removal of the bath Na+ (184). Others also showed the dependence of GHRH-induced Ca2+ influx on the depolarising Na+ conductance (42, 44). It has been reported that GHRH increases the total Nav current in somatotrophs, which was blocked by low levels of TTX (185). Others reported that GHRH facilitates a TTX-insensitive Nav current in cultured somatotrophs from GH-green fluorescent protein transgenic mice, but in a cAMP-independent and PKC-dependent manner (170). The relevance of Na+ conductance in the action of VIP-PACAP has also been suggested (186).
Non-selective Cation Channels
Contribution to the resting membrane potential
The resting membrane potential of −50 to −60 mV in the pituitary cells suggests that, in addition to the leaking K+ conductance, there is also a depolarising conductance due to other ions. The resting membrane potential rapidly reaches approximately −85 mV when extracellular Na+, but not Ca2+, is removed. This value is close to the equilibrium potential for K+, suggesting that a Na+-conducting channel has a constitutive activity, e.g. the Na+-conducting channel acts as a leak channel. Regarding the inhibition of all Nav channels, the addition of TTX in micromolar concentrations does not mimic the effect of the removal of bath Na+ on the resting membrane potential. This leak current is termed the background Na+ current (8, 21, 153, 180). Two other reports have suggested the presence of Ca2+-activated, non-selective cationic currents in GH3 cells (187) and gonadotrophs (188).
Structure and function
The nature of these channels is unclear at the present time. In general, the majority of transient receptor potential (TRP) channels are relatively non-selective cation-conducting channels. These channels were initially found in Drosophila, in which they contribute to phototransduction. Six protein families comprise the mammalian TRP superfamily: the “canonical” receptors (TRPCs), the vanilloid receptors (TRPVs), the melastatin receptors (TRPMs), the polysistins (TRPPs), the mucolpins (TRPMLs), and the ankyril transmembrane protein 1 (TRPA1). These channels resemble Kv channels in their overall structures. However, they show a limited conservation of the S4 positive charges and P loop sequences. The assembly of the channel subunits as homo- and heterotetramers results in the formation of cation-selective channels (189). The TRPM3 channel mRNA transcripts are present in pituitary cells (190), as well as in unidentified member(s) of the TRPC family of channels that are activated by phospholipase C (191). The mRNA transcripts for TRPC1, TRPC3, TRPC5, and TRPC7 have also been identified in human pituitary cells (192). Our quantitative RT-PCR analysis revealed that the mRNA transcripts for TRPC channels are expressed in the anterior pituitary cells in the following order: TRPC1 ≫ TRPC6 > TRPC4 > TRPC5 > TRPC3. Our pharmacological studies further indicate that the cation-conducting TRPC channels are good candidates for the background Na+ conductance; spontaneous electrical activity was abolished by the application of three blockers of these channels, including SKF96365, 2-APB, and Gd3+ (35).
PKA-dependent facilitation
The relevance of cation channels in the GHRH action observed in human GH-secreting adenoma cells was originally introduced by Takei et al (161). A subsequent study by the same group revealed that hGHRH stimulates Na+ conductance, with the reversal potential of −20 to 0 mV. The channel was also permeable to Li+ and K+, but not to TMA+. A similar nonselective cation current was activated by 8-Br-cAMP and forskolin, and the activation of the hGHRH-induced current was inhibited by PKA inhibitors, including (R)-p-adenosine 3′,5′-cyclic monophosphate, H-89, and PKA inhibitor peptide PKI-(5–24). These findings indicate that the hGHRH-induced current was activated by PKA. A cholera toxin pretreatment eliminated the hGHRH-induced current, suggesting that G is involved in the activation of this current (193). Our recent study indicated that forskolin was unable to facilitate Ca2+ influx in cells bathed in a medium containing SKF96365 and 2-APB, which are blockers of the TRPC channels. A high level of extracellular Mg2+, achieved with a blocker of variable channels, including the non-selective cationic channels (194), also inhibits the cAMP-dependent current in pituitary cells. We also observed that forskolin, 8-Br-cAMP, and GHRH stimulated an inward non-selective current, with larger amplitude at more negative potentials. The reversal potential of this current is consistent with the operation of a non-selective cation channel. The channel was inhibited by H89, further suggesting a role for PKA in the activation of these channels. Finally, in cells bathed in a medium containing NMDG+ instead of Na+, forskolin was unable to stimulate Ca2+ influx. This result indicates that the other cations present in the bath medium were unable to fully substitute Na+ in re-establishing the resting membrane potential, and in evoking the spontaneous and cAMP-PKA-stimulated Ca2+ influx (35).
Summary and Perspectives
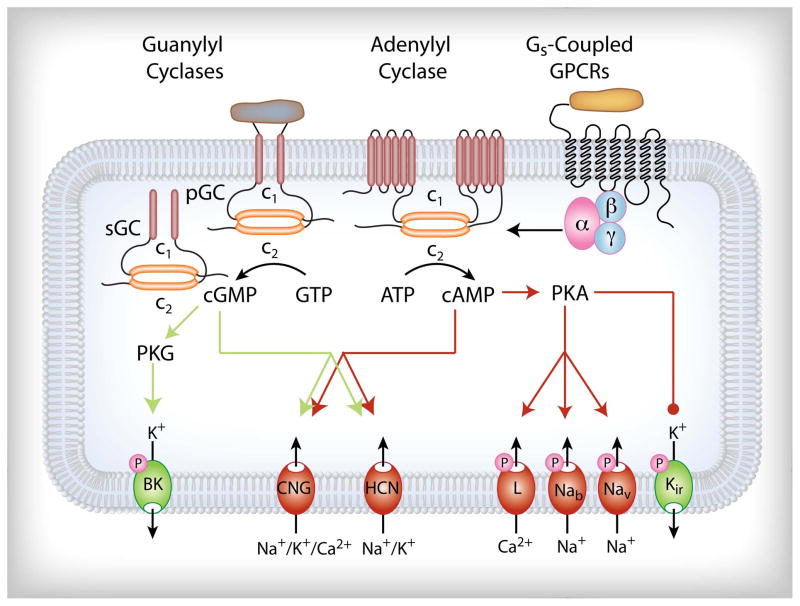
Pituitary cells express several subtypes of transmembrane ACs, and their basal and receptor-stimulated activity provides effective pathways for the generation of cAMP, whereas the expression and role of soluble AC has not been studied in this gland. Pituitary cells also express pGCs and sGC, which generate cGMP in an agonistic and NO-dependent manner, respectively. As summarised in Fig. 5, these messengers play important roles in the plasma membrane channel physiology, by altering the patterns of spontaneous electrical activity and the accompanying VGCI. cAMP and cGMP directly affect the gating of the CNG and HCN channels, and they indirectly affect the gating of BK-KCa, L-type Cav, Nav, Kir and voltage-insensitive Na+-conducting channels, through PKA and PKG-dependent phosphorylation. Although these channels are expressed in all pituitary cell types, this pattern does preclude the possibility that their modulation by the cyclic nucleotides may occur in a cell-type and/or receptor-type-specific manner.
Fig. 5.
Schematic representation of the expression and role of cyclic nucleotide signalling pathway in control of electrical activity in pituitary cells. BK, big calcium-activated potassium channels; CNG, cyclic nucleotide-gated channels; GPCR, G protein-coupled receptors; HCN, hyperpolarisation-activated nonselective cation channels; Kir, inward rectifier potassium channels; L, dihydropyridine-sensitive voltage-gated calcium channels; Nab, background Na+-conducting channels; Nav, TTX-resistant voltage-gated Na+ channels; PKA, protein kinase A; PKG, protein kinase G; pGc, particulate gyanylyl cyclase; sGC, soluble guanylyl cyclase.
In general, cAMP and cGMP could have a cooperative effect on membrane depolarisation and the facilitation of VGCI, by acting as co-agonists at CNG and HCN channels. However, in endocrine pituitary cells, these two intracellular messengers appear to have opposite roles in the control of endocrine pituitary cell excitability. Specifically, cAMP depolarises the cell membrane, leading to the facilitation of AP firing and VGCI, whereas cGMP hyperpolarises the cell membrane, causing the cessation of electrical activity in spontaneously firing cells. The hyperpolarising nature of cGMP could be explained by the expression and operation of BK type KCa channels in endocrine pituitary cells, whereas the stimulatory action of this messenger on hormone release could be independent of the electrical status of cells. cAMP facilitates the excitability of pituitary cells directly by activating HCN channels, which are expressed in all endocrine pituitary cell types, through PKA-dependent phosphorylation of several plasma membrane channels. It is interesting that the basal AC activity of cultured pituitary cells is of a sufficient amplitude to integrate HCN channels into electrical activity. In vivo, the removal of the dopaminergic and somatostatinergic inhibition of AC could also play a role in the integration of these channels in electrical activity. This hypothesis should be tested by studying effects of the withdrawal of dopamine and/or somatostatin on the status of the HCN channels in lactotrophs, melanotrophs and somatotrophs, in vitro. The mechanisms and physiological significance of GnRH-induced inhibition of the HCN channels in gonadotrophs should also be addressed.
The major action of cAMP on the excitability of pituitary cells appears to occur through a PKA-mediated phosphorylation of plasma membrane channels. The enlargement of the L-type Cav current, by GHRH in somatotrophs, is consistent with experiments with recombinant channels phosphorylated by PKA. Future work with this cell type should be focused on the changes in the pattern of electrical activity caused by the phosphorylation of these channels, because in somatotrophs, lactotrophs and probably corticotrophs, L-type Cav and BK channel interplay is important in the formation of the plateau bursting type of electrical activity. All other pituitary cell types also express L-type Cav channels, but at the present time, it is not clear whether their gating is enhanced by receptors coupled to AC signalling pathway. Solid evidence also exists for the relevance of Na+-conducting channels in PKA-mediated facilitation of electrical activity in pituitary cells. It is reasonable to suggest that the TTX-resistant, rather than TTX-sensitive, Na+ channels play a major role in PKA (and/or PKC) phosphorylation of these channels. Earlier investigations and our recent data indicate that the non-selective cation channels, presumably the TRPC family of these channels, could also contribute to cAMP-PKA-dependent facilitation of electrical activity. When considered with findings regarding the role of the Kir and TREK-1 channels in the PKA-mediated facilitation of electrical activity, these findings suggest the presence of multiple channel types for the PKA-dependent facilitation of pituitary cell excitability (Fig. 5).
Box 1.
Cyclic 3′,5′-adenosine monophosphate (cAMP) was the first historically identified intracellular messenger in 1957. cAMP is used in practically all types of organisms, from prokaryotes to mammals. It is synthesised from ATP by a family of enzymes consisting of transmembrane and soluble adenylyl cyclases (ACs). Molecular cloning has confirmed that several structurally related plasma membrane-bound ACs are expressed in the animal cells, and in mammals, these enzymes are encoded by nine different genes. ACs contain two hydrophobic domains, each composed of six transmembrane helices, and two cytoplasmic catalytic domains. All of the characterised isoforms of ACs produce cAMP in the absence of hormonal stimulation, and such basal activity is markedly enhanced upon the binding of Gs and is reduced upon the binding of Gi, Go, and Gz alpha subunits. The βγ dimers of Gi and Go proteins also contribute to the control of AC2, AC4, and AC7 activity. The Gq/11-coupled calcium-mobilising receptors modulate AC activity indirectly through the Ca2+ and protein kinase C (PKC) signalling pathways. Cytosolic Ca2+ exhibits direct inhibitory effects on the AC5 and AC6 isoforms and an indirect effect on AC9 (through the Ser/Thr protein phosphatase calcineurin) and AC3 (through Ca2+-calmodulin kinase II). Ca2+ also stimulates AC1 and AC8, via calmodulin. PKC activates the AC2, AC4, AC5, and AC7 isoforms. In contrast, cAMP-dependent protein kinase (PKA) inhibits the AC5 and AC6 isoforms, providing a form of negative feedback to the cAMP formation. Forskolin, a hypotensive agent, activates all isoforms except AC9, whereas several 3′-adenosine nucleotide analogues bind to the activated pyrophosphate complexes of ACs, thereby inhibiting the enzyme activity (195, 196). In 1999, a soluble AC was also identified, but their expression and potential roles in the pituitary gland have not been studied (197, 198).
Two families of enzymes, the particulate guanylyl cyclase (pGC) and soluble guanylyl cyclase (sGC), synthesise cyclic 3′,5′-guanosine monophosphate (cGMP) from GTP using a mechanism that is stereochemically analogous to that of the ACs. pGC contains an extracellular peptide receptor domain and an intracellular catalytic domain, connected by a single transmembrane domain. The active enzyme is now generally thought to be a homodimer of approximately 120 kDa subunits, and the dimerisation is mediated by the intracellular domain. Seven mammalian pGSs and a sea urchin enzyme have been identified and sequenced. Three of these have known peptide ligands (GC-A, atrial natriuretic peptide; GC-B, brain natriuretic peptide; and GC-C, the heat stable enterotoxin of Escherichia coli and endogenous intestinal peptide guanylin) (199–201). Based on cDNA cloning, four sGC subunits have been identified and termed α1, α2, β1, and β2. In the absence of nitric oxide (NO), sGC-derived cGMP production is negligible, and the three enzymes of NO synthases, including endothelial, neuronal and inducible NO, supply cells with NO by catalyzing the oxidation of L-arginine to L-citruline and NO (202). Many Gq/11 and Gs protein-coupled receptors also stimulate cGMP production in a Ca2+- and protein kinase-dependent manner (203).
As a family, ACs, pGCs, and sGCs are involved in a broad array of signal transduction pathways, mediated by the cyclic nucleotide monophosphates, including vision, blood pressure regulation, and responses to numerous hormones, neurotransmitters, and NO. Experimentally, the actions of cAMP and cGMP are mimicked by cell permeable analogues. Most of the physiologic actions of cAMP are mediated by PKA, which controls multiple aspects of cell function through the phosphorylation of protein substrates. cAMP also directly regulates certain guanine nucleotide exchange factors, the hyperpolarisation-activated cyclic nucleotide-regulated (HCN) cation channels, and the cyclic nucleotide-gated (CNG) channels. In contrast to cAMP, the intracellular messenger functions of cGMP are more restricted, and these functions are indirectly mediated through protein phosphorylation by cGMP-dependent protein kinase (PKG) and directly mediated by the activation of HCN and CNG channels (204).
PKAs are present in all eukaryotic cells. In the absence of cAMP, PKA is an inactive, asymmetric tetramer containing 2 regulatory and 2 catalytic subunits, which bind to each other with high affinity. The binding of cAMP to the regulatory subunit alters its affinity for the catalytic subunits, leading to the dissociation of the regulatory subunits and 2 active monomeric catalytic subunits. There are two categories of regulatory subunits, and several isoforms of each regulatory subunit are cloned and expressed in most cells. Each regulatory subunit has 2 high-affinity binding sites for cAMP per monomer, localised at the C-terminus, which differ in their structure and affinity for various analogues of cAMP. The catalytic PKA subunits are also encoded by at least three genes, giving rise to the α, β, and γ isoforms. The α-isoform appears to be expressed constitutively in most cells, whereas the expression of the β-isoform is tissue-specific. Because each regulatory subunit can associate with any of the catalytic subunits, a wide variety of AC holoenzymes can exist in various tissues (16, 205).
The marked amino acid sequence homologies between the PKAs and PKGs, as well as the predicted similarities in their structures, suggest that the 2 enzymes have evolved from an ancestral phosphotransferase. However, the PKGs have a more limited distribution in mammalian tissues than PKA. In high concentrations, they are present in cerebellar Purkinje cells, platelets, and intestinal epithelial cells. In other tissues, PKG is at a 10–100-fold lower concentration than PKA. In mammals, there are two forms of this enzyme. Type I kinase is a soluble protein, consisting of two spliced forms, and it mediates the effects of natriuretic peptides and NO in cardiovascular cells. On the other hand, type II kinase is a membrane-associated enzyme that transduces signals from the Escherichia coli heat-stable enterotoxin STa, and from the endogenous intestinal peptide, guanylin. PKGs operate as dimers, although each monomer seems to be self-sufficient in its regulatory and catalytic properties (206, 207).
Acknowledgments
The authors are thankful to all past and present members of their laboratories who have contributed to the understanding of the role of cyclic nucleotides and pituitary cells. This work is supported by the National Institute of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural grants.
Abbreviations
- AC
adenylyl cyclase
- ACTH
adrenocorticotropin hormone
- AP
action potential
- BK
high conductance
- [Ca2+]i
intracellular calcium concentration
- cAMP
Cav, voltage-gated calcium
- cyclic 3′
5′-adenosine monophosphate
- cGMP
3′5′-guanosine monophosphate
- CNG
cyclic nucleotide-gated
- CRH
corticotropin-releasing hormone
- GH
growth hormone
- GHRH
GH releasing hormone
- GnRH
gonadotropin-releasing hormone
- GPCR
G protein-coupled receptors
- H89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesuslvonamide dihydrochloride
- HCN
hyperpolarization-activated cyclic nucleotide-regulated
- KCa
calcium-controlled K+ channels
- Kir
inwardly rectifying potassium
- Nav
voltage-gated sodium
- NO
nitric oxide
- PACAP
pituitary adenylate cyclase-activating peptide
- PAC1
PACAP-preferring
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PKG
cGMP-dependent protein kinase
- pGC
particulate guanylyl cyclase
- Rp-cAMPS
3′,5′-(Rp)-phosphothioate
- sGC
soluble guanylyl cyclase
- TASK1, TASK3, and TEK-1
members of potassium leak channels with two-P-domain subunits
- TRH
thyrotropin-releasing hormone
- TRP
transient receptor potential
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
- VPAC2
PACAP/VIP-shared
- VGCI
voltage-gated calcium influx
References
- 1.Antoni FA, Sosunov AA, Haunso A, Paterson JM, Simpson J. Short-term plasticity of cyclic adenosine 3′,5′-monophosphate signaling in anterior pituitary corticotrope cells: the role of adenylyl cyclase isotypes. Mol Endocrinol. 2003;17(4):692–703. doi: 10.1210/me.2002-0369. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Iglesias AE, Jiang Y, Tomic M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS. Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol. 2006;20(9):2231–46. doi: 10.1210/me.2005-0363. [DOI] [PubMed] [Google Scholar]
- 3.Kostic TS, Andric SA, Stojilkovic SS. Spontaneous and receptor-controlled soluble guanylyl cyclase activity in anterior pituitary cells. Mol Endocrinol. 2001;15(6):1010–22. doi: 10.1210/mend.15.6.0648. [DOI] [PubMed] [Google Scholar]
- 4.Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31(6):845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persani L, Borgato S, Lania A, Filopanti M, Mantovani G, Conti M, Spada A. Relevant cAMP-specific phosphodiesterase isoforms in human pituitary: effect of Gs(alpha) mutations. J Clin Endocrinol Metab. 2001;86(8):3795–800. doi: 10.1210/jcem.86.8.7779. [DOI] [PubMed] [Google Scholar]
- 6.Ang KL, Antoni FA. Functional plasticity of cyclic AMP hydrolysis in rat adenohypophysial corticotroph cells. Cell Signal. 2002;14(5):445–52. doi: 10.1016/s0898-6568(01)00267-4. [DOI] [PubMed] [Google Scholar]
- 7.Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology. 2006;147(7):3435–45. doi: 10.1210/en.2006-0091. [DOI] [PubMed] [Google Scholar]
- 8.Kucka M, Kretschmannova K, Murano T, Wu CP, Zemkova H, Ambudkar SV, Stojilkovic SS. Dependence of multidrug resistance protein-mediated cyclic nucleotide efflux on the background sodium conductance. Mol Pharmacol. 2010;77(2):270–9. doi: 10.1124/mol.109.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochilla AJ, Angleson JK, Betz WJ. Differential regulation of granule-to-granule and granule-to-plasma membrane fusion during secretion from rat pituitary lactotrophs. J Cell Biol. 2000;150(4):839–48. doi: 10.1083/jcb.150.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikdar SK, Kreft M, Zorec R. Modulation of the unitary exocytic event amplitude by cAMP in rat melanotrophs. J Physiol. 1998;511(Pt 3):851–9. doi: 10.1111/j.1469-7793.1998.851bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikdar SK, Zorec R, Mason WT. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett. 1990;273(1–2):150–4. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- 12.Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of FSH{beta} by pulsatile GnRH is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2009 doi: 10.1128/MCB.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida M, Mitsui T, Yamakawa K, Sugiyama N, Takahashi W, Shimura H, Endo T, Kobayashi T, Arita J. Involvement of cAMP response element-binding protein in the regulation of cell proliferation and the prolactin promoter of lactotrophs in primary culture. Am J Physiol Endocrinol Metab. 2007;293(6):E1529–37. doi: 10.1152/ajpendo.00028.2007. [DOI] [PubMed] [Google Scholar]
- 14.Bertherat J. Nuclear effects of the cAMP pathway activation in somatotrophs. Horm Res. 1997;47 (4–6):245–50. doi: 10.1159/000185471. [DOI] [PubMed] [Google Scholar]
- 15.Boutillier AL, Gaiddon C, Lorang D, Roberts JL, Loeffler JP. Transcriptional activation of the proopiomelanocortin gene by cyclic AMP-responsive element binding protein. Pituitary. 1998;1(1):33–43. doi: 10.1023/a:1009966808106. [DOI] [PubMed] [Google Scholar]
- 16.Bossis I, Stratakis CA. Minireview: PRKAR1A: normal and abnormal functions. Endocrinology. 2004;145(12):5452–8. doi: 10.1210/en.2004-0900. [DOI] [PubMed] [Google Scholar]
- 17.Stojilkovic SS. Molecular mechanisms of pituitary endocrine cell calcium handling. Cell Calcium. 2012;51(3–4):212–21. doi: 10.1016/j.ceca.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Foll F, Castel H, Soriani O, Vaudry H, Cazin L. Gramicidin-perforated patch revealed depolarizing effect of GABA in cultured frog melanotrophs. J Physiol. 1998;507(Pt 1):55–69. doi: 10.1111/j.1469-7793.1998.055bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996;137(6):2269–77. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- 20.Guerineau N, Corcuff JB, Tabarin A, Mollard P. Spontaneous and corticotropin-releasing factor-induced cytosolic calcium transients in corticotrophs. Endocrinology. 1991;129(1):409–20. doi: 10.1210/endo-129-1-409. [DOI] [PubMed] [Google Scholar]
- 21.Liang Z, Chen L, McClafferty H, Lukowski R, MacGregor D, King JT, Rizzi S, Sausbier M, McCobb DP, Knaus HG, Ruth P, Shipston MJ. Control of hypothalamic-pituitary-adrenal stress axis activity by the intermediate conductance calcium-activated potassium channel, SK4. J Physiol. 2011;589(Pt 24):5965–86. doi: 10.1113/jphysiol.2011.219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic SS. Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol. 2007;98(1):131–44. doi: 10.1152/jn.00872.2006. [DOI] [PubMed] [Google Scholar]
- 23.Stojilkovic SS, Kukuljan M, Iida T, Rojas E, Catt KJ. Integration of cytoplasmic calcium and membrane potential oscillations maintains calcium signaling in pituitary gonadotrophs. Proc Natl Acad Sci U S A. 1992;89(9):4081–5. doi: 10.1073/pnas.89.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretschmannova K, Kucka M, Gonzalez-Iglesias AE, Stojilkovic SS. The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells. Mol Endocrinol. 2012;26(1):153–64. doi: 10.1210/me.2011-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Goor F, Zivadinovic D, Martinez-Fuentes AJ, Stojilkovic SS. Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. J Biol Chem. 2001;276(36):33840–6. doi: 10.1074/jbc.M105386200. [DOI] [PubMed] [Google Scholar]
- 26.Schlegel W, Winiger BP, Mollard P, Vacher P, Wuarin F, Zahnd GR, Wollheim CB, Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987;329(6141):719–21. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- 27.Chang JP, Habibi HR, Yu Y, Moussavi M, Grey CL, Pemberton JG. Calcium and other signalling pathways in neuroendocrine regulation of somatotroph functions. Cell Calcium. 2011 doi: 10.1016/j.ceca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102(46):16880–5. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodson DJ, Schaeffer M, Romano N, Fontanaud P, Lafont C, Birkenstock J, Molino F, Christian H, Lockey J, Carmignac D, Fernandez-Fuente M, Le Tissier P, Mollard P. Existence of long-lasting experience-dependent plasticity in endocrine cell networks. Nat Commun. 2012:3605. doi: 10.1038/ncomms1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaeffer M, Hodson DJ, Lafont C, Mollard P. Endocrine cells and blood vessels work in tandem to generate hormone pulses. J Mol Endocrinol. 2011;47(2):R59–66. doi: 10.1530/JME-11-0035. [DOI] [PubMed] [Google Scholar]
- 31.Hodson DJ, Romano N, Schaeffer M, Fontanaud P, Lafont C, Fiordelisio T, Mollard P. Coordination of calcium signals by pituitary endocrine cells in situ. Cell Calcium. 2011 doi: 10.1016/j.ceca.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Hezareh M, Schlegel W, Rawlings SR. Stimulation of Ca2+ influx in alpha T3–1 gonadotrophs via the cAMP/PKA signaling system. Am J Physiol. 1997;273(5 Pt 1):E850–8. doi: 10.1152/ajpendo.1997.273.5.E850. [DOI] [PubMed] [Google Scholar]
- 33.Cuttler L, Glaum SR, Collins BA, Miller RJ. Calcium signalling in single growth hormone-releasing factor-responsive pituitary cells. Endocrinology. 1992;130(2):945–53. doi: 10.1210/endo.130.2.1733736. [DOI] [PubMed] [Google Scholar]
- 34.Rawlings SR, Hoyland J, Mason WT. Calcium homeostasis in bovine somatotrophs: calcium oscillations and calcium regulation by growth hormone-releasing hormone and somatostatin. Cell Calcium. 1991;12(6):403–14. doi: 10.1016/0143-4160(91)90066-n. [DOI] [PubMed] [Google Scholar]
- 35.Tomic M, Kucka M, Kretschmannova K, Li S, Nesterova M, Stratakis CA, Stojilkovic SS. Role of nonselective cation channels in spontaneous and protein kinase A-stimulated calcium signaling in pituitary cells. Am J Physiol Endocrinol Metab. 2011;301(2):E370–9. doi: 10.1152/ajpendo.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee AK, Tse A. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol. 1997;504(Pt 2):367–78. doi: 10.1111/j.1469-7793.1997.367be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25(3):319–29. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004:44525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 39.Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulation of Ca2+ entry in corticotropes is partially dependent on protein kinase A. Endocrinology. 1995;136(9):3925–35. doi: 10.1210/endo.136.9.7649101. [DOI] [PubMed] [Google Scholar]
- 40.Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000:55237–66. discussion 66–7. [PubMed] [Google Scholar]
- 41.Tomic M, Koshimizu T, Yuan D, Andric SA, Zivadinovic D, Stojilkovic SS. Characterization of a plasma membrane calcium oscillator in rat pituitary somatotrophs. J Biol Chem. 1999;274(50):35693–702. doi: 10.1074/jbc.274.50.35693. [DOI] [PubMed] [Google Scholar]
- 42.Naumov AP, Herrington J, Hille B. Actions of growth-hormone-releasing hormone on rat pituitary cells: intracellular calcium and ionic currents. Pflugers Arch. 1994;427(5–6):414–21. doi: 10.1007/BF00374255. [DOI] [PubMed] [Google Scholar]
- 43.Kwiecien R, Tseeb V, Kurchikov A, Kordon C, Hammond C. Growth hormone-releasing hormone triggers pacemaker activity and persistent Ca2+ oscillations in rat somatotrophs. J Physiol. 1997;499(Pt 3):613–23. doi: 10.1113/jphysiol.1997.sp021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lussier BT, French MB, Moor BC, Kraicer J. Free intracellular Ca2+ concentration and growth hormone (GH) release from purified rat somatotrophs. III. Mechanism of action of GH-releasing factor and somatostatin. Endocrinology. 1991;128(1):592–603. doi: 10.1210/endo-128-1-592. [DOI] [PubMed] [Google Scholar]
- 45.Lussier BT, French MB, Moore BC, Kraicer J. Free intracellular Ca2+ concentration ([Ca2+]i) and growth hormone release from purified rat somatotrophs. I. GH-releasing factor-induced Ca2+ influx raises [Ca2+]i. Endocrinology. 1991;128(1):570–82. doi: 10.1210/endo-128-1-570. [DOI] [PubMed] [Google Scholar]
- 46.Lussier BT, Wood DA, French MB, Moor BC, Kraicer J. Free intracellular Ca2+ concentration ([Ca2+]i) and growth hormone release from purified rat somatotrophs. II. Somatostatin lowers [Ca2+]i by inhibiting Ca2+ influx. Endocrinology. 1991;128(1):583–91. doi: 10.1210/endo-128-1-583. [DOI] [PubMed] [Google Scholar]
- 47.Holl RW, Thorner MO, Leong DA. Intracellular calcium concentration and growth hormone secretion in individual somatotropes: effects of growth hormone-releasing factor and somatostatin. Endocrinology. 1988;122(6):2927–32. doi: 10.1210/endo-122-6-2927. [DOI] [PubMed] [Google Scholar]
- 48.Bonnefont X, Mollard P. Electrical activity in endocrine pituitary cells in situ: a support for a multiple-function coding. FEBS Lett. 2003;548(1–3):49–52. doi: 10.1016/s0014-5793(03)00727-0. [DOI] [PubMed] [Google Scholar]
- 49.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61(3):283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 50.Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev. 1996;17(1):4–29. doi: 10.1210/edrv-17-1-4. [DOI] [PubMed] [Google Scholar]
- 51.McArdle CA, Poch A, Schomerus E, Kratzmeier M. Pituitary adenylate cyclase-activating polypeptide effects in pituitary cells: modulation by gonadotropin-releasing hormone in alpha T3–1 cells. Endocrinology. 1994;134(6):2599–605. doi: 10.1210/endo.134.6.7515005. [DOI] [PubMed] [Google Scholar]
- 52.Schomerus E, Poch A, Bunting R, Mason WT, McArdle CA. Effects of pituitary adenylate cyclase-activating polypeptide in the pituitary: activation of two signal transduction pathways in the gonadotrope-derived alpha T3–1 cell line. Endocrinology. 1994;134(1):315–23. doi: 10.1210/endo.134.1.7903932. [DOI] [PubMed] [Google Scholar]
- 53.Koch B, Lutz-Bucher B. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates cyclic AMP formation as well as peptide output of cultured pituitary melanotrophs and AtT-20 corticotrophs. Regul Pept. 1992;38(1):45–53. doi: 10.1016/0167-0115(92)90071-2. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka K, Shibuya I, Harayama N, Nomura M, Kabashima N, Ueta Y, Yamashita H. Pituitary adenylate cyclase-activating polypeptide potentiation of Ca2+ entry via protein kinase C and A pathways in melanotrophs of the pituitary pars intermedia of rats. Endocrinology. 1997;138(10):4086–95. doi: 10.1210/endo.138.10.5442. [DOI] [PubMed] [Google Scholar]
- 55.Yada T, Vigh S, Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP) increases cytosolic-free calcium concentration in folliculo-stellate cells and somatotropes of rat pituitary. Peptides. 1993;14(2):235–9. doi: 10.1016/0196-9781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- 56.Canny BJ, Rawlings SR, Leong DA. Pituitary adenylate cyclase-activating polypeptide specifically increases cytosolic calcium ion concentration in rat gonadotropes and somatotropes. Endocrinology. 1992;130(1):211–5. doi: 10.1210/endo.130.1.1727697. [DOI] [PubMed] [Google Scholar]
- 57.Alarcon P, Garcia-Sancho J. Differential calcium responses to the pituitary adenylate cyclase-activating polypeptide (PACAP) in the five main cell types of rat anterior pituitary. Pflugers Arch. 2000;440(5):685–91. doi: 10.1007/s004240000368. [DOI] [PubMed] [Google Scholar]
- 58.Mollard P, Zhang Y, Rodman D, Cooper DM. Limited accumulation of cyclic AMP underlies a modest vasoactive-intestinal-peptide-mediated increase in cytosolic [Ca2+] transients in GH3 pituitary cells. Biochem J. 1992;284(Pt 3):637–40. doi: 10.1042/bj2840637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rawlings SR, Piuz I, Schlegel W, Bockaert J, Journot L. Differential expression of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptor subtypes in clonal pituitary somatotrophs and gonadotrophs. Endocrinology. 1995;136(5):2088–98. doi: 10.1210/endo.136.5.7720658. [DOI] [PubMed] [Google Scholar]
- 60.Perez FM, Malamed S, Scanes CG. Possible participation of calcium in growth hormone release and in thyrotropin-releasing hormone and human pancreatic growth hormone-releasing factor synergy in a primary culture of chicken pituitary cells. Gen Comp Endocrinol. 1989;75(3):481–91. doi: 10.1016/0016-6480(89)90184-6. [DOI] [PubMed] [Google Scholar]
- 61.Perez FM, Malamed S, Scanes CG. Growth hormone release from chicken anterior pituitary cells in primary culture: TRH and hpGRF synergy, protein synthesis, and cyclic adenosine 3′5′-monophosphate. Gen Comp Endocrinol. 1989;73(1):12–20. doi: 10.1016/0016-6480(89)90050-6. [DOI] [PubMed] [Google Scholar]
- 62.Lieste JR, Scheenen WJ, Willems PH, Jenks BG, Roubos EW. Calcium oscillations in melanotrope cells of Xenopus laevis are differentially regulated by cAMP-dependent and cAMP-independent mechanisms. Cell Calcium. 1996;20(4):329–37. doi: 10.1016/s0143-4160(96)90038-x. [DOI] [PubMed] [Google Scholar]
- 63.Luque RM, Rodriguez-Pacheco F, Tena-Sempere M, Gracia-Navarro F, Malagon MM, Castano JP. Differential contribution of nitric oxide and cGMP to the stimulatory effects of growth hormone-releasing hormone and low-concentration somatostatin on growth hormone release from somatotrophs. J Neuroendocrinol. 2005;17(9):577–82. doi: 10.1111/j.1365-2826.2005.01345.x. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Pacheco F, Luque RM, Tena-Sempere M, Malagon MM, Castano JP. Ghrelin induces growth hormone secretion via a nitric oxide/cGMP signalling pathway. J Neuroendocrinol. 2008;20(3):406–12. doi: 10.1111/j.1365-2826.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- 65.Hartt DJ, Ogiwara T, Ho AK, Chik CL. Cyclic GMP stimulates growth hormone release in rat anterior pituitary cells. Biochem Biophys Res Commun. 1995;214(3):918–26. doi: 10.1006/bbrc.1995.2374. [DOI] [PubMed] [Google Scholar]
- 66.Willmott NJ, Asselin J, Galione A. Calcium store depletion potentiates a phosphodiesterase inhibitor- and dibutyryl cGMP-evoked calcium influx in rat pituitary GH3 cells. FEBS Lett. 1996;386(1):39–42. doi: 10.1016/0014-5793(96)00413-9. [DOI] [PubMed] [Google Scholar]
- 67.Cataldi M, Secondo A, D’Alessio A, Sarnacchiaro F, Colao AM, Amoroso S, Di Renzo GF, Annunziato L. Involvement of phosphodiesterase-cGMP-PKG pathway in intracellular Ca2+ oscillations in pituitary GH3 cells. Biochim Biophys Acta. 1999;1449(2):186–93. doi: 10.1016/s0167-4889(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 68.Duvilanski BH, Velardez MO, Gonzalez Iglesias A, Theas S, Seilicovich A, Becu-Villalobos D. Nitric oxide donors modify free intracellular calcium levels in rat anterior pituitary cells. Mol Cell Endocrinol. 1998;146(1–2):19–26. doi: 10.1016/s0303-7207(98)00203-2. [DOI] [PubMed] [Google Scholar]
- 69.Ceccatelli S, Hulting AL, Zhang X, Gustafsson L, Villar M, Hokfelt T. Nitric oxide synthase in the rat anterior pituitary gland and the role of nitric oxide in regulation of luteinizing hormone secretion. Proc Natl Acad Sci U S A. 1993;90(23):11292–6. doi: 10.1073/pnas.90.23.11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duvilanski BH, Zambruno C, Seilicovich A, Pisera D, Lasaga M, Diaz MC, Belova N, Rettori V, McCann SM. Role of nitric oxide in control of prolactin release by the adenohypophysis. Proc Natl Acad Sci U S A. 1995;92(1):170–4. doi: 10.1073/pnas.92.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andric SA, Gonzalez-Iglesias AE, Van Goor F, Tomic M, Stojilkovic SS. Nitric oxide inhibits prolactin secretion in pituitary cells downstream of voltage-gated calcium influx. Endocrinology. 2003;144(7):2912–21. doi: 10.1210/en.2002-0147. [DOI] [PubMed] [Google Scholar]
- 72.Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006:68375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- 73.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2001;281(5):H1835–62. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- 74.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem. 1998;273(49):32950–6. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- 75.Ding C, Potter ED, Qiu W, Coon SL, Levine MA, Guggino SE. Cloning and widespread distribution of the rat rod-type cyclic nucleotide-gated cation channel. Am J Physiol. 1997;272(4 Pt 1):C1335–44. doi: 10.1152/ajpcell.1997.272.4.C1335. [DOI] [PubMed] [Google Scholar]
- 76.Khan S, Perry C, Tetreault ML, Henry D, Trimmer JS, Zimmerman AL, Matthews G. A novel cyclic nucleotide-gated ion channel enriched in synaptic terminals of isotocin neurons in zebrafish brain and pituitary. Neuroscience. 165(1):79–89. doi: 10.1016/j.neuroscience.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003:65453–80. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 78.Tian L, Shipston MJ. Characterization of hyperpolarization-activated cation currents in mouse anterior pituitary, AtT20 D16:16 corticotropes. Endocrinology. 2000;141(8):2930–7. doi: 10.1210/endo.141.8.7617. [DOI] [PubMed] [Google Scholar]
- 79.Kretschmannova K, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells. J Neuroendocrinol. 2006;18(7):484–93. doi: 10.1111/j.1365-2826.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- 80.Simasko SM, Sankaranarayanan S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am J Physiol. 1997;272(3 Pt 1):E405–14. doi: 10.1152/ajpendo.1997.272.3.E405. [DOI] [PubMed] [Google Scholar]
- 81.Mei YA, Soriani O, Castel H, Vaudry H, Cazin L. Adenosine potentiates the delayed-rectifier potassium conductance but has no effect on the hyperpolarization-activated Ih current in frog melanotrophs. Brain Res. 1998;793(1–2):271–8. doi: 10.1016/s0006-8993(98)00184-x. [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez-Iglesias AE, Kretschmannova K, Tomic M, Stojilkovic SS. ZD7288 inhibits exocytosis in an HCN-independent manner and downstream of voltage-gated calcium influx in pituitary lactotrophs. Biochem Biophys Res Commun. 2006;346(3):845–50. doi: 10.1016/j.bbrc.2006.05.194. [DOI] [PubMed] [Google Scholar]
- 83.Liu YC, Wang YJ, Wu PY, Wu SN. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(2):127–35. doi: 10.1007/s00210-008-0353-0. [DOI] [PubMed] [Google Scholar]
- 84.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30(40):13373–83. doi: 10.1523/JNEUROSCI.1687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52(6):1027–36. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Pian P, Bucchi A, Decostanzo A, Robinson RB, Siegelbaum SA. Modulation of cyclic nucleotide-regulated HCN channels by PIP(2) and receptors coupled to phospholipase C. Pflugers Arch. 2007;455(1):125–45. doi: 10.1007/s00424-007-0295-2. [DOI] [PubMed] [Google Scholar]
- 87.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato M, Hattori MA, Suzuki M. Inhibition by extracellular Na+ replacement of GRF-induced GH secretion from rat pituitary cells. Am J Physiol. 1988;254(4 Pt 1):E476–81. doi: 10.1152/ajpendo.1988.254.4.E476. [DOI] [PubMed] [Google Scholar]
- 89.Ritchie AK, Kuryshev YA, Childs GV. Corticotropin-releasing hormone and calcium signaling in corticotropes. Trends Endocrinol Metab. 1996;7(10):365–9. doi: 10.1016/s1043-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 90.Rawlings SR, Canny BJ, Leong DA. Pituitary adenylate cyclase-activating polypeptide regulates cytosolic Ca2+ in rat gonadotropes and somatotropes through different intracellular mechanisms. Endocrinology. 1993;132(4):1447–52. doi: 10.1210/endo.132.4.8384987. [DOI] [PubMed] [Google Scholar]
- 91.Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65(11):4506–14. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 92.Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, Cheadle C, Watkins T, Wen F, Starost MF, Bossis I, Nesterova M, Stratakis CA. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19(8):1387–98. doi: 10.1093/hmg/ddq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos S, Robinson-White A, Lenherr S, Weinberg FD, Claflin E, Meoli E, Cho-Chung YS, Stratakis CA. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res. 2004;64(24):8811–5. doi: 10.1158/0008-5472.CAN-04-3620. [DOI] [PubMed] [Google Scholar]
- 94.Pedarzani P, Stocker M. Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65(20):3196–217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59 (6):873–81. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Van Goor F, Li YX, Stojilkovic SS. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J Neurosci. 2001;21 (16):5902–15. doi: 10.1523/JNEUROSCI.21-16-05902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tabak J, Tomaiuolo M, Gonzalez-Iglesias AE, Milescu LS, Bertram R. Fast-activating voltage- and calcium-dependent potassium (BK) conductance promotes bursting in pituitary cells: a dynamic clamp study. J Neurosci. 2011;31(46):16855–63. doi: 10.1523/JNEUROSCI.3235-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Goor F, Zivadinovic D, Stojilkovic SS. Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol. 2001;15(7):1222–36. doi: 10.1210/mend.15.7.0668. [DOI] [PubMed] [Google Scholar]
- 99.Vacher P, Vacher AM, Mollard P. Tubocurarine blocks a calcium-dependent potassium current in rat tumoral pituitary cells. Mol Cell Endocrinol. 1998;139(1–2):131–42. doi: 10.1016/s0303-7207(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 100.Shipston MJ, Kelly JS, Antoni FA. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem. 1996;271(16):9197–200. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- 101.Lang DG, Ritchie AK. Tetraethylammonium ion sensitivity of a 35-pS CA2(+)-activated K+ channel in GH3 cells that is activated by thyrotropin-releasing hormone. Pflugers Arch. 1990;416(6):704–9. doi: 10.1007/BF00370618. [DOI] [PubMed] [Google Scholar]
- 102.Lang DG, Ritchie AK. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2(+)-activated K+ channels in a pituitary cell line. J Physiol. 1990:425117–32. doi: 10.1113/jphysiol.1990.sp018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shipston MJ, Armstrong DL. Activation of protein kinase C inhibits calcium-activated potassium channels in rat pituitary tumour cells. J Physiol. 1996;493(Pt 3):665–72. doi: 10.1113/jphysiol.1996.sp021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kehl SJ, Wong K. Large-conductance calcium-activated potassium channels of cultured rat melanotrophs. J Membr Biol. 1996;150(3):219–30. doi: 10.1007/s002329900046. [DOI] [PubMed] [Google Scholar]
- 105.Kanyicska B, Freeman ME, Dryer SE. Endothelin activates large-conductance K+ channels in rat lactotrophs: reversal by long-term exposure to dopamine agonist. Endocrinology. 1997;138(8):3141–53. doi: 10.1210/endo.138.8.5299. [DOI] [PubMed] [Google Scholar]
- 106.Shipston MJ, Duncan RR, Clark AG, Antoni FA, Tian L. Molecular components of large conductance calcium-activated potassium (BK) channels in mouse pituitary corticotropes. Mol Endocrinol. 1999;13(10):1728–37. doi: 10.1210/mend.13.10.0355. [DOI] [PubMed] [Google Scholar]
- 107.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22(10):505–12. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 108.Gragasin FS, Michelakis ED, Hogan A, Moudgil R, Hashimoto K, Wu X, Bonnet S, Haromy A, Archer SL. The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB J. 2004;18(12):1382–91. doi: 10.1096/fj.04-1978com. [DOI] [PubMed] [Google Scholar]
- 109.Lim I, Yun J, Kim S, Lee C, Seo S, Kim T, Bang H. Nitric oxide stimulates a large-conductance Ca-activated K+ channel in human skin fibroblasts through protein kinase G pathway. Skin Pharmacol Physiol. 2005;18(6):279–87. doi: 10.1159/000088013. [DOI] [PubMed] [Google Scholar]
- 110.Luedders DW, Muenz BM, Li F, Rueckleben S, Tillmanns H, Waldecker B, Wiecha J, Erdogan A, Schaefer CA, Kuhlmann CR. Role of cGMP in sildenafil-induced activation of endothelial Ca2+-activated K+ channels. J Cardiovasc Pharmacol. 2006;47(3):365–70. doi: 10.1097/01.fjc.0000206438.35477.f2. [DOI] [PubMed] [Google Scholar]
- 111.Fellner SK, Arendshorst WJ. Complex interactions of NO/cGMP/PKG systems on Ca2+ signaling in afferent arteriolar vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2010;298(1):H144–51. doi: 10.1152/ajpheart.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274(16):10927–35. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 113.Nara M, Dhulipala PD, Ji GJ, Kamasani UR, Wang YX, Matalon S, Kotlikoff MI. Guanylyl cyclase stimulatory coupling to K(Ca) channels. Am J Physiol Cell Physiol. 2000;279(6):C1938–45. doi: 10.1152/ajpcell.2000.279.6.C1938. [DOI] [PubMed] [Google Scholar]
- 114.Klyachko VA, Ahern GP, Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron. 2001;31(6):1015–25. doi: 10.1016/s0896-6273(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 115.Stanfield PR, Nakajima S, Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol. 2002:14547–179. doi: 10.1007/BFb0116431. [DOI] [PubMed] [Google Scholar]
- 116.Gregerson KA, Flagg TP, O’Neill TJ, Anderson M, Lauring O, Horel JS, Welling PA. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142(7):2820–32. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- 117.Wulfsen I, Hauber HP, Schiemann D, Bauer CK, Schwarz JR. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J Neuroendocrinol. 2000;12 (3):263–72. doi: 10.1046/j.1365-2826.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- 118.Kuzhikandathil EV, Yu W, Oxford GS. Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Mol Cell Neurosci. 1998;12(6):390–402. doi: 10.1006/mcne.1998.0722. [DOI] [PubMed] [Google Scholar]
- 119.Einhorn LC, Gregerson KA, Oxford GS. D2 dopamine receptor activation of potassium channels in identified rat lactotrophs: whole-cell and single-channel recording. J Neurosci. 1991;11(12):3727–37. doi: 10.1523/JNEUROSCI.11-12-03727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tomic M, Van Goor F, He ML, Zivadinovic D, Stojilkovic SS. Ca(2+)-mobilizing endothelin-A receptors inhibit voltage-gated Ca(2+) influx through G(i/o) signaling pathway in pituitary lactotrophs. Mol Pharmacol. 2002;61(6):1329–39. doi: 10.1124/mol.61.6.1329. [DOI] [PubMed] [Google Scholar]
- 121.Sims SM, Lussier BT, Kraicer J. Somatostatin activates an inwardly rectifying K+ conductance in freshly dispersed rat somatotrophs. J Physiol. 1991:441615–37. doi: 10.1113/jphysiol.1991.sp018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tomic M, Zivadinovic D, Van Goor F, Yuan D, Koshimizu T, Stojilkovic SS. Expression of Ca(2+)-mobilizing endothelin(A) receptors and their role in the control of Ca(2+) influx and growth hormone secretion in pituitary somatotrophs. J Neurosci. 1999;19(18):7721–31. doi: 10.1523/JNEUROSCI.19-18-07721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas P, Smith PA. Tetrabutylammonium: a selective blocker of the somatostatin-activated hyperpolarizing current in mouse AtT-20 corticotrophs. Pflugers Arch. 2001;441(6):816–23. doi: 10.1007/s004240000488. [DOI] [PubMed] [Google Scholar]
- 124.Kozasa T, Kaziro Y, Ohtsuka T, Grigg JJ, Nakajima S, Nakajima Y. G protein specificity of the muscarine-induced increase in an inward rectifier potassium current in AtT-20 cells. Neurosci Res. 1996;26 (3):289–97. doi: 10.1016/s0168-0102(96)01111-x. [DOI] [PubMed] [Google Scholar]
- 125.Tallent M, Dichter MA, Reisine T. Evidence that a novel somatostatin receptor couples to an inward rectifier potassium current in AtT-20 cells. Neuroscience. 1996;73(3):855–64. doi: 10.1016/0306-4522(96)00079-6. [DOI] [PubMed] [Google Scholar]
- 126.Takano K, Yasufuku-Takano J, Kozasa T, Nakajima S, Nakajima Y. Different G proteins mediate somatostatin-induced inward rectifier K+ currents in murine brain and endocrine cells. J Physiol. 1997;502(Pt 3):559–67. doi: 10.1111/j.1469-7793.1997.559bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takano K, Yasufuku-Takano J, Teramoto A, Fujita T. Gi3 mediates somatostatin-induced activation of an inwardly rectifying K+ current in human growth hormone-secreting adenoma cells. Endocrinology. 1997;138(6):2405–9. doi: 10.1210/endo.138.6.5185. [DOI] [PubMed] [Google Scholar]
- 128.Charles AC, Piros ET, Evans CJ, Hales TG. L-type Ca2+ channels and K+ channels specifically modulate the frequency and amplitude of spontaneous Ca2+ oscillations and have distinct roles in prolactin release in GH3 cells. J Biol Chem. 1999;274(11):7508–15. doi: 10.1074/jbc.274.11.7508. [DOI] [PubMed] [Google Scholar]
- 129.Barros F, Villalobos C, Garcia-Sancho J, del Camino D, de la Pena P. The role of the inwardly rectifying K+ current in resting potential and thyrotropin-releasing-hormone-induced changes in cell excitability of GH3 rat anterior pituitary cells. Pflugers Arch. 1994;426(3–4):221–30. doi: 10.1007/BF00374775. [DOI] [PubMed] [Google Scholar]
- 130.Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270(11):5691–4. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 131.Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. J Physiol. 1997;502(Pt 2):265–79. doi: 10.1111/j.1469-7793.1997.265bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu R, Zhao Y, Chen C. Growth hormone-releasing peptide-2 reduces inward rectifying K+ currents via a PKA-cAMP-mediated signalling pathway in ovine somatotropes. J Physiol. 2002;545(Pt 2):421–33. doi: 10.1113/jphysiol.2002.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barros F, del Camino D, Pardo LA, Palomero T, Giraldez T, de la Pena P. Demonstration of an inwardly rectifying K+ current component modulated by thyrotropin-releasing hormone and caffeine in GH3 rat anterior pituitary cells. Pflugers Arch. 1997;435(1):119–29. doi: 10.1007/s004240050491. [DOI] [PubMed] [Google Scholar]
- 134.Corrette BJ, Bauer CK, Schwarz JR. An inactivating inward-rectifying K current present in prolactin cells from the pituitary of lactating rats. J Membr Biol. 1996;150(2):185–95. doi: 10.1007/s002329900043. [DOI] [PubMed] [Google Scholar]
- 135.Dedman A, Sharif-Naeini R, Folgering JH, Duprat F, Patel A, Honore E. The mechano-gated K(2P) channel TREK-1. Eur Biophys J. 2009;38(3):293–303. doi: 10.1007/s00249-008-0318-8. [DOI] [PubMed] [Google Scholar]
- 136.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29(11):566–75. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee AK, Smart JL, Rubinstein M, Low MJ, Tse A. Reciprocal regulation of TREK-1 channels by arachidonic acid and CRH in mouse corticotropes. Endocrinology. 2011;152(5):1901–10. doi: 10.1210/en.2010-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000:16521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 139.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 140.Mudado MA, Rodrigues AL, Prado VF, Beirao PS, Cruz JS. CaV 3.1 and CaV 3.3 account for T-type Ca2+ current in GH3 cells. Braz J Med Biol Res. 2004;37(6):929–35. doi: 10.1590/s0100-879x2004000600020. [DOI] [PubMed] [Google Scholar]
- 141.Glassmeier G, Hauber M, Wulfsen I, Weinsberg F, Bauer CK, Schwarz JR. Ca2+ channels in clonal rat anterior pituitary cells (GH3/B6) Pflugers Arch. 2001;442(4):577–87. doi: 10.1007/s004240100567. [DOI] [PubMed] [Google Scholar]
- 142.Fiordelisio T, Jimenez N, Baba S, Shiba K, Hernandez-Cruz A. Immunoreactivity to neurofilaments in the rodent anterior pituitary is associated with the expression of alpha 1A protein subunits of voltage-gated Ca2+ channels. J Neuroendocrinol. 2007;19(11):870–81. doi: 10.1111/j.1365-2826.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 143.Marchetti C, Childs GV, Brown AM. Membrane currents of identified isolated rat corticotropes and gonadotropes. Am J Physiol. 1987;252(3 Pt 1):E340–6. doi: 10.1152/ajpendo.1987.252.3.E340. [DOI] [PubMed] [Google Scholar]
- 144.Stutzin A, Stojilkovic SS, Catt KJ, Rojas E. Characteristics of two types of calcium channels in rat pituitary gonadotrophs. Am J Physiol. 1989;257(5 Pt 1):C865–74. doi: 10.1152/ajpcell.1989.257.5.C865. [DOI] [PubMed] [Google Scholar]
- 145.Tse A, Hille B. Role of voltage-gated Na+ and Ca2+ channels in gonadotropin-releasing hormone-induced membrane potential changes in identified rat gonadotropes. Endocrinology. 1993;132(4):1475–81. doi: 10.1210/endo.132.4.8384989. [DOI] [PubMed] [Google Scholar]
- 146.Mason WT, Sikdar SK. Characteristics of voltage-gated Ca2+ currents in ovine gonadotrophs. J Physiol. 1989:415367–91. doi: 10.1113/jphysiol.1989.sp017726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Goor F, Goldberg JI, Chang JP. Electrical membrane properties and ionic currents in cultured goldfish gonadotrophs. Can J Physiol Pharmacol. 1996;74(6):729–43. [PubMed] [Google Scholar]
- 148.Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701–11. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- 149.Bosma MM, Hille B. Electrophysiological properties of a cell line of the gonadotrope lineage. Endocrinology. 1992;130(6):3411–20. doi: 10.1210/endo.130.6.1317783. [DOI] [PubMed] [Google Scholar]
- 150.Lewis DL, Goodman MB, St John PA, Barker JL. Calcium currents and fura-2 signals in fluorescence-activated cell sorted lactotrophs and somatotrophs of rat anterior pituitary. Endocrinology. 1988;123(1):611–21. doi: 10.1210/endo-123-1-611. [DOI] [PubMed] [Google Scholar]
- 151.Yu Y, Ali DW, Chang JP. Characterization of ionic currents and electrophysiological properties of goldfish somatotropes in primary culture. Gen Comp Endocrinol. 2010;169(3):231–43. doi: 10.1016/j.ygcen.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 152.Zhang H, Langeslag M, Breukels V, Jenks BG, Roubos EW, Scheenen WJ. Calcium channel kinetics of melanotrope cells in Xenopus laevis depend on environmental stimulation. Gen Comp Endocrinol. 2008;156(1):104–12. doi: 10.1016/j.ygcen.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 153.Kwiecien R, Robert C, Cannon R, Vigues S, Arnoux A, Kordon C, Hammond C. Endogenous pacemaker activity of rat tumour somatotrophs. J Physiol. 1998;508(Pt 3):883–905. doi: 10.1111/j.1469-7793.1998.883bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simasko SM, Weiland GA, Oswald RE. Pharmacological characterization of two calcium currents in GH3 cells. Am J Physiol. 1988;254(3 Pt 1):E328–36. doi: 10.1152/ajpendo.1988.254.3.E328. [DOI] [PubMed] [Google Scholar]
- 155.Kunze DL, Ritchie AK. Multiple conductance levels of the dihydropyridine-sensitive calcium channel in GH3 cells. J Membr Biol. 1990;118(2):171–8. doi: 10.1007/BF01868474. [DOI] [PubMed] [Google Scholar]
- 156.Ritchie AK. Estrogen increases low voltage-activated calcium current density in GH3 anterior pituitary cells. Endocrinology. 1993;132(4):1621–9. doi: 10.1210/endo.132.4.8462461. [DOI] [PubMed] [Google Scholar]
- 157.Kuryshev YA, Childs GV, Ritchie AK. Three high threshold calcium channel subtypes in rat corticotropes. Endocrinology. 1995;136(9):3916–24. doi: 10.1210/endo.136.9.7649100. [DOI] [PubMed] [Google Scholar]
- 158.Li YX, Rinzel J, Vergara L, Stojilkovic SS. Spontaneous electrical and calcium oscillations in unstimulated pituitary gonadotrophs. Biophys J. 1995;69(3):785–95. doi: 10.1016/S0006-3495(95)79952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen C, Israel JM, Vincent JD. Electrophysiological responses to somatostatin of rat hypophysial cells in somatotroph-enriched primary cultures. J Physiol. 1989:408493–510. doi: 10.1113/jphysiol.1989.sp017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen C, Clarke IJ. Modulation of Ca2+ influx in the ovine somatotroph by growth hormone-releasing factor. Am J Physiol. 1995;268(2 Pt 1):E204–12. doi: 10.1152/ajpendo.1995.268.2.E204. [DOI] [PubMed] [Google Scholar]
- 161.Takei T, Takano K, Yasufuku-Takano J, Fujita T, Yamashita N. Enhancement of Ca2+ currents by GHRH and its relation to PKA and [Ca2+]i in human GH-secreting adenoma cells. Am J Physiol. 1996;271(5 Pt 1):E801–7. doi: 10.1152/ajpendo.1996.271.5.E801. [DOI] [PubMed] [Google Scholar]
- 162.Chen C, Clarke IJ. Effects of growth hormone-releasing peptide-2 (GHRP-2) on membrane Ca2+ permeability in cultured ovine somatotrophs. J Neuroendocrinol. 1995;7(3):179–86. doi: 10.1111/j.1365-2826.1995.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 163.Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79(2):511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- 164.Xie J, Nagle GT, Childs GV, Ritchie AK. Expression of the L-type Ca(2+) channel in AtT-20 cells is regulated by cyclic AMP. Neuroendocrinology. 1999;70(1):1–9. doi: 10.1159/000054454. [DOI] [PubMed] [Google Scholar]
- 165.Dominguez B, Avila T, Flores-Hernandez J, Lopez-Lopez G, Martinez-Rodriguez H, Felix R, Monjaraz E. Up-regulation of high voltage-activated Ca(2+) channels in GC somatotropes after long-term exposure to ghrelin and growth hormone releasing peptide-6. Cell Mol Neurobiol. 2008;28(6):819–31. doi: 10.1007/s10571-007-9234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 167.Schwab Y, Jahke R, Jover E. Expression of tetrodotoxin-sensitive and resistant sodium channels by rat melanotrophs. Neuroreport. 2004;15(7):1219–23. doi: 10.1097/00001756-200405190-00028. [DOI] [PubMed] [Google Scholar]
- 168.Vega AV, Espinosa JL, Lopez-Dominguez AM, Lopez-Santiago LF, Navarrete A, Cota G. L-type calcium channel activation up-regulates the mRNAs for two different sodium channel alpha subunits (Nav1.2 and Nav1.3) in rat pituitary GH3 cells. Brain Res Mol Brain Res. 2003;116(1–2):115–25. doi: 10.1016/s0169-328x(03)00279-1. [DOI] [PubMed] [Google Scholar]
- 169.Morinville A, Fundin B, Meury L, Jureus A, Sandberg K, Krupp J, Ahmad S, O’Donnell D. Distribution of the voltage-gated sodium channel Na(v)1.7 in the rat: expression in the autonomic and endocrine systems. J Comp Neurol. 2007;504(6):680–9. doi: 10.1002/cne.21484. [DOI] [PubMed] [Google Scholar]
- 170.Yang SK, Wang K, Parkington H, Chen C. Involvement of tetrodotoxin-resistant Na+ current and protein kinase C in the action of growth hormone (GH)-releasing hormone on primary cultured somatotropes from GH-green fluorescent protein transgenic mice. Endocrinology. 2008;149(9):4726–35. doi: 10.1210/en.2008-0405. [DOI] [PubMed] [Google Scholar]
- 171.Kehl SJ. Voltage-clamp analysis of the voltage-gated sodium current of the rat pituitary melanotroph. Neurosci Lett. 1994;165(1–2):67–70. doi: 10.1016/0304-3940(94)90711-0. [DOI] [PubMed] [Google Scholar]
- 172.Valentijn JA, Valentijn K. Two distinct Na+ currents control cytosolic Ca2+ pulsing in Xenopus laevis pituitary melanotrophs. Cell Calcium. 1997;21(3):241–51. doi: 10.1016/s0143-4160(97)90048-8. [DOI] [PubMed] [Google Scholar]
- 173.Galas L, Garnier M, Lamacz M. Calcium waves in frog melanotrophs are generated by intracellular inactivation of TTX-sensitive membrane Na+ channel. Mol Cell Endocrinol. 2000;170(1–2):197–209. doi: 10.1016/s0303-7207(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 174.Waring DW, Turgeon JL. Estradiol inhibition of voltage-activated and gonadotropin-releasing hormone-induced currents in mouse gonadotrophs. Endocrinology. 2006;147(12):5798–805. doi: 10.1210/en.2006-1112. [DOI] [PubMed] [Google Scholar]
- 175.Heyward PM, Chen C, Clarke IJ. Inward membrane currents and electrophysiological responses to GnRH in ovine gonadotropes. Neuroendocrinology. 1995;61(6):609–21. doi: 10.1159/000126887. [DOI] [PubMed] [Google Scholar]
- 176.Mason WT, Sikdar SK. Characterization of voltage-gated sodium channels in ovine gonadotrophs: relationship to hormone secretion. J Physiol. 1988:399493–517. doi: 10.1113/jphysiol.1988.sp017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Price CJ, Goldberg JI, Chang JP. Voltage-activated ionic currents in goldfish pituitary cells. Gen Comp Endocrinol. 1993;92(1):16–30. doi: 10.1006/gcen.1993.1139. [DOI] [PubMed] [Google Scholar]
- 178.Tiwari JK, Sikdar SK. Voltage gated Na+ channels contribute to membrane voltage fluctuation in alphaT3–1 pituitary gonadotroph cells. Neurosci Lett. 1998;242(3):167–71. doi: 10.1016/s0304-3940(98)00046-9. [DOI] [PubMed] [Google Scholar]
- 179.Horta J, Hiriart M, Cota G. Differential expression of Na channels in functional subpopulations of rat lactotropes. Am J Physiol. 1991;261(5 Pt 1):C865–71. doi: 10.1152/ajpcell.1991.261.5.C865. [DOI] [PubMed] [Google Scholar]
- 180.Sankaranarayanan S, Simasko SM. A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. Am J Physiol. 1996;271(6 Pt 1):C1927–34. doi: 10.1152/ajpcell.1996.271.6.C1927. [DOI] [PubMed] [Google Scholar]
- 181.Avila G, Monjaraz E, Espinosa JL, Cota G. Downregulation of voltage-gated sodium channels by dexamethasone in clonal rat pituitary cells. Neurosci Lett. 2003;339(1):21–4. doi: 10.1016/s0304-3940(02)01460-x. [DOI] [PubMed] [Google Scholar]
- 182.Xu SH, Cooke IM. Voltage-gated currents of tilapia prolactin cells. Gen Comp Endocrinol. 2007;150(2):219–32. doi: 10.1016/j.ygcen.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 183.Cobbett P, Ingram CD, Mason WT. Sodium and potassium currents involved in action potential propagation in normal bovine lactotrophs. J Physiol. 1987:392273–99. doi: 10.1113/jphysiol.1987.sp016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kato M, Suzuki M. Growth hormone releasing factor depolarizes rat pituitary cells in Na+-dependent mechanism. Brain Res. 1989;476(1):145–8. doi: 10.1016/0006-8993(89)91547-3. [DOI] [PubMed] [Google Scholar]
- 185.Kato M. Growth hormone-releasing hormone augments voltage-gated Na+ current in cultured rat pituitary cells. Am J Physiol. 1996;270(1 Pt 1):C125–30. doi: 10.1152/ajpcell.1996.270.1.C125. [DOI] [PubMed] [Google Scholar]
- 186.Murakami Y, Tanaka J, Koshimura K, Kato Y. Involvement of tetrodoxin-sensitive sodium channels in rat growth hormone secretion induced by pituitary adenylate cyclase-activating polypeptide (PACAP) Regul Pept. 1998;73(2):119–21. doi: 10.1016/s0167-0115(97)01075-6. [DOI] [PubMed] [Google Scholar]
- 187.Wu SN, Li HF, Jan CR. Regulation of Ca2+-activated nonselective cationic currents in rat pituitary GH3 cells: involvement in L-type Ca2+ current. Brain Res. 1998;812(1–2):133–41. doi: 10.1016/s0006-8993(98)00964-0. [DOI] [PubMed] [Google Scholar]
- 188.Vergara L, Rojas E, Stojilkovic SS. A novel calcium-activated apamin-insensitive potassium current in pituitary gonadotrophs. Endocrinology. 1997;138(7):2658–64. doi: 10.1210/endo.138.7.5220. [DOI] [PubMed] [Google Scholar]
- 189.Clapham DE, Julius D, Montell C, Schultz G International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57(4):427–50. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 190.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26(3):159–78. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 191.Yamashita M, Oki Y, Iino K, Hayashi C, Yogo K, Matsushita F, Sasaki S, Nakamura H. The role of store-operated Ca2+ channels in adrenocorticotropin release by rat pituitary cells. Regul Pept. 2009;156(1–3):57–64. doi: 10.1016/j.regpep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 192.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem. 2002;277(14):12302–9. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- 193.Takano K, Takei T, Teramoto A, Yamashita N. GHRH activates a nonselective cation current in human GH-secreting adenoma cells. Am J Physiol. 1996;270(6 Pt 1):E1050–7. doi: 10.1152/ajpendo.1996.270.6.E1050. [DOI] [PubMed] [Google Scholar]
- 194.Nakajima T, Iwasawa K, Hazama H, Asano M, Okuda Y, Omata M. Extracellular Mg2+ inhibits receptor-mediated Ca(2+)-permeable non-selective cation currents in aortic smooth muscle cells. Eur J Pharmacol. 1997;320(1):81–6. doi: 10.1016/s0014-2999(96)00873-4. [DOI] [PubMed] [Google Scholar]
- 195.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87(3):965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 196.Hanoune J, Pouille Y, Tzavara E, Shen T, Lipskaya L, Miyamoto N, Suzuki Y, Defer N. Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Mol Cell Endocrinol. 1997;128(1–2):179–94. doi: 10.1016/s0303-7207(97)04013-6. [DOI] [PubMed] [Google Scholar]
- 197.Zippin JH, Levin LR, Buck J. CO(2)/HCO(3)(−)-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab. 2001;12(8):366–70. doi: 10.1016/s1043-2760(01)00454-4. [DOI] [PubMed] [Google Scholar]
- 198.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011;79(12):1277–88. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52(3):375–414. [PubMed] [Google Scholar]
- 200.Garbers DL. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 201.Yuen PS, Garbers DL. Guanylyl cyclase-linked receptors. Annu Rev Neurosci. 1992:15193–225. doi: 10.1146/annurev.ne.15.030192.001205. [DOI] [PubMed] [Google Scholar]
- 202.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int. 2004;45(6):813–9. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 203.Kostic TS, Tomic M, Andric SA, Stojilkovic SS. Calcium-independent and cAMP-dependent modulation of soluble guanylyl cyclase activity by G protein-coupled receptors in pituitary cells. J Biol Chem. 2002;277(19):16412–8. doi: 10.1074/jbc.M112439200. [DOI] [PubMed] [Google Scholar]
- 204.Stojilkovic SS. Cyclic nucleotides and their regulation. In: Izzo JL, Sica DA, Black HR, editors. The Hypertension Primer. Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 205.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990:59971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 206.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–63. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22(8):307–12. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]